Abstract
Animals utilize specialized sensory neurons enabling the detection of a wide range of environmental stimuli from the presence of toxic chemicals to that of touch. However, how these neurons discriminate between different kinds of stimuli remains poorly understood. By combining in vivo calcium imaging and molecular genetic manipulation, here we investigate the response patterns and the underlying mechanisms of the C. elegans phasmid neurons PHA/PHB to a variety of sensory stimuli. Our observations demonstrate that PHA/PHB neurons are polymodal sensory neurons which sense harmful chemicals, hyperosmotic solutions and mechanical stimulation. A repulsive concentration of IAA induces calcium elevations in PHA/PHB and both OSM-9 and TAX-4 are essential for IAA-sensing in PHA/PHB. Nevertheless, the PHA/PHB neurons are inhibited by copper and post-synaptically activated by copper removal. Neuropeptide is likely involved in copper removal-induced calcium elevations in PHA/PHB. Furthermore, mechanical stimulation activates PHA/PHB in an OSM-9-dependent manner. Our work demonstrates how PHA/PHB neurons respond to multiple environmental stimuli and lays a foundation for the further understanding of the mechanisms of polymodal signaling, such as nociception, in more complex organisms.
Similar content being viewed by others
Introduction
Animals employ sensory neurons to detect external stimuli such as the presence of chemicals or aspects of temperature or touch. These specialized neurons are often polymodal where a single type of sensory neuron may respond to a number of different kinds of stimuli1. Polymodal nociceptive neurons in the mammalian skin, for example, have the ability to respond to heat, noxious chemicals and mechanical stimuli2. How these neurons discriminate between these different kinds of stimuli remains largely unknown.
Simple organisms such as C. elegans provide useful platforms to tease out how signaling molecules and neuronal circuits generate complex behaviors. C. elegans is equipped with a small nervous system consisting of 302 neurons. Many of these neurons may be multifunctional3,4. 32 presumed chemosensory neurons in the amphid, phasmid and inner labial organs are either directly or indirectly exposed to the environment5. The amphids in the head are the largest sensory organs in C. elegans. They consist of 12 pairs of sensory neurons capable of sensing numerous sensory stimuli1,5. The phasmids are bilateral sensory organs located in the tail of the worm. They contain similar structures to those of the amphids3,6. A previous study has reported that the antagonistic activity of the amphid neurons, mainly ASH, and the phasmid neurons, PHA and PHB, integrate to generate avoidance behaviors6. In Hilliard’s study, the PHA/PHB neurons were suggested to probably act as the initial chemo-sensors in detecting detergent (SDS) where the decision to initiate avoidance behavior were considered to also incorporate information from the ASH neurons. In this way the worms were enabled to avoid or escape from noxious SDS stimulus6,7. Ablation of the PHA/PHB neurons also caused significant deficiencies in the avoidance responses to harsh touch. This indicates that these neurons also play a specific role in the noxious touch sensation8. However, the nature of such responses of phasmid neurons to environmental stimuli has yet to be determined on a cellular level and the underlying molecular mechanisms remain unclear.
In this study, we show that the PHA/PHB neurons respond to a wide range of aversive stimuli including aversive odors, copper, alkaline solution, hyperosmotic solution, and harsh touch. We further identify critical roles for the TRPV protein OSM-9, the CNG channel protein TAX-4 and the post-synaptic neuropeptide in the sensory transduction of PHA/PHB. Our data suggests that the PHA/PHB neurons are polymodal neurons employing an elaborate combination of intracellular and intercellular signaling pathways to detect and process environmental stimuli.
Results
The PHA/PHB neurons respond to a wide range of aversive stimuli
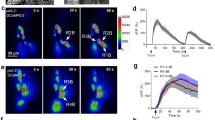
To monitor the activities of the PHA/PHB neurons, we generated a transgenic strain in which the calcium indicator protein GCaMP5.0 was transcribed under the control of the ocr-2 promoter9. Previous studies have reported that PHA/PHB neurons are required for detergent (SDS)-evoked avoidance behavior6,7. Consistent with these studies, we observed reliable calcium elevations in both the soma and the processes of the PHA/PHB neurons upon perfusion of 1% SDS to the tail of the worm (Fig. 1a–c).
(a) Calcium transients in the PHA/PHB neurons visualized with GCaMP5.0. A red fluorescent protein DsRED was co-expressed as a reference. Individual frames taken before, during and after application of 1:100 SDS are shown. (b) Fluorescence intensities reflect an increase in the calcium intracellular level in PHA/PHB neurons. (c) Calcium transients in the cell body and dendrites of PHA/B have similar profiles in response to a 60 s stimulus of 1:100 SDS.
We then sought to discover whether the PHA/PHB neurons could be activated by other chemical and physical stimuli. We observed robust calcium transients in the PHA/PHB neurons during the stimulation of the worms with aversive odors such as isoamyl alcohola (1:100 IAA) and 1-octanol (1:1000) and an alkaline solution of pH 12. Harsh touch (20 μm displacement) and hyperosmotic solution (2 M glycerol) also induced robust calcium transients in the PHA/PHB neurons. However, no such response was observed with the perfusion of the bath solution, alkaloid quinine (20 mM), or an acidic solution of pH 3 (Fig. 2a and b). Interestingly, the calcium levels of the PHA/PHB neurons decreased upon the application of copper heavy metal ions and were increased by copper removal (Fig. 2a,b). No detectable calcium variation was observed with the application of attractive odorants such as butanone. Notably, we did not observed any differences between the responses of PHA and PHB to these stimuli. These observations suggest that PHA/PHB are polymodal neurons responding to noxious chemical and physical stimuli.
(a) A variety of noxious chemical stimuli triggered calcium transients in the PHA/PHB neurons. The solid line shows the average fluorescence change and the shading around it indicate the ± SEM. Horizontal lines indicate the duration of application for each stimulus. Notably, 20 mM Cu2+ induced a decrease of calcium level in the PHA/PHB neurons. For 1-Ocanol induced-calcium elevations, both the soma and the progress were shown. (b) Calcium transients in the PHA/PHB neurons in response to physical stimuli. (c) Maximal calcium changes of the PHA/PHB neurons to the application of noxious stimuli. N > = 6 for each experiment, Data are mean ± SEM.
PHA/PHB neurons function as primary sensory neurons for sensing odorants
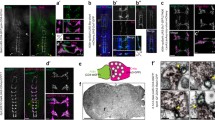
One possibility is that calcium elevations in the PHA/PHB neurons upon exposure to sensory stimuli occur post-synaptically and are induced by other neurons. Therefore, we tested the IAA-induce responses in PHA/PHB in unc-13 mutant worms and unc-31 mutant worms. In this unc-13 encodes the ortholog of the mammalian Munc13 which is required for neurotransmitter release from synaptic vesicle10,11 and unc-31 encodes the ortholog of the mammalian CAPS proteins and is essential for neuropeptide release from dense core vesicles (DCVs)10,11. Notably, IAA-induced calcium elevations in PHA/PHB in unc-13 and unc-31 background were similar to those of wild-type worms. This seems to confirm that PHA and PHB are the primary sensory neurons for sensing IAA (Fig. 3a,b).
(a,b) 1:100IAA induced-calcium transients in the PHA/PHB neurons in wild-type worms, unc-13 mutants and unc-31 mutants. (a) Fluorescence changes (mean ± SEM); (b) Bar graphs. (c,d) 1:100IAA induced-calcium transients in the PHA/PHB neurons in wild-type worms, osm-9 mutants and tax-4 mutants. (c) Fluorescence changes (mean ± SEM); (d) Bar graphs. Error bars indicate mean ± S.E.M.; ns, not significant, P > 0.05; *P < 0.05; **P < 0.01; two-tailed paired t-test.
IAA-sensing of the PHA/PHB neurons is dependent in TAX-4 and OSM-9
We then investigated the molecular mechanisms of IAA-sensing in PHA/PHB. TAX-4, a subunit of a cyclic nucleotide gated channel involved in chemotaxis mediated by the AWC neurons, has been implicated as required for PHA/PHB-mediated avoidance response to SDS6,12. We found that IAA-induce responses in PHA/PHB were dramatically diminished in tax-4 mutant worms (Fig. 3c,d). Sensory transduction in the ASH neurons in response to noxious osmotic shock, heavy metal ions and volatile chemical and alkaline solutions have all been noted to be mediated by OSM-9, a TRPV-related cation channel1,13. OSM-9 is expressed in PHA/PHB as well as in some amphid sensory neurons such ASH and AWA14. Interestingly, IAA-induced responses in PHA/PHB were also significantly weaker in osm-9 mutants than in wild-type worms (Fig. 3c,d). This demonstrates that both TAX-4 and OSM-9 are required for IAA-sensing in the PHA/PHB neurons.
Copper inhibits the PHA/PHB neurons
Both IAA and copper activates ASH neurons1,15. Unexpectedly, we found that the calcium levels in the PHA/PHB neurons were decreased by the application of copper (an “ON” response), and were increased by copper removal (an “OFF” response) (Fig. 4a,4b). Neither the “ON” response nor the “OFF” response was affected by the loss of UNC-13 (Fig. 4a,b). However, the “OFF” response was abolished in unc-31 mutant worms (Fig. 4a,b). These results indicate that copper autonomously inhibits PHA/PHB at a cellular level. Meanwhile, the PHA/PHB neurons may be post-synaptically activated by copper removal via neuropeptides. The Cu2+ -induced “ON” response in PHA/PHB was diminished in osm-9 mutant worms. Nevertheless, TAX-4 was required for “OFF” responses (Fig. 4c,d). These data suggests that copper inhibits PHA/PHB in an OSM-9-dependent manner, and both TAX-4 and neuropeptides are involved in copper removal-induced calcium elevations in PHA/PHB.
(a,b) Cu2+ (20 mM) induced-calcium variations in the PHA/PHB neurons in wild-type worms, unc-13 mutants and unc-31 mutants. (a) Fluorescence changes (mean ± SEM); (b) Bar graphs. (c,d) Cu2+ (20 mM) induced-calcium variations in the PHA/PHB neurons in wild-type worms, osm-9 mutants and tax-4 mutants. (c) Fluorescence changes (mean ± SEM); (d) Bar graphs. Error bars indicate mean ± S.E.M.; ns, not significant, P > 0.05; **P < 0.01; ***P < 0.001; two-tailed paired Student t-test.
PHA/PHB neurons sense mechanical stimulation in an OSM-9-dependent manner
Laser ablation of the PHA/PHB neurons reduces response to harsh touch. This shows that these neurons are also involved in mechano-sensation8. Consistent with the behavioral phenotype, we observed robust touch-induced calcium elevations in PHA/PHB (Fig. 5a,b). Touch-induced calcium elevations in PHA/PHB were not reduced in unc-13 mutant worms and were only slightly smaller in unc-31 mutant worms than those in the wild-type, which indicates that PHA and PHB are likely mechano-receptor cells (Fig. 5a,b).
(a,b) Touch induced-calcium transients in the PHA/PHB neurons in wild-type worms, unc-13 mutants and unc-31 mutants. (a) Fluorescence changes (mean ± SEM); (b) Bar graphs. (c,d) Touch induced-calcium transients in the PHA/PHB neurons in wild-type worms, wild-type worms treated with amiloride, osm-9 mutants, and deg-1 mutants. (c) Fluorescence changes (mean ± SEM); (d) Bar graphs. Error bars indicate mean ± S.E.M.; ns, not significant, P > 0.05; *P < 0.05; ***P < 0.001; two-tailed paired t-test.
Three mechano-gated channels have been identified in C. elegans, the two amiloride-sensitive sodium channel (ENaC) proteins MEC-4 and DEG-1, and the TRPN (nomPC) protein TRP-416,17,18. Since MEC-4 and TRP-4 are not expressed in the PHA/PHB neurons17,18,19, we examined touch-induced response in PHA/PHB in deg-1 mutant worms. We found that the touch-induced calcium elevations in the PHA/PHB neurons were normal in deg-1 mutant worms (Fig. 5c,d). Furthermore, the ENaC blocker amiloride failed to affect the touch-induced calcium elevations in PHA/PHB (Fig. 5c,d). This demonstrates that ENaC channels are not involved in mechano-transduction in PHA/PHB. OSM-9 is required for touch-evoked responses in the ASH neurons1. We found touch-induced calcium elevations in PHA/PHB were dramatically reduced in osm-9 mutant worms, indicating that OSM-9 plays a role in mediating PHA/PHB excitation in response to mechanical stimulation.
Discussion
In this study, we demonstrate that the C. elegans phasmid neurons PHA and PHB are polymodal sensory neurons responding to harmful chemicals and mechanical stimulation. We show that the TRPV channel OSM-9 is essential for both IAA-sensation and touch-sensation, but not for copper-induced calcium variations in PHA/PHB. The CNG channel TAX-4 is especially required for chemo-sensation in these neurons. In addition, neuropeptides are likely required for the copper removal induced-calcium elevations in PHA/PHB.
In C. elegans, two GPCR-related signal transduction systems are prominent in chemo-sensation. One relies upon CNG channel and another is mediated by TRPV channels5,15. In the AWC neurons, odorants bind to the GPCR receptor and activate Gα proteins. This leads to drop of the intracellular level of cGMP, thereby closing the CNG channels TAX-2/TAX-4 and hyperpolarizing the cell5,20. The CNG channels also mediate thermo-sensation in the AFD neurons and photo-sensation in the ASJ neurons21,22. The TRPV channel OSM-9 has been proposed to mediate depolarization following all chemical stimuli sensed by the ASH neurons1,13. Interestingly, here we found both TAX-4 and OSM-9 were essential for IAA-sensation in the PHA/PHB neurons, while OSM-9 and TAX-4 were involved in the copper-induced “ON” and “OFF” responses, respectively, in PHA/PHB. These observations suggest that the PHA/PHB neurons represent distinct mechanisms of chemo-transduction from the amphid sensory neurons. Additionally, we found that copper removal post-synaptically activated the PHA/PHB neurons via neuropeptides. This indicates that the activities of PHA/PHB can also be modulated by other neurons.
Two classes of mechano-gated channels have been identified in C. elegans. The first is the amiloride-sensitive ENaC channel subfamily and includes MEC-4 and DEG-1. The second is the TRP subfamily which includes TRP-416,17,18. MEC-4 is expressed in the six touch receptor neurons including ALM, AVM, PLM, and PVM. MEC-4 may form a heteromeric mechano-transduction channel with MEC-1018. These proteins interact with the paraoxonase-like MEC-6 and the cholesterol-binding stomatin-like MEC-2 protein which are required to sense gentle mechanical touch along the body wall18,23. TRP-4 is an N-type TRP channel which is a close homolog of NOMPC/TRPN1 in Drosophila24. TRP-4 is expressed in dopaminergic neurons such as CEP, PDE and DVA, and is involved in slowing the basal response, proprioception and in sensing ultrasound stimulus17,19,25. In the ASH neurons, loss of OSM-9 abolishes touch-evoked calcium elevations1. However, DEG-1, but not OSM-9, is required for the touch-receptor currents in ASH. This suggests that OSM-9 may act as a calcium modulator, but not as a touch receptor16. Here we found that OSM-9 was required for touch-induced calcium responses in the PHA/PHB neurons. Nevertheless, our data excludes a role of either DEG-1 or other ENaC in touch-induced calcium responses in PHA/PHB. Further efforts might be expended to identify which mechano-gated channel(s) function as the mechano-receptor(s) in these neurons. The answer to this question may shed new insights into the long-lasting attempt to identify the mechno-gated channels mediating hearing, touch-sensation and pain in mammals23,26,27.
A single type of sensory neuron responding to different kinds of stimuli represents an intriguing problem in neurology. Our data suggests that the combined approach of CNG signaling, TRP signaling, and neuropeptide signaling are responsible for encoding and discriminating between different kinds of stimuli in the PHA/PHB neurons. This observation may help us to uncover the other mechanisms of polymodal signaling such as nociception in more complex organisms.
Materials and Methods
Strains
C. elegans strains were maintained under standard conditions28. We generated a transgenic strain kanEx178 [Pocr-2::dsRed + Pocr-2::GCaMP5] to monitor intracellular activities in the PHA/PHB neurons. Mutant strains included: osm-9(ky10) kanEx178; tax-4(ky11) kanEx178; deg-1(u38) kanEx178; unc-13(e51) kanEx178; unc-31(e928) kanEx178.
Calcium Imaging
A drop of bath solution containing a D2 adult worm was placed on a coverslip. Then the worm was glued to the pad with a cyanoacrylate-based glue (Gluture Topical Tissue Adhesive, Abbott Laboratories). The anus segment was exposed to chemical and mechanical stimuli. The calcium indicator GCaMP5 was used to measure the intracellular calcium signals. Imaging was acquired in an Olympus microscope (BX51WI) with a 60 × objective lens on an Andor DL-604M EMCCD camera. Data was collected using the Macro-manager software. GCaMP5 was excited by a Lambda XL light source and fluorescent signals were collected at a rate of 1 Hz. The average GCaMP5 signal from the first 3 s before stimulus was taken as F0, and ΔF/F0was calculated for each data point. The data was analyzed using Imaging J. The bath solution contained (in mM):145 NaCl,2.5 KCl, 1 MgCl2, 5 CaCl2,10 HEPES, 20 glucose. (325~335 mOsm, pH adjusted to 7.3 with NaOH).
Mechanical Stimulation
Touch stimuli was delivered to the cell using a tip diameter of ~1 μm borosilicate glass capillary driven by a piezoelectric actuator (PI) mounted on a micromanipulator (Sutter). The needle was placed perpendicular to the worm’s body. In the “on” phase, the needle was moved toward the worm’s tail so that it could probe into the worm’s tail on the cilia and then held on the cilia for 500 ms. In the “OFF” phase the needle was returned to its original position.
Statistical analysis
Data analysis was performed using Excel 2010 and Image J.Error bars were mean ± SEM. N represents the number of cells. P values were determined by Student’s t test. P < 0.05 was regarded as statistically significant.
Additional Information
How to cite this article: Zou, W. et al. Polymodal Responses in C. elegans Phasmid Neurons Rely on Multiple Intracellular and Intercellular Signaling Pathways. Sci. Rep. 7, 42295; doi: 10.1038/srep42295 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Hilliard, M. A. et al. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. The EMBO journal 24, 63–72, doi: 10.1038/sj.emboj.7600493 (2005).
Lee, Y., Lee, C. H. & Oh, U. Painful channels in sensory neurons. Molecules and cells 20, 315–324 (2005).
White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314, 1–340 (1986).
Li, Z., Liu, J., Zheng, M. & Xu, X. Z. Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell 159, 751–765, doi: 10.1016/j.cell.2014.09.056 (2014).
Bargmann, C. I. Chemosensation in C. elegans . WormBook: the online review of C. elegans biology, 1–29, doi: 10.1895/wormbook.1.123.1 (2006).
Hilliard, M. A., Bargmann, C. I. & Bazzicalupo, P. C. Elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol 12, 730–734 (2002).
Oren-Suissa, M., Bayer, E. A. & Hobert, O. Sex-specific pruning of neuronal synapses in Caenorhabditis elegans. Nature 533, 206–211, doi: 10.1038/nature17977 (2016).
Li, W., Kang, L., Piggott, B. J., Feng, Z. & Xu, X. Z. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nature communications 2, 315, doi: 10.1038/ncomms1308 (2011).
Tobin, D. M. et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35, 307–318 (2002).
Yang, X. et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nature structural & molecular biology 22, 547–554, doi: 10.1038/nsmb.3038 (2015).
Zhou, K. M. et al. PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56, 657–669, doi: 10.1016/j.neuron.2007.09.015 (2007).
Harris, G. et al. Dissecting the signaling mechanisms underlying recognition and preference of food odors. The Journal of neuroscience: the official journal of the Society for Neuroscience 34, 9389–9403, doi: 10.1523/JNEUROSCI.0012-14.2014 (2014).
Wang, X., Li, G., Liu, J., Liu, J. & Xu, X. Z. TMC-1 Mediates Alkaline Sensation in C. elegans through Nociceptive Neurons. Neuron 91, 146–154, doi: 10.1016/j.neuron.2016.05.023 (2016).
Colbert, H. A., Smith, T. L. & Bargmann, C. I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. The Journal of neuroscience: the official journal of the Society for Neuroscience 17, 8259–8269 (1997).
Yoshida, K. et al. Odour concentration-dependent olfactory preference change in C. elegans . Nature communications 3, 739, doi: 10.1038/ncomms1750 (2012).
Geffeney, S. L. et al. DEG/ENaC but not TRP channels are the major mechanoelectrical transduction channels in a C. elegans nociceptor. Neuron 71, 845–857, doi: 10.1016/j.neuron.2011.06.038 (2011).
Kang, L., Gao, J., Schafer, W. R., Xie, Z. & Xu, X. Z. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 67, 381–391, doi: 10.1016/j.neuron.2010.06.032 (2010).
O’Hagan, R., Chalfie, M. & Goodman, M. B. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature neuroscience 8, 43–50, doi: 10.1038/nn1362 (2005).
Li, W., Feng, Z., Sternberg, P. W. & Xu, X. Z. A. C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440, 684–687, doi: 10.1038/nature04538 (2006).
Chalasani, S. H. et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70, doi: 10.1038/nature06292 (2007).
Wang, D., O’Halloran, D. & Goodman, M. B. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J Gen Physiol 142, 437–449, doi: 10.1085/jgp.201310959 (2013).
Liu, J. et al. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nature neuroscience 13, 715–722, doi: 10.1038/nn.2540 (2010).
Delmas, P. & Coste, B. Mechano-gated ion channels in sensory systems. Cell 155, 278–284, doi: 10.1016/j.cell.2013.09.026 (2013).
Zhang, W. et al. Ankyrin Repeats Convey Force to Gate the NOMPC Mechanotransduction Channel. Cell 162, 1391–1403, doi: 10.1016/j.cell.2015.08.024 (2015).
Ibsen, S., Tong, A., Schutt, C., Esener, S. & Chalasani, S. H. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nature communications 6, 8264, doi: 10.1038/ncomms9264 (2015).
Wu, Z. & Muller, U. Molecular Identity of the Mechanotransduction Channel in Hair Cells: Not Quiet There Yet. The Journal of neuroscience: the official journal of the Society for Neuroscience 36, 10927–10934, doi: 10.1523/JNEUROSCI.1149-16.2016 (2016).
Corey, D. P. & Holt, J. R. Are TMCs the Mechanotransduction Channels of Vertebrate Hair Cells? The Journal of neuroscience: the official journal of the Society for Neuroscience 36, 10921–10926, doi: 10.1523/JNEUROSCI.1148-16.2016 (2016).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Acknowledgements
We thank the Caenorhabditis Genetic Center which is supported by the National Institutes of Health - Office of Research Infrastructure Programs (P40 OD010440). This work was supported by grants from the Major National Scientific Research Projects of the Ministry of Science and Technology of China (2013CB945603, to L.J.K.), National High-tech R&D Program of China (2015AA020512), the National Foundation of Natural Science of China (31271180, 31471023, to L.J.K.), and the Zhejiang Natural Science Funds for Distinguished Young Scholars of China (LR14C090001, to L.J.K.).
Author information
Authors and Affiliations
Contributions
Wenjuan Zou and Lijun Kang designed the experiments. Wenjuan Zou, Hankui Cheng, Sitian Li, Yadan Xue and Xiaomin Yue conducted the experiments. Wenjuan Zou, Hankui Cheng, Sixi Chen and Lijun Kang analyzed and interpreted the results. Wenjuan Zou, Sixi Chen and Lijun Kang wrote the manuscript and modification was provided by all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zou, W., Cheng, H., Li, S. et al. Polymodal Responses in C. elegans Phasmid Neurons Rely on Multiple Intracellular and Intercellular Signaling Pathways. Sci Rep 7, 42295 (2017). https://doi.org/10.1038/srep42295
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42295
- Springer Nature Limited
This article is cited by
-
Polymodal Functionality of C. elegans OLL Neurons in Mechanosensation and Thermosensation
Neuroscience Bulletin (2021)
-
Serotonergic neuron ADF modulates avoidance behaviors by inhibiting sensory neurons in C. elegans
Pflügers Archiv - European Journal of Physiology (2019)
-
OSM-9 and an amiloride-sensitive channel, but not PKD-2, are involved in mechanosensation in C. elegans male ray neurons
Scientific Reports (2018)