Abstract
The processes of excitation and quenching of electronically excited molecular oxygen O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) in the Earth’s atmosphere at nightglow sky heights are considered. The Herzberg I bands in the range of 250–360 nm have a wide spectrum of luminescence in the nightglow of the Earth. The volume intensity profiles of the Herzberg I bands of molecular oxygen in the Earth’s atmosphere are calculated at night using data from a semiempirical model of the temperature response of the middle atmosphere depending on altitude, season, and solar activity at the Earth’s midlatitudes. The calculations of the luminescence intensities of the Herzberg I bands are compared with the experimental data obtained from the Space Shuttle Discovery (STS-53) and from standard SP-48, SP-49, and SP-50 spectrographs from the 1950s–1960s. It is shown that the calculation results are in good agreement with the experimental data obtained from both the space shuttle and ground-based observations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
The upper atmosphere of the Earth (above 80 km) is a very rarefied gaseous medium, the main components of which are atoms and molecules of nitrogen and oxygen, as well as hydrogen and helium. The so-called minor components—nitric oxide, carbon monoxide, etc., as well as metastable atoms and molecules, are important for the photochemistry and radiation of the upper atmosphere. As a result of exposure to the ionizing ultraviolet radiation of the Sun, numerous photochemical processes occur in the upper atmosphere, the consequence of which is the atmosphere’s own radiation [1]. In this altitude region, molecular oxygen is dissociated by solar UV radiation. The processes of recombination of atomic oxygen lead to the formation of electronically excited O2 molecules, which cause many emissions and affect the radiative balance of this region [1]. It is known that one of the sources of the glow of the Earth’s night atmosphere is the electronically excited molecular oxygen O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)), which is formed during triple collisions in the Earth’s atmosphere with the participation of two O atoms and a third particle
where ν' are the vibrational levels of the \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) state and M is the third particle in the collision. Oxygen atoms are formed in the Earth’s atmosphere during the daytime during the photodissociation of O2 molecules by solar UV radiation O2 + hν → O + O. Triple collisions (1) with the formation of O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) are most effective in a layer of the Earth’s atmosphere about 10 km in thickness with a center at an altitude of about 90 km [1, 2]. Subsequently, the electronically excited oxygen molecule passes from the \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) state to the ground state \({{{\text{X}}}^{3}}\Sigma _{g}^{ - }\) while emitting Herzberg I bands. In this work, the processes of excitation and quenching of electronically excited molecular oxygen O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) are considered. At the same time, it should be noted that the Herzberg I bands in the range of 250–360 nm have a wide emission spectrum in the own radiation of the Earth’s upper atmosphere at night.
This paper uses experimental data on the characteristic of [O] concentrations in the aforementioned layer based on the luminescence characteristics of atomic oxygen O for different months of the year under low (F10.7 = 75, 1976 and 1986) and high (F10.7 = 203, 1980 and 1981) solar activity at midlatitudes (55.7° N; 36.8° E), Zvenigorod Observatory at the Obukhov Institute of Atmospheric Physics, Russian Academy of Sciences (IAP RAS). Regular data on the luminescence of atomic oxygen were obtained from a semiempirical model integrating several types of different midlatitude measurements, regression relations, and theoretical calculations over several decades by the staff at the IAP RAS [1]. At middle latitudes, the 557.7 nm emission is excited mainly in the altitudinal region of 85–115 km with an intensity maximum at ~97 km. An increase in solar activity leads to an increase in the O concentration at the layer maximum and to a lowering of its lower boundary [4]. Results [1, 3] showed a significant scatter in the absolute concentration values of atomic oxygen at the maximum of the layer, the height of which was also not constant. The results of model calculations for the 557.7 nm emission revealed that there is a negative correlation between the height of the maximum concentrations of atomic oxygen and their values. Moreover, a negative correlation is clearly observed between the emission intensity of 557.7 nm and the height of the maximum of the emitting layer, both for seasonal variations and for the dependence on solar activity [5, 6]. As a result of changes in the concentration profiles of atomic oxygen, the rate profiles of the formation of electronically excited molecular oxygen \({\text{O}}_{2}^{*}\) in the Earth’s atmosphere inevitably change as a result of the process of triple collisions (1) and the emission intensity of various bands of molecular oxygen. Therefore, the glow intensity of the Herzberg I bands will depend both on the time of a year and on solar activity. In addition, paper [1] also presented the results of an analysis of the response of monthly average temperature values of the middle atmosphere on solar activity based on long-term data obtained using rockets and spectrophotometry of a number of emissions of its own radiation during several cycles of 11-year solar activity. The analysis was carried out in [7]. Based on these data, using the temperature differences for different heights of the profiles corresponding to the years of high and low solar activity, in a linear approximation it is possible to find the rate of temperature increase under the influence of solar activity:
where δТF(Z) = dt/df is the change in temperature at height Z at ΔF10.7 = 100 sfu. After determining the values of δТF(Z), seasonal temperature variations were plotted for individual altitude levels [1]. The altitude profiles of the rates of change in the temperatures of the middle atmosphere at different altitudes from solar activity indicate their pronounced altitude nonlinearity. Significant seasonal difference in the influence of solar activity in the mesopause region is obviously due to the difference in the altitude distributions of temperature depending on the time of a year [1]. Figure 1 presents the results of studies [1] based on an empirical model of temperature response to solar activity on height and season; the months are indicated by numbers: 1 is January, 4 is April, 7 is July, and 10 is October. On the abscissa: δT/δ F10.7, K (100 sfu)—1 are values of the atmospheric temperature response to solar activity at F10.7 = 100 sfu; along the y axis, altitude values are in km. Thus, the altitude distributions of the temperature response to solar activity at altitudes of 30–100 km indicate that significant seasonal temperature variations are observed at altitudes of ≈80–95 km and minimal ones are at altitudes of ≈55–70 km. This is clearly seen from Fig. 1.
Model altitude distributions of the temperature response to solar activity for 4 months of the year (1 is January, 4 is April, 7 is July, and 10 is October) at altitudes of 30–100 km [1]. On the abscissa axis: δT/δF10.7, K (100 sfu)—1—the values of the atmospheric temperature response to solar activity at F10.7 = 100 sfu; y axis: height values in kilometers.
The purpose of this work is to compare the results of theoretical calculations of the luminescence intensities of the Herzberg I bands in the range of 250–360 nm with the experimental data on the intensity of the night-glow of molecular oxygen \({\text{O}}_{2}^{*}\) in the own radiation of the Earth’s upper atmosphere at night. Particular attention is paid to the peculiarities of the formation of different vibrational levels ν' of the electronically excited state \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) of the oxygen molecule as a result of triple collisions (1).
2 DESCRIPTION OF THE CALCULATION OF THE EXCITED OXYGEN O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) CONCENTRATION
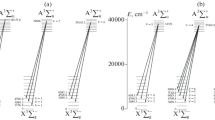
Figure 2 schematically shows several spontaneous radiative transitions from different vibrational levels of the electronically excited state \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) to different vibrational levels of the ground state \({{{\text{X}}}^{3}}\Sigma _{g}^{ - }\) at which the Herzberg I bands are emitted. All of the above states are below the dissociation energy of the O2 molecule ~41300 cm—1 (8065 cm—1 = 1 eV). Wavelength λ of the Herzberg I bands can be calculated using the formula
where EA(ν') (cm–1) is the energy of the vibrational level ν' of the state \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\); EX(ν'') (cm–1) is the energy of the vibrational level ν'' of the state \({{{\text{X}}}^{3}}\Sigma _{g}^{ - }\). Since the transitions between the considered states are dipole-forbidden, then the characteristic radiative times of the states \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) are of the order of 1 and 0.1 s, respectively [8]. Therefore, when calculating the concentrations of electronically excited oxygen, it is necessary to take into account the quenching of the О2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) molecule not only during radiative transitions (2), but also during collisions with the main atmospheric components N2 and О2 in this altitude range [9]:
Since the concentrations of N2 at altitudes of 90–100 km exceed 1013 cm–3 and the quenching constants of the \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) state are greater than ~10–12 cm3 s–1 [9, 10], the collisional lifetimes of the considered vibrational levels of these states are either comparable or are much smaller than the radiative Herzberg I bands at night-glow heights. This means that the kinetics of Herzberg I states in the considered range of atmospheric altitudes is largely determined by collisional processes. Calculations of the concentration of excited oxygen O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) at the heights of the Earth’s upper atmosphere for vibrational levels ν' = 3–9 of this state for October 1976 and 1986 were carried out (low solar activity, F10.7 = 75) [12]. The concentration of electronically excited oxygen О2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) was calculated according to the formula:
where αA is the quantum yield of state \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) in triple collisions (1); \(q_{{v'}}^{{\text{A}}}\) are the quantum yields of the vibrational levels ν' of this state; k1 is the rate constant of the recombination reaction in triple collisions (1); k5a, and k5b are the rate constants of reactions (5a), (5b); and \({\text{A}}_{{v'}}^{{\text{A}}}\) is the sum of the Einstein coefficients for all spontaneous radiative transitions from the vibrational levels v' of the state \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\). The recombination reaction rate constant k1(cm6 s—1) was used as a calculated value depending on the temperature of the atmosphere in the considered height interval according to [1]; the quenching constants of electronically excited oxygen in collisions of molecular oxygen О2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) with atmospheric components N2 and О2, k5а(cm3 s–1), k5b(cm3 s–1), were taken into account according to [9, 10]; quantum yields αA' and αA were taken into account according to [13], and Einstein coefficients for all spontaneous transitions were taken into account according to [8]. An analytical formula for calculating quantum yields \(q_{{v'}}^{{\text{A}}}\) was presented in [9]:
where E0 = 40 000 cm–1, β = 1500 cm–1 are the parameters determined by the least squares method by comparing the calculated vibrational populations of the \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) state with the results of ground-based observations. However, in [12], the quantum yields \(q_{{v'}}^{{\text{A}}}\) were corrected based on a comparison of the calculated intensities of the Herzberg I bands measured from the Space Shuttle Discovery (STS-53). In this paper, we use \(q_{{v'}}^{{\text{A}}}\) according to [11].
3 RESULTS OF CALCULATING THE EMISSION INTENSITY OF THE HERZBERG I BANDS
According to formula (6), the vertical distribution profiles of the concentrations of electronically excited molecular oxygen \({\text{O}}_{2}^{*}\) were calculated for the \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) state in the Earth’s upper atmosphere. When calculating the concentrations of electronically excited oxygen, we used altitude temperature profiles compiled on the basis of long-term (1960–2000) measurements of temperature profiles at altitudes of 30–110 km [7]. The method developed by these authors for calculating altitude profiles of temperature and total concentration of the atmosphere makes it possible to determine the temperature and density of the atmosphere at middle latitudes for given heliogeophysical conditions (altitude, solar activity level, and year number). The values of the volume intensities of the radiation bands corresponding to the transitions (2) were calculated by the formula
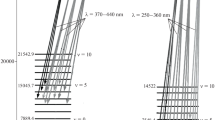
where \([{\text{O}}_{2}^{*}]\) (cm–3) is the calculated concentration of electronically excited oxygen \({\text{O}}_{2}^{*}\) depending on height h [12]; Av'v'' (s–1) is the Einstein coefficient corresponding to the spontaneous radiative transition from vibrational level v' of the upper state to the vibrational level v'' of the lower state in (2) [8]. Figure 3 shows the calculated height distributions of the volume emission intensities of the bands associated with the spontaneous transition \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) (v' = 6) → \({{{\text{X}}}^{3}}\Sigma _{g}^{ - }\) (v'' = 3) (3a, 3b) for low (F10.7 = 75, 1976 and 1986) (3a) and high (F10.7 = 203, 1980 and 1981) (3b) solar activity at the middle latitudes of the Earth. The numbers represent the months of the year: 1 is January, 2 is April, 3 is July, and 4 is October. The calculations used data on atomic oxygen concentrations and temperatures for the average months of each season. The abscissa axes show the values of the volume radiation intensity i (cm–3 s–1); the ordinate axes show heights in kilometers. Figure 4a shows a fragment of the average night sky glow spectrum in the range of 250–360 nm, respectively, measured by the spectrograph from the Space Shuttle Discovery (STS-53) in the range from 115 to 900 nm during its 12-day mission in January 1995 (low solar activity conditions) [2]. The ordinate axes show the intensities in Rayleighs/angstroms (R/Å) (1 R = 106 photon/cm2 s); the abscissa axes show the wavelengths in angstroms (λ(Å)). Both numbers above the luminescence peaks denote vibrational levels (v'–v'') during radiative transitions (2). The calculated values of the radiation intensity I (cm–2 s–1) (histograms) for various Herzberg I bands due to radiative transitions (2) were obtained for October 1976 and 1986 (conditions of low solar activity F10.7 = 75) in the same wavelength range. The results of the calculations are shown in Fig. 4b. While recalculating the volumetric radiation intensity iv'v'' into the radiation intensity Iv'v'', the approximation of an optically thin layer is used; i.e., the absorption of photons inside the layer is neglected. In this case, in contrast to the results of [12], the radiative transitions from the ninth vibrational level v' = 9 of the \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) state are taken into account and the intensities of the Herzberg I bands (9-1) and (9-2) located in the range of 255–270 nm are given. As can be seen from Fig. 4, there is a good agreement between the calculated intensities of the excited oxygen emission bands \({\text{O}}_{2}^{*}\) (\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\), v' = 3–9) and the spectrum obtained from the shuttle [2]—the experimental data of the night sky glow in the range of 250–360 nm. Figure 5a shows the results obtained by ground-based long-term measurements of the emission spectrum of the nighttime upper atmosphere in the UV wavelength range of 305–395 nm, i.e., the Herzberg I band [14]. The observations were carried out during the period of high solar activity using standard Soviet SP-48, SP-49, and SP-50 spectrographs of the 1950s–1960s [1]. The theoretically calculated intensities of the Herzberg I bands for the period of high solar activity are shown in Fig. 5b. As can be seen from the comparison of Fig. 5a and Fig. 5b, the calculated intensities of the Herzberg I bands are in good agreement with the experimental data. A comparison of the theoretically calculated intensities of the Herzberg I bands with the experimental data [14] makes it possible to identify the maxima in the obtained spectra: the maximum at 315 nm is due to the band (4-4); 321–323 nm, bands (3-4) and (5-5); 327–332 nm, bands (7-6), (4-5), and (6-6); 337 nm, bands (3-5) and (5-6); 342–348 nm, bands (7-7), (4-6), and (6-7); 355 nm, bands (3-6) and (5-7).
Altitude distributions of the volume radiation intensity iv'v'' (cm–3 s–1) of Herzberg I (a) for high solar activity and (b) for low solar activity for different months of the year (1 is January, 2 is April, 3 is July, and 4 is October) at the middle latitudes of the Earth. The abscissa axes show the values of the volume radiation intensity i (cm–3 s–1); the ordinate axes show heights in kilometers.
(a) Fragment of the average night sky glow spectrum in the range of 250–360 nm measured by a spectrograph from the space shuttle [2]: the Rayleigh/angstrom values (R/Å) are along the ordinate axis; the wavelengths λ (Å) are along the abscissa axis; and the numbers above the peaks are (v'–v'') for radiative transitions (2). (b) Emission intensities for various Herzberg I bands.
(a) Radiation spectrum of the nighttime upper atmosphere in the UV wavelength range of 305–395 nm, Herzberg I bands, obtained by ground-based observations [14]. (b) Values of the emission intensity of the Herzberg I bands.
4 CONCLUSIONS
Several spontaneous radiative transitions are schematically presented (Fig. 2) from different vibrational levels of the electronically excited state \({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\) to different vibrational levels of the ground state \({{{\text{X}}}^{3}}\Sigma _{g}^{ - }\) at which Herzberg I bands are emitted. Calculations of the concentration of excited oxygen O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) at the altitudes of the Earth’s upper atmosphere for vibrational levels v' = 3 – 9 of this state have been carried out. When calculating the concentrations of electronically excited oxygen, the quenching of the O2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) molecule is taken into account not only during radiative transitions (2), but also during collisions with the main atmospheric components N2 and O2 in a given altitude range: 85–100 km [9]. The values of the emission intensity of the Herzberg I bands due to radiative transitions from the vibrational levels v' = 3–9 of electronically excited oxygen О2(\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\)) for low (F10.7 = 75, 1976 and 1986) and high (F10. 7 = 203, 1958 and 1959) solar activity for middle latitudes are obtained. The emission intensity of the Herzberg I bands under conditions of low solar activity is compared with the experimental data obtained in the wavelength range of 250–360 nm by a spectrograph from the space shuttle during its 12-day STS 53 mission in September 1995 (years of low solar activity) [2]. The result of a comparison of the calculated values with the experimental data is a good agreement between the calculated intensities of the excited oxygen emission bands \({\text{O}}_{2}^{*}\) (\({{{\text{A}}}^{3}}\Sigma _{{\text{u}}}^{ + }\), v' = 3–9) and the spectrum obtained from the shuttle [2] the experimental data of the night sky glow in the range of 250–360 nm, which can be seen from Fig. 4. The calculated values of the emission intensity of the Herzberg I bands under conditions of high solar activity were also compared with experimental data obtained in the wavelength range of 305–395 nm by standard ground-based Soviet spectrographs of the 50s–60s [14]. The results obtained by these long-term measurements of the emission spectrum of the night-time upper atmosphere are in good agreement with the calculated values of the emission intensity of the Herzberg I bands. A comparison of the theoretically calculated intensities of the Herzberg I bands with experimental data [14] allows us to identify the maxima in the obtained spectra: the maximum at 315 nm is due to the band (4-4); that at 321–323 nm is due to bands (3-4) and (5-5); at 327–332 nm to bands (7-6), (4-5), and (6-6); at 337 nm to bands (3-5) and (5-6); at 342–348 nm to bands (7-7), (4-6), and (6-7); and at 355 nm to the bands (3-6) and (5-7).
REFERENCES
N. N. Shefov, A. I. Semenov, and V. Yu. Khomich, Radiation of the Upper Atmosphere as an Indicator of its Structure and Dynamics (GEOS, Moscow, 2006) [in Russian].
A. L. Broadfoot and P. J. Bellaire, Jr., “Bridging the gap between ground-based and space-based observations of the night airglow,” J. Geophys. Res. 104 (A8), 17127–17138 (1999).
V. I. Perminov, A. I. Semenov, and N. N. Shefov, “Deactivation of hydroxyl molecule vibrational states by atomic and molecular oxygen in the mesopause region,” Geomagn. Aeron. (Engl. Transl.) 38 (6), 761–764 (1998).
A. I. Semenov and N. N. Shefov, “Variations in the temperature and atomic-oxygen concentration of the mesopause–lower thermosphere region according to variations in solar activity,” Geomagn. Aeron. (Engl. Transl.) 39 (4), 484–487 (1999).
A. I. Semenov and N. N. Shefov, “An empirical model of nocturnal variations in the 557.7-nm emission of atomic oxygen: 1. Intensity,” Geomagn. Aeron. (Engl. Transl.) 37 (2), 215–221 (1997).
N. N. Shefov, A. I. Semenov, and N. N. Pertsev, “Dependencies of the amplitude of the temperature enhancement maximum and atomic oxygen concentration in the mesopause region on seasons and solar activity level,” Phys. Chem. Earth, Part B 25 (5–6), 537–539 (2000).
A. I. Semenov, N. N. Pertsev, N. N. Shefov, V. I. Perminov, and V. V. Bakanas, “Calculation of the vertical profiles of the atmospheric temperature and number density at altitudes of 30–110 km,” Geomagn. Aeron. (Engl. Transl.) 44 (6), 773–778 (2004).
D. R. Bates, “Oxygen band system transition arrays,” Planet. Space Sci. 37 (7), 881–887 (1989).
A. S. Kirillov, “Model of vibrational level populations of Herzberg states of oxygen molecules at heights of the lower thermosphere and mesosphere,” Geomagn. Aeron. (Engl. Transl.) 52 (2), 242–247 (2012).
A. S. Kirillov, “Electronic kinetics of main atmospheric components in high-latitude lower thermosphere and mesosphere,” Ann. Geophys. 28 (1), 181–192 (2010).
A. S. Kirillov, “The calculation of quenching rate coefficients of O2 Herzberg states in collisions with CO2, CO, N2, O2 molecules,” Chem. Phys. Lett. 592, 103–108 (2014).
O. V. Antonenko and A. S. Kirillov, “Modeling the Earth’s nightglow spectrum for systems of bands emitted at spontaneous transitions between different states of electronically excited oxygen molecules,” Bull. Russ. Acad. Sci.: Phys. 85 (3), 219–223 (2021).
V. A. Krasnopolsky, “Excitation of the oxygen nightglow on the terrestrial planets,” Planet. Space Sci. 59 (8), 754–766 (2011).
V. I. Krassovsky, N. N. Shefov, and V. T. Yarin, “Atlas of the airglow spectrum 3000–12400 Å,” Planet. Space Sci. 9 (12), 883–915 (1962).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Selikhanovich
Rights and permissions
About this article
Cite this article
Antonenko, O.V., Kirillov, A.S. Study of the Earth’s Own Radiation of the Upper Atmosphere (Herzberg I Bands) as a Function of Solar Activity, Atmospheric Temperature, and Seasons of the Year. Izv. Atmos. Ocean. Phys. 58, 578–584 (2022). https://doi.org/10.1134/S0001433822060020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001433822060020