Abstract—
Materials collected in different seasons in the region of the Yenisei estuary, as well as previously published data, were used to analyze the scale and patterns of the seasonal variability of microbial communities. In all seasons concerned, three zones could be distinguished in the estuarine area based on the salinity values. The abundance and activity of bacterioplankton in these zones varied by an order of magnitude. In waters with salinities less than 2.5 PSU, the mean bacterial abundance values were 254 × 103, (2741 ± 394) × 103, and (2069 ± 185) × 103 cells/mL in spring, summer, and fall, respectively. At salinities greater than 18 PSU, the bacterial abundance in the same seasons was (196 ± 50) × 103, (683 ± 184) × 103, and (125 ± 24) × 103 cells/mL, respectively. Similar patterns were observed in the biomass distribution. Production and activity characteristics were unstable, with maximum values observed in the summer period. Within the zones identified by salinity indices, the distribution of bacterial abundance was apparently associated with small-scale patchiness in the distribution of organic matter, phytoplankton, and SPM. The contribution of the heterotrophic component of the microbial community was dominant in early spring. Despite the observed correlations between bacterial abundance and activity, phytoplankton, and organic carbon, their distribution patterns differed along the salinity gradient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Although ongoing climate change and advances in exploration of shelf regions have spurred growing interest in microbial communities in Arctic seas, there is currently no general consensus on the dynamics of microorganism growth and factors that affect it. Nevertheless, under conditions of strongly pronounced seasonal changes, prolonged polar night, and strict spatial and temporal limitations on phytoplankton vegetation typical of Siberian Arctic seas, the microbial loop [18], and bacterioplankton, in particular, is a central element of the pelagic community. Estuaries are regions characterized by high activity of Arctic microbial communities. Although the volume of the Arctic Ocean constitutes only 1% of the total volume of the World Ocean, it annually receives nearly 11% of global river discharge [20]. The Kara Sea receives 1300–1400 km3 of fresh water per year, which is 41% of the total freshwater flow into the Arctic Ocean [7]. In particular, the Gulf of Ob and the Yenisei River account for 82% of this amount [32]; the average runoff volume of the Yenisei is 630 km3 per year [31]. That is, processes occurring in the river–sea mixing zone affect not only coastal ecosystems, but also the entire Arctic Basin [28]. The runoff of Siberian rivers, in particular, the Yenisei, exhibits pronounced seasonal variation. From May to June, it discharges approximately 284 km3 of water, which is 45% of the total annual runoff and affects the amounts of organic matter and nutrients delivered to the estuary [22].
Bacterioplankton plays a central role in the remineralization of nutrients and transformation of organic matter even in low-temperature environments [16, 25]. Plankton microbial activity is a key mechanism determining the amount and composition of matter that flows into the sea or sediments in the marginal filter zone [6]. However, data available for Siberian Arctic seas on the microbial distribution patterns in the river–sea mixing zone, their seasonal development dynamics, and effects of abiotic factors and other components of planktonic communities is rather scarce [see, e.g., 3, 11, 14, 29, 34]. The goal of the present study was to assess the scale and patterns of seasonal variation in microbial communities of the Yenisei estuary based on data obtained in original field research and previous publications.
MATERIALS AND METHODS
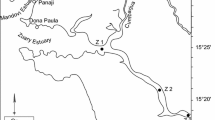
Materials used in this study were collected during three expeditions: cruise 59 of the R/V Akademik Mstislav Keldysh on September 17–22, 2011; a cruise of the ice-class containership Norilsk Nikel from March 29 to April 4, 2016; and cruise 66 of the R/V Akademik Mstislav Keldysh on July 25–28, 2016 (Fig. 1). Water samples were collected with Niskin bottles from horizons selected according to the hydrophysical profile determined by an SBE 911 Plus (SeaBird Electronics, United States) or from the surface water layer (March–April 2016).
Quantification of bacterioplankton. To determine the total bacterial abundance and bacterioplankton biomass, water samples were fixed immediately after collection by adding neutral formaldehyde solution to a final concentration of 1% and stored until further analysis. In fall 2011 and spring 2016, the bacterial abundance was determined by fluorescence microscopy (Leica DM 5000B) using DAPI staining on black Nucleopore filters with 0.17-µm pores [33] at least 3 months after sampling [38]. In summer 2016, the bacterial abundance was quantified by flow cytometry. Samples were stained with SYBR GREEN I fluorescent dye for 15 min and analyzed on a BD Accuri C6 cytometer within a day after collection [24]. To improve the accuracy of bacterial abundance estimates, samples filtered through 0.17-µm filters were also analyzed. The cytometry results were used to calculate the proportion of cells with high and low nucleic acid content, which presumably describes the active cell fraction [27]. Linear cell sizes were measured using ImageScopeColor М software. Bacterial biomass was calculated in carbon units as proposed in [12].
Bacterioplankton production. Bacterial production and bacterioplankton consumption by grazers were evaluated with direct method using antibiotics to inhibit bacterial growth [35] in a modification proposed for natural habitats [39]. Immediately after collection, water samples were distributed into 100-mL flasks. To estimate bacterioplankton grazing by nano- and micrograzers, water samples were supplemented with antibiotics (benzylpenicillin to 1 mg/L and vancomycin to 200 mg/L), which suppress bacteria growth but do not affect their consumers [35]. Control samples were incubated without antibiotics. The prepared samples were incubated for 8–10 h at surface water temperature. The time of exposition was selected based on the results of previous experiments that determined the dynamics of antibiotic effects in polar waters. All experiments were performed in two replicates. Correlations between the characteristics of the communities were evaluated using Spearman’s rank correlation coefficient. Factors that affect the parameters of the microbial community were identified by forward stepwise multiple regression analysis (P < 0.05). Hereinafter, we denote the pairwise correlation coefficient as r and the multiple correlation coefficient as R. The calculations were performed using PAST 3.14 and STATISTICA 6.0.
RESULTS
In early spring 2016, the bacterial abundance in the surface water layer along the Yenisei estuary profile ranged from 149 to 354 × 103 cells/mL, with mean values of (196 ± 50) × 103 cells/mL in the marine zone (at salinities of >25 PSU) and (314 ± 60) × 103 cells/mL in waters with salinities less than 18 PSU (Fig. 2). At the same time, no vectored change along the salinity gradient could be observed within either zone: the total bacterial abundance ranged from 149 to 268 ×103 cells/mL at salinities higher than 25 PSU, and from 254 to 354 × 103 cells/mL at lower salinities. The average bacterial biomass in these two regions also differed nearly twofold: 2.7 ± 0.68 mgC/m3 and 5.13 ± 1.44 mgC/m3 in the marine and river part of the transect, respectively. The variation of specific growth rate along the profile was more than fivefold; in comparison to other seasons, the mean production level was fairly high: 0.79 ± 0.43 day–1. However, in the zone with the highest salinity gradient, bacterial production was close to zero, while the total bacterial abundance was relatively high.
In the summer of 2016, bacterial abundance in the surface layer ranged from 3.1 × 106 cells/mL in the Yenisei estuary to 214 × 103 cells/mL in the marine part of the transect (Fig. 3). The distribution of bacterial abundance in the surface layer correlated with the chlorophyll concentration (r = 0.8, P < 0.05) but did not correspond to the areas of distribution of phytoplankton species complexes or to variations of phytoplankton abundance [15]. The maximum and minimum values of bacterial abundance were observed in the surface water layer. Bacterial abundance (except in the bottom water layer) correlated with the salinity profile (r = –0.84, P < 0.05) and exhibited discontinuous variation: the average bacterial abundance was (2741 ± 394), (1805 ± 333), and (683 ± 184) ×103 cells/mL in salinity ranges of <1.5, 2.5–15, and >18 PSU, respectively.
The bottom layer of saline water to the south of St. 5333, which reaches upstream the Yenisei estuary, is worthy special discussion. At salinities greater than 21 PSU, the bacterial abundance in this layer exhibited little variation within the range of 1025–1211 ×103 cells/mL, with a mean value of (1152 ± 104) ×103 cells/mL. Considering that the relative dissolved oxygen content was rather low (73 ± 2%), it seems likely that these waters are characterized by active transformation of organic matter carried by river runoff.
Bacterial abundance in the bottom water layer at St. 5333 and seawards did not exceed 416 × 103 cells/mL and decreased towards the shelf edge, with a mean of (333 ± 73) ×103 cells/mL. The mean level of water saturation with oxygen was 83 ± 4%.
The share of cells with a high nucleic acid content ranged from 17 to 83% of the total bacterial abundance. It was the lowest in freshened waters and the highest at northern shelf stations of the transect with salinity levels greater than 30 PSU. In the surface water layer, the share of cells with a high nucleic acid content decreased with increasing pheophytin concentration (r = –0.65, P < 0.05).
Bacterial biomass in the surface layer ranged from 1.56 to 18.25 mgC/m3. The highest and lowest bacterial biomass values along the Yenisei estuary profile were observed in the surface water layer. The distribution of average bacterial biomass in the water column did not exhibit significant differences among the salinity ranges: 5.26, 9.56 ± 2.91, and 4.32 ± 1.51 mgC/m3 for waters with salinities of less than 1.5, 2.5 to 15, and more than 18 PSU, respectively. In the bottom layer of saline water, bacterioplankton biomass was 5.61 ± 2.87 and 2.12 ± 0.37 mgC/m3 to the south and to north of St. 5333, respectively.
Levels of specific growth rate ranged broadly from values below the detection limit of the method to 11.7 day–1. There was no common pattern in their distribution along the transect. However, at the southernmost station, this parameter decreased with depth (from 11.7 to 1.1 day–1), whereas at seaward sites, the pattern was the opposite: the level of specific growth rate increased from 1 day–1 in the surface layer to 3.2 day–1 in the bottom layer.
Bacterial abundance in the surface water layer observed in fall 2011 also decreased from 2.2 × 106 cells/mL in the river to 143 × 103 cells/mL in the shelf part of the Yenisei estuarine zone (Fig. 4).
Distribution of bacterial abundance (N, total abundance, ×103 cells/mL; B, biomass, mgC/m3) and share of actively respiring cells (CTCpositive cells, %; according to [9]) on the transect along the Yenisei estuarine zone, September 2016.
Bacterial abundance levels along the transect varied by an order of magnitude depending on salinity. For instance, at the southernmost station, where the salinity was below 1 PSU, this level was (2069 ± 185) ×103 cells/mL, while at salinities greater than 26 PSU, the average bacterial abundance decreased to (125 ± 24) ×103 cells/mL. In the salinity range of 5–17 PSU, bacterial abundance decreased from 1045 to 440 ×103 cells/mL. A similar pattern was observed for biomass values. At the southernmost station of the profile (salinity <1 PSU), this parameter decreased with depth from 47.5 to 23.6 mgC/m3. In contrast to the abundance values, it remained stable with increasing salinity in the range of 5–17 PSU, averaging 13.9 ± 3.2 mgC/m3. At salinities greater than 26 PSU, the mean biomass was 4.8 ± 1 mgC/m3. The highest production values were observed at the southernmost river site of the transect (salinity <0.1 PSU). The specific growth rate exhibited little variation with depth (1.89–2.18 day–1). At most northern sites, this parameter dropped below the detection limit of the method and did not exceed 0.45 day–1.
DISCUSSION
As illustrated by the annual chart of river discharge [21] (Fig. 5), water samples were collected before the seasonal high flood, immediately after it, and in the fall period characterized by relatively low freshwater runoff. Quantification of bacterioplankton in the surface layer indicated that major seasonal variations occurred in the freshened area of the estuarine zone. In summer, the bacterial abundance in the freshened zone (salinity <15 PSU) increased by nearly an order of magnitude in comparison to spring levels, whereas in the marine area, the bacterial abundance remained stable during all three seasons.
Annual variation of daily discharge in Yenisei estuary in 2011 and 2016 [21].
To analyze the data on the distribution of bacterial abundance and activity based on conditionally defined salinity ranges, it is important to take into account that waters of different salinity are associated with different depths. Nevertheless, in both summer and in fall, conditionally defined freshened and marine waters were present at different stations of the transect both in the upper photic layer and in the layer below the pycnocline, while the values of bacterial abundance were similar irrespective of depth.
In spring 2016, the zone of a twofold decrease in bacterial abundance coincided with an area with an elevated share of autotrophic organisms in the microplankton community [30]. In the conventional fluvial zone (2.6–14.8 PSU), the share of autotrophic microplankton was only 23%, whereas outside the river estuary (27–31 PSU), it was 51% on average [30]. This supports the notion that the microbial loop is strongly involved in the functioning of the estuarine microbial community in early spring.
In summer 2016, the bacterial abundance decreased with depth, increasing salinity, and decreasing relative dissolved oxygen concentration (R2 = 0.88, P < 0.05). In contrast, the share of cells with a high nucleic acid content increased with depth, salinity, and dissolved oxygen concentration (R2 = 0.52, P < 0.05). No specific pattern in the distribution of bacterial production could be identified.
According to [9], in the samples collected in fall 2011, the share of bacteria with an active electron transport chain ranged from 1.8 to 78.4% of the total bacterial abundance. In the Yenisei estuary, it was 17.4% of the total abundance on average, whereas on the shelf, where the absolute values of bacterial abundance were low, it reached 36%. The average share of potentially viable cells (cells with intact membranes) along the transect was 89.5% of the total bacterial abundance; it was lowest in the Yenisei mouth: 71.8–88.9% [9]. Although just a few sites were available for analysis, it could be observed that the distribution of bacterial biomass correlated with water temperature in waters with salinities greater than 5 PSU (r = 0.63, P < 0.05), while the share of actively respiring cells declined as the share of phaeophytin increased (r = –0.65, P < 0.05).
According to the data from spring 2016 [26], the number of bacterial cells with attached viral particles ranged from 27 to 110 × 103 cells/mL, and their share in the total bacterial abundance increased from 14.4% in the river part of the transect to 24.3% in the estuary and dropped to 7.6% at the outmost marine station of the transect. Bacterial viral-induced mortality rate varied from 2 to 18% of bacterial production.
At the end of the vegetation season, the highest number of cells with attached viral particles was observed in the Yenisei river: 293–331 ×103 cells/mL; in the estuary region, these values decreased to 11–139 ×103 cells/mL [5]. However, the difference in their shares in the total bacterial abundance was less pronounced: 13.2–16.5% vs. 8.5–15.6%. The rate of viral-induced mortality of bacterioplankton was 9.5–16.1 and 1.4–9.5% of total bacterial production in the river and the estuary zone, respectively [5]. That is, the contribution of bacteriophage viruses to regulation of the bacterial abundance and production in different seasons was relatively low.
Data obtained on the August–September expedition 2001 also confirmed the correlation between bacterial abundance in the surface layer of the Yenisei and the adjacent shelf and salinity [29]. The mean values of bacterial abundance were 1.5 × 106 cells/mL in the river, 1.8 × 106 cells/mL in the estuary (salinity of 5–15 PSU), and decreased to 460 × 103 cells/mL in marine waters with salinities greater than 25 PSU. A similar pattern was observed for bacterial production in the surface layer: 11.27, 6.83, and 3.31 mgC/m3 per day in the river, estuary, and marine part of the transect, respectively [29]. In the bottom layer, the production levels were 25% lower than on the surface. It was found that bacterial production correlated with the chlorophyll a concentration and surface water temperature. Experiments showed that addition of glucose caused an increase in bacterial production by 17–43%, while addition of filtered river water did not stimulate bacterial production. This probably suggests that the growth of bacterioplankton in late summer is limited by the availability of labile carbon sources [29].
In summer 2016, the quantitative distribution of bacterioplankton significantly correlated both with concentrations of dissolved organic carbon (DOC) (r = 0.8, P < 0.05) and with colloidal matter content (r = 0.75, P < 0.05) [2]. The DOC concentration decreased with decreasing salinity; in slightly freshened waters, its concentration exhibited considerable variation. It was found that relative water enrichment with DOC increased in the bottom layer. Despite this, the colloidal fraction was relatively low, indirectly suggesting that these waters had been enriched with DOC during an earlier period [2]. Spring and fall samples of surface waters were shown to contain predominantly terrigenous organic matter. In spring 2016, the colored DOC fraction in the surface water layer was mainly represented by humic substances of terrigenous origin [13]. Analysis of the carbon isotope ratio in water samples collected in fall 2011 also showed that the major share of SPM in the surface layer was of terrigenous origin, whereas the share of 13C isotope in the bottom layers increased due to autochthonous organic matter [4]. Despite few observations, it is possible to notice similarity in the distribution of bacterial biomass and DOC concentrations [1]. A study performed in the fall of 2001 also found that only 6–16% of dissolved organic matter in the estuary region was of planktonic origin [17].
Data on bacterial productivity and seasonal variations in other regions of the Kara Sea and Arctic estuaries are rather scarce. For example, bacterial abundance values in the Ob River estuary observed in fall 1993 were very low: 206 × 103 cells/mL in the river and 173 × 103 cells/mL in the shelf region [8]. In August–September 2001, the mean bacterial abundance in the surface water layer of the Ob estuary was 1.4, 2.1, and less than 0.5 × 106 cells/mL in fresh (<1 PSU), freshened (5–15 PSU), and marine (>20 PSU) waters, respectively [29]. Bacterial production values decreased seaward, averaging 7.14, 5.06, and 3.21 µgC L–1 day–1 in these three zones, respectively.
The bacterial abundance in water samples collected in the same region in fall 2007 was determined immediately after sampling [14] and after more than 3 months of storage [11] (accounting for potentially viable cells; [38]). In the first study, relatively high abundance values were recorded on the southern station of the profile at a salinity of 0.05 PSU (1950 and 3170 × 103 cells/mL in the surface and bottom layers, respectively), but no specific bacterioplankton distribution pattern was observed in the estuary region. The levels of bacterial abundance ranged from 700 to 2540 ×103 cells/mL in the surface horizon and from 580 to 3170 in the bottom layer, while the biomass values ranged from 11.9 to 33 mgC/m3 on the surface and 9 to 57.1 mgC/m3 in the bottom layer. The share of particle-associated bacteria increased with depth from 20 to 80% of the total abundance [14]. Analysis of the stored samples revealed a different pattern. In the layer above the pycnocline, the bacterial abundance was slightly lower in the river part of the transect with salinities less than 5 PSU: 505 ± 197 × 103 cells/mL vs. 723 ± 146 × 103 cells/mL in the marine part of the estuarine zone. A similar pattern was observed in the distribution of bacterial biomass: 14.2 ± 6.5 and 21.3 ± 3.8 mgC/m3, respectively. Below the pycnocline, the bacterial abundance dropped to 376 ± 64 × 103 cells/mL (8.1 ± 4.6 mgC/m3). Above the pycnocline, the level of specific growth rate was significantly higher in river waters than in the marine part of the estuarine zone: 0.54 and 0.18 day–1, respectively. Below the pycnocline, production levels were close to zero, and it was only in the bottom layer that specific growth rate reached 0.06 day–1 [11].
For comparison, in fall 1991, the bacterial abundance in the upper freshened water layer of Tiksi Bay (Laptev Sea), which receives fresh waters from the Lena River, exceeded 1.5 × 106 cells/mL. In the area unaffected by freshening, the bacterial abundance was only 200–400 × 103 cells/mL even in the surface layer and further decreased two- to fourfold below the pycnocline. The levels of specific growth rate in the Lena River and its delta in most cases did not exceed 0.1 day–1. In Tiksi Bay and in the open sea, they ranged from 0.08 to 0.3 day–1 [36].
For comparison, according to the data obtained in summer 2004 in the Mackenzie River estuary in the Canadian Arctic, the bacterial abundance ranged from 570 to 1360 × 103 cells/mL. The level of bacterial production was 0.82 mgC m–3 day–1 and did not differ significantly between the river, estuary, and marine zone, nor did it exhibit a correlation with the environmental factors. However, it was found that the contribution of particle-associated bacterial cells to the total production differed significantly between the zones, 74% in river water, but it decreased to 26% in the estuary and sea. The contribution of free-living bacteria decreased with increasing suspended organic matter content with particles larger than 3 µm [19]. Another study performed in the Mackenzie estuary in summer 2006 did not detect significant differences in bacterial abundance between the three zones determined by salinity, either in the surface or bottom layers [37]. The levels of bacterial production in the surface layer did not differ among the zones, while in the bottom layer of the transition zone, they were significantly higher and exceeded nearly twofold the production level in the surface layer. Further differences were observed for the contribution of particle-associated bacteria to the total production in the surface layer: this parameter decreased seaward from 94 to 31%. In summer samples, bacteria were found to respond readily to the addition of glucose. The effect was most pronounced in river waters: bacterioplankton production increased 3.9- and 2.5-fold in the river and the transition zone, respectively, but in the marine zone, the ratio of production levels did not differ significantly from 1 [37].
The currently available data do not include the seasonal high flood period, although some studies suggest that the share of labile dissolved carbon in river discharge waters during this period can reach as high as 20–40% [23], whereas during the most of the vegetation season, the major carbon source for heterotrophic bacterioplankton of estuarine areas of Siberian coastal seas is organic matter synthesized by primary producers. Therefore, the pattern of the seasonal bacterioplankton dynamics needs to be complemented with data for this particular period.
CONCLUSIONS
Based on field research data and previously published results, the Yenisei estuary can be divided into three zones distinguished by salinity (Fig. 6). The bacterial abundance and activity in these zones can differ by an order of magnitude. The distribution of bacterial abundance within the zones is mostly due to small-scale patchiness, which can be observed in the distribution of organic matter, phytoplankton, and suspended material [10]. However, the limits determining the variation in qualitative and quantitative characteristics of different groups of planktonic organisms do not necessarily coincide.
Furthermore, the field research data and previously published results indicate that major seasonal variations in the quantitative characteristics of bacterioplankton population are associated with the freshened area of the Yenisei estuary. Microbial production parameters exhibit an uneven distribution, with maximum values observed in summer. The contribution of the heterotrophic component of the microbial community was dominant in the early spring period. Despite the observed correlations in the distribution of bacterial abundance and activity, phytoplankton, and organic carbon, their distribution patterns along the salinity gradient were different. It was found that bacteriophages had relatively little effect on regulation of the bacterial abundance and production during the spring and fall periods. Further research should specifically evaluate the contribution of particle-associated and free-living bacteria to the total microbial abundance and activity. Moreover, analysis of the seasonal dynamics of bacterioplankton growth needs to include the high flood period proper, which dramatically differ from other seasons in the composition and availability of organic matter transported by river waters. Unfortunately, no such studies have been performed to date.
REFERENCES
N. A. Belyaev, M. S. Ponyaev, and A. M. Kiriutin, “Organic carbon in water, particulate matter, and upper layer of bottom sediments of the central part of the Kara Sea,” Oceanology (Engl. Transl.) 55, 508–520 (2015).
N. A. Belyaev, A. V. Kolokolova, and A. M. Kiriutin, “A report of biogeochemical squad,” in Scientific Report of Expedition of 66th Cruise of R/V Akademik Mstislav Keldysh in the Kara Sea in July 13–August 21,2016 (Shirshov Institute of Oceanology, Russian Academy of Sciences, Moscow, 2016), pp. 240–255.
M. A. Boltenkova, T. I. Shirokolobova, P. R. Makarevich, and N. D. Romanova, “Community of planktonic bacteria and viruses of the Ob’ Bay and adjacent shelf of the Kara Sea,” Nauka Yuga Ross. 14, 78–87 (2018).
M. V. Ivanov, A. Yu. Lein, A. S. Savvichev, et al., “Abundance and activity of microorganisms at the water-sediment interface and their effect on the carbon isotopic composition of suspended organic matter and sediments of the Kara Sea,” Microbiology (Moscow) 82, 735–742 (2013).
A. I. Kopylov, A. F. Sazhin, E. A. Zabotkina, and N. D. Romanova, “Virioplankton in the Kara Sea: the impact of viruses on mortality of heterotrophic bacteria,” Oceanology (Engl. Transl.) 55, 561–572 (2015).
A. P. Lisitsyn, “Marginal filter of oceans,” Okeanologiya (Moscow) 34, 735–737 (1994).
P. N. Makkaveev, Z. G. Melnikova, A. A. Polukhin, et al., “Hydrochemical characteristics of the waters in the western part of the Kara Sea,” Oceanology (Engl. Transl.) 55, 485–496 (2015).
I. N. Mitskevich and B. B. Namsaraev, “Numbers and distribution of bacterioplankton in the Kara Sea in September 1993,” Okeanologiya (Moscow) 34, 704–708 (1994).
I. V. Mosharova, V. V. Il’inskii, and S. A. Mosharov, “State of heterotrophic bacterioplankton of Yenisei estuary and the zone of Ob–Yenisei discharge in autumn in relation with environmental factors,” Water Resour. 43, 341–352 (2016).
V. V. Pelevin, P. O. Zavjalov, N. A. Belyaev, et al., “Spatial variability of concentrations of chlorophyll a, dissolved organic matter and suspended particles in the surface layer of the Kara Sea in September 2011 from lidar data,” Oceanology (Engl. Transl.) 57, 165–173 (2017).
N. D. Romanova and A. F. Sazhin, “Bacterioplankton of the Kara Sea shelf,” Oceanology (Engl. Transl.) 55, 858–862 (2015).
N. D. Romanova and A. F. Sazhin, “Relationships between the cell volume and the carbon content of bacteria,” Oceanology (Engl. Transl.) 50, 522–530 (2010).
A. F. Sazhin, S. A. Mosharov, N. D. Romanova, et al., “The plankton community of the Kara Sea in early spring,” Oceanology (Engl. Transl.) 57, 222–224 (2017).
A. S. Savvichev, E. E. Zakharova, E. F. Veslopolova, et al., “Microbial processes of the carbon and sulfur cycles in the Kara Sea,” Oceanology (Engl. Transl.) 50, 893–908 (2010).
I. N. Sukhanova, “A report of phytoplanktonic squad,” in Scientific Report of Expedition of 66th Cruise of R/V Akademik Mstislav Keldysh in the Kara Sea in July 13–August 21,2016 (Shirshov Institute of Oceanology, Russian Academy of Sciences, Moscow, 2016), pp. 132–141.
N. G. Teplinskaya, “Bacterial transformation of the compounds of nitrogen, sulfur, and phosphorous in subantarctic bottom sediments,” Ekol. Bezop. Pribrezhnoi Shel’fovoi Zon Morya, No. 15, 581–589 (2007).
R. M. Amon and B. Meon, “The biogeochemistry of dissolved organic matter and nutrients in two large Arctic estuaries and potential implications for our understanding of the Arctic Ocean system,” Mar. Chem. 92, 311–330 (2004).
T. Fenchel, “The microbial loop—25 years later,” J. Exp. Mar. Biol. Ecol. 366, 99–103 (2008).
M.-E. Garneau, W. F. Vincent, R. Terrado, and C. Lovejoy, “Importance of particle-associated bacterial heterotrophy in a coastal Arctic ecosystem,” J. Mar. Syst. 75, 185–197 (2009).
V. Gordeev, J. Martin, I. Sidorov, and M. Sidorova, “A reassessment of the Eurasian river input of water, sediment, major elements, and nutrients to the Arctic Ocean,” Am. J. Sci. 296, 664–691 (1996).
R. M. Holmes, J. W. McClelland, S. E. Tank, et al., Water Quality Dataset, Arctic Great Rivers Observatory, 2018. https://www.arcticgreatrivers.org/data.
R. M. Holmes, J. W. McClelland, B. J. Peterson, et al., “Seasonal and annual fluxes of nutrients and organic matter from large rivers to the Arctic Ocean and surrounding seas,” Estuaries Coasts 35, 369–382 (2012).
R. M. Holmes, J. W. McClelland, P. A. Raymond, et al., “Lability of DOC transported by Alaskan rivers to the Arctic Ocean,” Geophys. Res. Lett. 35, (2008).
E. Kamiya, S. Izumiyama, M. Nishimura, et al., “Effects of fixation and storage on flow cytometric analysis of marine bacteria,” J. Oceanogr. 63, 101–112 (2007).
D. L. Kirchman, R. R. Malmstrom, and M. T. Cottrell, “Control of bacterial growth by temperature and organic matter in the Western Arctic,” Deep Sea Res., Part II 52, 3386–3395 (2005).
A. I. Kopylov, A. F. Sazhin, E. A. Zabotkina, et al., “Virioplankton of the Kara Sea and the Yenisei River estuary in early spring,” Estuarine, Coastal Shelf Sci. 217, 37–44 (2019).
P. Lebaron, P. Servais, A.-C. Baudoux, et al., “Variations of bacterial-specific activity with cell size and nucleic acid content assessed by flow cytometry,” Aquat. Microb. Ecol. 28, 131–140 (2002).
J. W. McClelland, R. M. Holmes, K. H. Dunton, and R. W. Macdonald, “The Arctic Ocean estuary,” Estuaries Coasts 35, 353–368 (2012).
B. Meon and R. M. Amon, “Heterotrophic bacterial activity and fluxes of dissolved free amino acids and glucose in the Arctic rivers Ob, Yenisei and the adjacent Kara Sea,” Aquat. Microb. Ecol. 37, 121–135 (2004).
S. A. Mosharov, A. F. Sazhin, E. I. Druzhkova, and P. V. Khlebopashev, “Structure and productivity of the phytocenosis in the Southwestern Kara Sea in early spring,” Oceanology (Engl. Transl.) 58, 396–404 (2018).
A. A. Osadchiev, A. S. Izhitskiy, P. O. Zavialov, et al., “Structure of the buoyant plume formed by Ob and Yenisei river discharge in the southern part of the Kara Sea during summer and autumn,” J. Geophys. Res.: Oceans 122, 5916–5935 (2017).
A. A. Polukhin and P. N. Makkaveev, “Features of the continental runoff distribution over the Kara Sea,” Oceanology (Engl. Transl.) 57, 19–30 (2017).
K. G. Porter and Y. S. Feig, “The use of DAPI for identifying and counting aquatic microflora,” Limnol. Oceanogr. 25, 943–948 (1980).
A. Saliot, G. Cauwet, G. Cahet, et al., “Microbial activities in the Lena River delta and Laptev Sea,” Mar. Chem. 53, 247–254 (1996).
B. F. Sherr, E. B. Sherr, T. L. Andrew, et al., “Trophic interactions between heterotrophic protozoa and bacterioplankton in estuarine water analyzed with selective metabolic inhibitors,” Mar. Ecol.: Prog. Ser. 32, 169–179 (1986).
Y. I. Sorokin and P. Y. Sorokin, “Plankton and primary production in the Lena River estuary and in the south-eastern Laptev Sea,” Estuarine, Coastal Shelf Sci. 43, 399–418 (1996).
C. Vallières, L. Retamal, P. Ramlal, et al., “Bacterial production and microbial food web structure in a large arctic river and the coastal Arctic Ocean,” J. Mar. Syst. 74, 756–773 (2008).
J. H. Vosjan and G. J. van Noort, “Enumerating nucleoid-visible marine bacterioplankton: bacterial abundance determined after storage of formalin fixed samples agrees with isopropanol rinsing method,” Aquat. Microb. Ecol. 14, 149–154 (1998).
T. Weisse, “The microbial loop in the Red Sea: dynamics of pelagic bacteria and heterotrophic nanoflagellates,” Mar. Ecol.: Prog. Ser. 55, 241–250 (1989).
Funding
Analysis of field samples was supported by the Russian Science Foundation, project no. 17-77-10 138; data analysis was supported by the Russian Foundation for Basic Research, project no. 18-05-00326.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Timchenko
Rights and permissions
About this article
Cite this article
Romanova, N.D., Boltenkova, M.A. Seasonal Variability of Bacterioplankton of the Yenisei Estuary. Oceanology 60, 74–82 (2020). https://doi.org/10.1134/S0001437020010191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001437020010191