Abstract
The role of circulating tumour cells (CTCs) in advanced oesophageal cancer (EC) patients undergoing concurrent chemoradiotherapy (CCRT) remains uncertain. A negative selection protocol plus flow cytometry was validated to efficiently identify CTCs. The CTC number was calculated and analysed for survival impact. The protocol’s efficacy in CTC identification was validated with a recovery rate of 44.6 ± 9.1% and a coefficient of variation of 20.4%. Fifty-seven patients and 20 healthy donors were enrolled. Initial staging, first response to CRT, and surgery after CRT were prognostic for overall survival, with P values of <0.0001, <0.0001, and <0.0001, respectively. The CTC number of EC patients is significantly higher (P = 0.04) than that of healthy donors. Multivariate analysis for disease-specific progression-free survival showed that surgery after response to CCRT, initial stage, and CTC number (≥21.0 cells/mL) played independent prognostic roles. For overall survival, surgery after CCRT, performance status, initial stage, and CTC number were significant independent prognostic factors. In conclusion, a negative selection plus flow cytometry protocol efficiently detected CTCs. The CTC number before CCRT was an independent prognostic factor in patients with unresectable oesophageal squamous cell carcinoma. Further large-scale prospective studies for validation are warranted.
Similar content being viewed by others
Introduction
Oesophageal cancer (EC) is the 7th–8th most common cancer and is the 7th most common cause of death related to cancer in the United States1 and Europe2,3, and also in Asia, including Taiwan4. There are two main histological types of EC: squamous cell carcinoma (ESCC) and adenocarcinoma (EAC). Currently, the former type accounts for the majority of cases of EC in African American, southern European, and Asian populations, whereas the incidence of the latter has tended to show gradual increases in the United States and northern Europe1,2,3,4. In EC that is unresectable or at locally advanced stages, regardless of the type, concurrent chemoradiotherapy (CCRT) has been the golden standard in treatment for decades5,6. Even in metastatic settings, palliative CCRT remains the main method of relieving symptoms resulting from cancer7,8. In recent years, several prognostic factors, such as the decreased number of lymph nodes after CCRT9, pathologic complete remission after CCRT and surgery10, and a history of heavy smoking11, have been found to be clinically prognostic in EC patients scheduled for CCRT. However, several biomarkers, such as microRNA (miRNA)12,13, NY-ESO-1 autoantibody14, and anti-P16 antibody15, are under investigation and awaiting large-scale clinical trials for evaluation.
Circulating epithelial or tumour cells (CECs or CTCs), identified and of interest since 186916, are defined as cells expressing epithelial cell surface markers and/or tumour specific marker(s) and must simultaneously be excluded from red/white blood cells (RBCs/WBCs) in the circulation. These cells have been thought to be live cells shed from the primary tumour mass, which are cultivable17,18, have the potential to metastasise into distant organs19, promote thrombosis12, acquire resistance to anticancer drugs20,21,22,23, and have been proven to be prognostic and predictive in patients with various kinds of solid tumours24,25,26,27,28,29. Even more, CTCs could also potentially guide anticancer therapies22,30. Development of a reliable method of detection or isolation of CTCs could represent a good biomarker or predictor before the initiation of anticancer treatment in cancer patients. The major limitation in the efficacy of CTC isolation has spurred advances in nanoscience31, biochips18,32, physiology31,33, chemistry, and novel surface markers34,35,36, as well as many new methods or devices37. Although the CellSearch® system was developed and approved by the US Food and Drug Administration (FDA) in 2004, there is still no standard method or protocol to identify or isolate CTCs because of the relatively low efficiency of detection to date. In our opinion, a cheap and easy-to-access protocol or device is urgently needed.
For EC patients, the role of CTCs remains unclear in the literature. Therefore, to elucidate the clinical relevance of CTCs in patients with locally advanced ESCC, which is the most common type of EC at diagnosis, we prospectively designed and conducted a trial in a single medical centre in Taiwan. In addition, we attempted to report the efficacy of a relatively easy-to-perform method for CTC detection to enhance the advances in the field of CTCs.
Material and Methods
Study Design
The study was designed to be a prospective observational study. We aimed to elucidate the clinical significance of baseline CTCs before CCRT of unresectable ESCC patients. To determine a cutoff of CTC number for further survival analysis, we designed to use the cutoff found by ROC curves with Youden test (Supplementary Table S1) in EC patients (n = 57) and healthy donors (n = 20) in this pilot study. The endpoints of the study were to find the correlations among baseline CTCs, progression-free survival (PFS), and overall survival (OS). Following treatment response, surgery, disease progression and death from any causes were documented for survival analysis. The analysis was done only after when more than half of the events have occurred. A score combining treatment response and baseline CTC categories was designed to be analysed for its prediction ability of cancer death.
Patient Enrolment
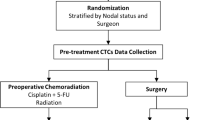
This study was conducted in a single medical centre, Chang Gung Memorial Hospital, in Linkou, Taiwan. The study protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval ID: 101-2161C). All patients provided written informed consent for the ethically approved protocols. Eligible patients with histologically or cytopathologically confirmed ESCC were all medically unfit for surgery or had surgically unresectable, locally advanced (stage IIb–IV, American Joint Committee on Cancer [AJCC] 7th edition), or metastatic cancer at initial presentation. Other enrolment criteria included: (1) age ≥ 20 years old; (2) patients who could understand and sign the informed consent by their own will; and (3) patients with adequate liver and renal function and WBC counts for anticancer therapies, especially for CCRT. Patients with synchronous cancer or prior cancers within 5 years, except for non-melanoma skin cancers and in situ cervical cancers, were excluded at enrolment. Disease staging and management followed the standard treatment protocols according to institutional guidelines. Blood samples were drawn within 7 days before the first dose of chemotherapy. Study results were reported following the REporting recommendations for tumour MARKer prognostic studies (REMARK) guidelines38. All the methods were carried out in accordance with the relevant guidelines, including any relevant details. Examinations for initial staging and response evaluation included computed tomography (CT), pan-endoscopy, endoscopic ultrasonography (EUS), positron emission tomography (PET), and/or bronchoscopy, except in patients who initially had metastatic lesions on CT scans. EUS was performed with an ultrasonic miniprobe (UM2R/12 MHz or UM3R/20 MHz; Olympus, Inc., Tokyo, Japan). Following the standard treatment guidelines, CCRT and/or surgery were scheduled and delivered by the medical oncologists, radiation oncologists, and chest surgeons on the oesophageal tumour board at Chang Gung Memorial Hospital. Based on the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0 guidelines, the treatment response, including complete remission (CR), partial response (PR), stable disease (SD), and progressive disease (PD), was determined by the multidisciplinary oesophageal tumour board.
Chemotherapy Regimens of Chemoradiotherapy
Two chemotherapy regimens of CCRT were scheduled. One was 5-fluorouracil (1,000 mg/m2/day, administered as a continuous infusion over a period of 96 h on days 1–4 and 29–33) plus cisplatin (75 mg/m2, administered as an intravenous infusion for 3 h on days 1–29), which was called the PF regimen. The other regimen was carboplatin (area under the curve of 2, weekly) plus paclitaxel (50–60 mg/m2, weekly) for 6 consecutive doses, which was called the TC regimen. Radiotherapy was administered either sequentially to PF chemotherapy on days 8–29 to a total dose of 30 Gy (200 cGy/fraction) or concurrently with TC chemotherapy to a total dose of 41.4 Gy (180 cGy/fraction).
Clinical Assessment of Chemoradiotherapy Effectiveness and Consolidation Therapy for Patients Who Did Not Undergo Oesophagectomy
The clinical response to CCRT was determined based on the results of endoscopy and imaging findings (CT and oesophagography) at 5–6 weeks post-treatment. As of 2007, PET scans were used for both staging and restaging workups. The response was considered complete (cCR) in the presence of the following criteria: (1) no evidence of disease on CT scans and no increased tracer uptake on PET images; and (2) no stricture, no residual tumour nor ulcer identified by panendoscopy, and negative biopsy results. Endoscopic findings in cCR patients were further classified into three categories: (1) Scar: healing ulcer; (2) Other finding: other abnormal mucosal findings (e.g., mucosa tag, polypoid lesion, granular protruded lesions, erosion, and lugol-voiding lesions); and (3) Normal: patients who did not show any mucosal abnormality. In the absence of contraindications, oesophagectomy was scheduled in all patients. Eligibility for surgery was based on the following: (1) medical fitness for surgery, with absence of liver cirrhosis and/or heart failure (New York Heart Association class III or IV); (2) absence of tracheoesophageal fistula; and (3) no evidence of recurrent laryngeal nerve invasion. For patients who refused scheduled surgery after neoadjuvant chemoradiotherapy (nCRT), another course of CRT, consisting of PF or TC chemotherapy, was given as consolidation therapy. The total radiation dose was 60 Gy.
Experiments for Efficiency of Detection and Patients’ Sample Analysis
CTC analysis was performed using a protocol with combined negative selection and positive detection strategies. Briefly, the methods were (1) a negative selection protocol for effective RBC and leukocyte depletion with Ficoll isolation and a CD45 depletion kit; and (2) flow cytometry to quantitatively identify the number of CTCs. The concept of the protocol is illustrated in Fig. 1a. For control and validation experiments with head and neck cancer cell lines, the details were described in the Supplementary file.
Statistical Analysis
The numbers of pre-CCRT CTCs in advanced EC patients and healthy donors were compared using box plots and the Mann-Whitney U test with two-sided significance. Factors influencing the survival of patients with EC were assessed by univariate and multivariate Cox proportional hazards regression analysis. Parameters with significance in univariate analysis were subjected to multivariate Cox regression analysis. Disease-specific PFS was calculated from the date of CTC sampling, within 7 days before the start of CCRT, to the date of cancer-specific progression. OS was defined as the period from the date of CTC sampling to the date of death from any cause. The circulating tumour cell plus response (CTCR) score is defined as the summation of the CTC score (zero for CTC number less than 21.0 cells/mL; 1 for CTC number ≥ 21.0 cells/mL) and the score of the CCRT response (zero for CR; 1 for PR; 2 for SD; and 3 for PD). Statistical analysis was performed using SPSS for Windows (version 18, SPSS Inc., Chicago, IL, USA). A P value of 0.05 was considered statistically significant.
Results
The Protocol Efficiency, Recovery Rate, and Purity
The efficacy of the protocol in identifying CTCs was validated with a recovery rate of 44.6 ± 9.1% and a % coefficient of variation (CV) of 20.4% in cell line spiking experiments (n = 20). Blood samples from healthy individuals were used as a control group, and the recovery rate of flow cytometry was 71.1 ± 12.0% with a %CV of 16.8% (experiment n = 43, 20 individuals in total).
Patient Enrolment
Between December 2012 and December 2014, a total of 57 patients were enrolled after detailed introduction of the trial design, scientific goals, and inconvenience/risks of participation in the Division of Haematology-Oncology, Chang Gung Memorial Hospital in Linkou. Meanwhile, 20 healthy donors were also recruited as controls for the CTC analysis. Basic characteristics of the enrolled patients are shown in Table 1. Clinical information and survival data were updated until August 2015. The median age of the patients was 54 (36–78) years old. The majority of tumours were of the moderately differentiated type (68.4%), and the most frequent tumour locations in our population were the middle (36.8%) and upper part (36.1%) of the oesophagus. In addition, most patients scored an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 (61.4%), whereas the other 38.6% scored ≥2.
The Impacts of Initial Staging and Surgery After Chemoradiotherapy
Figure 2a–d demonstrates the prognostic survival impact of the initial AJCC (7th edition) staging, first treatment response to CRT, surgery after CRT, and undergoing surgery, with the exception of patients with initial T4b and M1 status, with P values of <0.0001, <0.0001, <0.0001, and 0.005, respectively. In patients with imaging CR, PR, SD, and PD to CCRT, the median OS values were not reached, 15.9, 7.7, and 4.2 months, respectively, with a log rank test P value of <0.001.
Panel A demonstrates that initial TNM staging correlates with overall survival (OS). Treatment response after concurrent chemoradiotherapy (CCRT) also correlates with OS (Panel B). Residual tumour with or without surgery is also highly prognostic for OS (Panel C); patients with initial T4b and M1 status are excluded (Panel D).
Clinical Relevance of Circulating Tumour Cells Before Chemoradiotherapy
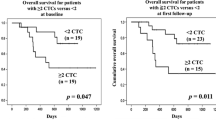
The CTC numbers of patients with EC (n = 57) were significantly higher (Mann-Whitney T test; P = 0.04) than those of healthy donors (n = 20) (Fig. 3a). A cutoff of 21.0 cells/mL with a sensitivity of 52.6% and a specificity of 80.0% was obtained by ROC curve (details in Supplementary Table S1) to differentiate EC patients from healthy individuals. After cox-proportional hazards model was examined, the following analysis showed that the CTC number before CCRT can serve as a prognostic factor for disease-specific PFS and OS in patients with advanced squamous cell carcinoma with log rank test P values of 0.041 and 0.021, respectively (Fig. 3b,c). In multivariate analysis for disease-specific progression, surgery after response to CCRT, initial AJCC TNM stage, and CTC number (≥21.0 cells/mL) were found to be independent prognostic factors with P values of 0.001, <0.001, and 0.004, respectively (Table 2). In multivariate analysis for OS, surgery after response to CCRT, ECOG performance status, initial TNM stage (AJCC 7th edition), and CTC number were found to be independent prognostic factors with P values of <0.001, <0.001, 0.003, and 0.028, respectively (Table 2). Cancer location and chemotherapy regimen did not impact the OS (P = 0.515 and 0.136, respectively; Supplementary Figure S1A–C). The baseline CTC number group is predictive for response to CCRT and locoregional or distant failure with P values of 0.001 and 0.027, respectively, by the chi-square test (Supplementary Table S2). In further analysis, the CTCR score was found to be able to significantly differentiate patients with advanced EC into groups with distinct OS after first response evaluation (Fig. 3d and Supplementary Figure S1D). However, there was no clear correlation among CTC number and clinical T stage (P = 0.088), N stage (P = 0.164), and initial M status (P = 0.400) by the chi-square test (Table not shown).
The method of circulating tumour cell (CTC) detection can differentiate healthy individuals from patients with advanced oesophageal cancer with a P value of 0.04 using the Mann-Whitney U test (Panel A). Panels B and C show that patients with a lower pre-treatment CTC number have longer disease-specific progression-free survival or overall survival. Given the CTC status (score zero for CTC number less than 21.0 cells/mL; 1 for CTC number ≥ 21.0 cells/mL) and response after concurrent chemoradiotherapy (CCRT, score zero for complete remission; 1 for partial response; 2 for stable disease, and 3 for progressive disease), the summation of the CTC score and the score of response to CCRT is defined as the circulating tumour cell plus response (CTCR) score, and this score highly correlates with overall survival with a log rank test P value of <0.0001.
Discussion
This study firstly demonstrated an overall recovery rate of 44.6 ± 9.1% and a %CV of 20.4% using a simple, cheap, and widely available CTC analysis method with Ficoll isolation and CD45 depletion by magnetic beads followed by flow cytometry for detection. The detection rate of CTCs was 100.0% and a cutoff of 21.0 cells/mL was obtained by ROC curve (P = 0.04). Not surprisingly, the initial staging, surgery after CCRT, and the response to CCRT contributed to the OS of ESCC patients with statistical significance. (P < 0.0001, <0.0001, and <0.0001, respectively) Taking into consideration of all the above factors, the pre-CCRT CTC number showed its independent prognostic impact on disease-specific PFS and OS with hazard ratios (HRs) (95% confidence interval [CI]) of 3.113 (1.427–6.791) and 1.002 (1.000–1.004), respectively. In addition, the CTCR score, which was first proposed in the literature, could clearly separate the OS with statistical significance (P < 0.001), by considering both the CTC category (low, high) and responses (CR, PR, SD + PD). The CTCR score might be able to provide group selection in future clinical trials. To the best of our knowledge, this is the first report using a negative selection strategy combined with flow cytometry to perform CTC analysis in advanced ESCC patients who underwent CCRT that proved the independent prognostic role of CTCs.
In the literature, there are only a few studies that address the role of CTCs in EC patients. Table 3 summarizes the most important information from these studies. In 2002, Koike, M. et al. reported the first work using reverse transcription-polymerase chain reaction (RT-PCR) to detect tumour-specific carcinoembryonic antigen (CEA) messenger RNA (mRNA) in the circulation and concluded that the CEA mRNA measured by RT-PCR could be more sensitive than conventional serum tumour markers (CEA and squamous cell carcinoma [SCC])39. Also utilizing RT-PCR, Nakashima, S. et al. found in 2003 that CEA mRNA detection could predict cancer recurrence40. In the following year, Ito et al. found that CEA combined with cytokeratin 20 (CK20) mRNA (CEA assay) could also predict tumour recurrence better than serum tumour markers41. In 2007, Ikoma, D. et al. used multiple mRNAs, including CEA, p16, E-cadherin, and retinoic acid receptor (RAR) to monitor EC cancer after curative surgery. In the same year, Liu, Z. et al. used CEA mRNA from peripheral blood cells in a Chinese population to prove that the CEA mRNA expression detected by RT-PCR could predict distant metastasis in EC patients who underwent curative surgery42. In addition to patients with resectable tumours, Yin, X. D. et al. chose EC patients scheduled to undergo radical radiotherapy, and reported that the detection of CEA+CK19+ survivin mRNA by RT-PCR could be a promising biomarker for radiation efficiency and assessment of prognosis. Until 2012, RT-PCR for tumour-specific mRNA was the main method for CTC detection. However, cancer cells were not actually captured in these studies, and cancer-specific mRNA expression might not truly correlate with the cancer cell count in the circulation. In 2014, Bobek, V. et al. from the Czech Republic reported on an interesting device utilising a size-based mechanism to literally capture CTCs43. The report concluded that the method could capture CTCs and prove that these cells are alive for further cultivation. This was a novel and important finding, but no clinical relevance was reported with the use of this device. The CellSearch® system (Janssen Diagnostics, LLC, Raritan, NJ, USA), an FDA-approved device using a positive selection strategy to identify CTCs, was used by three groups, Matsushitam et al. Reeh et al. and Tanaka et al., and the correlations with treatment response and an independent prognostic role of CTCs were impressive26,44,45. However, one of the drawbacks of the Cellsearch® system was its known detection rate, suggesting a possible loss of CTC information. Furthermore, performing CTCs analysis with the CellSearch® system is relatively costly and device-dependent. Theoratically, our method, utilising negative depletion of CD45+ cells and positive selection of EpCAM and cytokeratins with flow cytometry, is capable of providing clinicians and medical researchers a much easier and cheaper platform to conduct CTC-related clinical trials. Moreover, our study focused on patients with unresectable or metastatic status, a group seldom investigated in the literature.
One subject we should particularly discuss is that the survival of enrolled patients in our study was relatively short when compared with that of the general EC population. Several plausible reasons include, firstly, the fact that the study only enrolled Asian patients with ESCC and that 38.6% of the patients were classified as ECOG PS ≥ 2, which means that the status of the population could be worse than that of general, locally advanced EC patients. Secondly, there are some differences amongst Western and Asian patients with EC, including major histological types, risk factors, male-to-female ratio, and clinical outcomes. In comparison with Western countries, the major histology in Asian EC patients is still SCC, and this accounts for 91.5% of EC patients in Taiwan46, while in some areas of the United States, up to 60% of patients are diagnosed with AC1. Poor outcomes in Taiwanese patients could be explained as a result of relatively low socioeconomic status and exposure to multiple carcinogens, including cigarettes, alcohol, and betel nut chewing47. Thirdly, the male-to-female ratio (27.5 to 1) in our study is not comparable to that of the general EC population, which might partially explain the short survival in this study. More specifically, the male-to-female ratio is approximately 13.57 to 14 according to the Taiwan Cancer Registry database, whereas a ratio of 3 to 1 is commonly noted in Western countries1,2. The phenomenon of male populations harbouring worse outcomes than those for women has also been noticed in some southern European countries2. These reasons could explain the short survival, but would not confuse the role of CTCs after statistical adjustment.
Although the current study successfully demonstrated the correlations among the number of CTCs, the disease-specific PFS and OS, and the cutoff of CTCs with clinical impact, these could change with different methods of CTC detection. In fact, different methods would yield different recovery rates and purity. We should note that the CTC number obtained by different methods should not be compared for clinical significance. Furthermore, the cutoff value with one type of CTC detection method is different from another. That is, the concept of a “low” or “high” CTC number would be relatively adequate rather than setting a definite CTC number cutoff for all solid tumours. In the present study, an easy-to-perform, controlled, cheap, and commonly available method is proposed to help foster the availability of CTC studies in every medical laboratory. Sample loss could be a problem with this method, but this could be overcome through careful procedures conducted by well-trained technicians. Although we found a cutoff to separate EC patients into two groups with survival difference, the relatively small sampling size limited the accuracy of the cutoff in this pilot study. In the near future, a satisfactory cutoff value will possibly be decided upon after the collection of large-scale information using one single method with similar controls. Possible reasons that could explain the false positivity of this method include the background noise or non-significant epithelial cells in the circulation, and further long-term observations for these patients are still needed. Another confirmation method may be possible with the sequencing of cancer-specific DNA mutations on CTCs to avoid bias. Although the technique of DNA mutation detection on CTCs has been proposed, it still seems to be highly technique-dependent48,49.
There are still some limitations of this study to discuss. Firstly, patients with T4b cancer and those with cervical EC were enrolled in this study, which could contribute some bias. These patients would not undergo surgery due to their disease status even with the achievement of PR after CCRT. One other problem was the fact that some patients, mostly of cT4b status, who died of sudden massive tumour bleeding and/or sepsis from tracheoesophageal fistula during CCRT, caused shortening of the survival in this study. Secondly, the Cellsearch® system can generate a detection rate (≧5 cells/7.5 mL blood) of 0~48%50, a recovery rate of 80~82%51 and an inter-laboratory % CV of 45~64%52. We admit that our recovery rateof our protocol was lower than Cellsearch®; however, our protocol was designed to be very easily obtained and performed. The relatively low recovery was probably resulted from Ficoll separation procedure which lost a part of CTCs53, but it could separate red blood cells completely and allow further molecular analysis to proceed. Nevertheless, given consideration of these conditions, CTCs could still play an important role in patients with EC. Further studies on females or patients with EAC in the Asian population are warranted.
In conclusion, we attempted to develop, validate, and report on an easy-to-use platform for CTC analysis that is mostly available in medical laboratories. The CTC number before CCRT was proven to be an independent prognostic factor in patients with unresectable ESCC. However, as with many proof-of-concept reports, further large-scale prospective studies to determine a better cutoff value are warranted.
Additional Information
How to cite this article: Su, P.-J. et al. Circulating Tumour Cells as an Independent Prognostic Factor in Patients with Advanced Oesophageal Squamous Cell Carcinoma Undergoing Chemoradiotherapy. Sci. Rep. 6, 31423; doi: 10.1038/srep31423 (2016).
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J Clin 65, 5–29 (2015).
Castro, C. et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann Oncol 25, 283–290 (2014).
Bosetti, C. et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer 122, 1118–1129 (2008).
World Health Organization Statistical Information System. WHO Mortality Database. Available at http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index.html (Accessed: 25th November 2015).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Esophageal cancer, version 3. Available at http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. (Accessed: 30th December 2015).
Versteijne, E. et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus 28, 453–459 (2015).
Ikeda, E. et al. Efficacy of concurrent chemoradiotherapy as a palliative treatment in stage IVB esophageal cancer patients with dysphagia. Jpn J Clin Oncol 41, 964–972 (2011).
Akl, F. M., Elsayed-Abd-Alkhalek, S. & Salah, T. Palliative concurrent chemoradiotherapy in locally advanced and metastatic esophageal cancer patients with dysphagia. Ann Palliat Med 2, 118–123 (2013).
Aiko, S. et al. Reduction rate of lymph node metastasis as a significant prognostic factor in esophageal cancer patients treated with neoadjuvant chemoradiation therapy. Dis Esophagus 20, 94–101 (2007).
Park, J. W. et al. Prognosis of esophageal cancer patients with pathologic complete response after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 81, 691–697 (2011).
Shitara, K. et al. Heavy smoking history interacts with chemoradiotherapy for esophageal cancer prognosis: a retrospective study. Cancer Sci 101, 1001–1006 (2010).
Jiang, Z. et al. Serum microRNA-218 is a potential biomarker for esophageal cancer. Cancer Biomark 15, 381–389 (2015).
Wang, C. et al. Clinical potential of miR-3651 as a novel prognostic biomarker for esophageal squamous cell cancer. Biochem Biophys Res Commun 465, 30–34 (2015).
Oshima, Y. et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: screening in 1969 patients with various cancers. J Gastroenterol 51, 30–34 (2016).
Jin, Y. et al. Anti-p16 autoantibodies may be a useful biomarker for early diagnosis of esophageal cancer. Asia Pac J Clin Oncol 11, e37–e41 (2015).
Ashworthm, T. R. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australian Med J. 14, 146 (1869).
Yu, M. et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Sheng, W. et al. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab on a chip 14, 89–98 (2014).
Cheng, M., Liu, L., Yang, H. S. & Liu, G. F. Circulating tumor cells are associated with bone metastasis of lung cancer. Asian Pac J Cancer Prev 15, 6369–6374 (2014).
Nadal, R. et al. CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int J Cancer 133, 2398–2407 (2013).
Yu, K. H. et al. Pharmacogenomic modeling of circulating tumor and invasive cells for prediction of chemotherapy response and resistance in pancreatic cancer. Clin Cancer Res 20, 5281–5289 (2014).
Abdallah, E. A. et al. Thymidylate synthase expression in circulating tumor cells: A new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients. Int J Cancer 137, 1397–1405 (2015).
Gradilone, A. et al. How circulating tumor cells escape from multidrug resistance: translating molecular mechanisms in metastatic breast cancer treatment. Am J Clin Oncol 34, 625–627 (2011).
Lu, C. Y. et al. Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br J Cancer 108, 791–797 (2013).
Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 29, 1556–1563 (2011).
Matsushita, D. et al. Clinical Significance of Circulating Tumor Cells in Peripheral Blood of Patients with Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 22, 3674–3680 (2015).
Ma, X. et al. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: a systematic review and meta-analysis. Tumour Biol 35, 5551–5560 (2014).
Hsieh, J. C. et al. Prognostic value of circulating tumor cells with podoplanin expression in patients with locally advanced or metastatic head and neck squamous cell carcinoma. Head & neck 37, 1448–1455 (2015).
Friedlander, T. W. et al. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int J Cancer 134, 2284–2293 (2014).
Musella, V. et al. Circulating tumor cells as a longitudinal biomarker in patients with advanced chemorefractory, RAS-BRAF wild-type colorectal cancer receiving cetuximab or panitumumab. Int J Cancer 137, 1467–1474 (2015).
Stec, M. et al. Isolation and characterization of circulating micro(nano)vesicles in the plasma of colorectal cancer patients and their interactions with tumor cells. Oncol Rep 34, 2768–2775 (2015).
Alix-Panabieres, C. & Pantel, K. Technologies for detection of circulating tumor cells: facts and vision. Lab on a chip 14, 57–62 (2014).
Huang, S. B. et al. High-purity and label-free isolation of circulating tumor cells (CTCs) in a microfluidic platform by using optically-induced-dielectrophoretic (ODEP) force. Lab on a chip 13, 1371–1383 (2013).
Chiu, T. K. et al. Development of a microfluidic-based optical sensing device for label-free detection of circulating tumor cells (CTCs) through their lactic acid metabolism. Sensors (Basel) 15, 6789–6806 (2015).
Barbazan, J. et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer 135, 2633–2643 (2014).
Zhang, Y. et al. Patterns of circulating tumor cells identified by CEP8, CK and CD45 in pancreatic cancer. Int J Cancer 136, 1228–1233 (2015).
Andreopoulou, E. et al. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int J Cancer 130, 1590–1597 (2012).
McShane, L. M. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93, 387–391 (2005).
Koike, M. et al. Molecular detection of circulating esophageal squamous cell cancer cells in the peripheral blood. Clin Cancer Res 8, 2879–2882 (2002).
Nakashima, S. et al. Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery 133, 162–169 (2003).
Ito, H. et al. Detection and quantification of circulating tumor cells in patients with esophageal cancer by real-time polymerase chain reaction. J Exp Clin Cancer Res 23, 455–464 (2004).
Liu, Z., Jiang, M., Zhao, J. & Ju, H. Circulating tumor cells in perioperative esophageal cancer patients: quantitative assay system and potential clinical utility. Clin Cancer Res 13, 2992–2997 (2007).
Bobek, V. et al. Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem Cytobiol 52, 171–177 (2014).
Reeh, M. et al. Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients With Esophageal Cancer. Ann Surg 261, 1124–1130 (2015).
Tanaka, M. et al. Prognostic significance of circulating tumor cells in patients with advanced esophageal cancer. Esophagus 12, 352–359 (2015).
Bureau of Health Promotion, Department of Health, the Executive Yuan, Taiwan. Cancer Registry Annual Report in 2012, Taiwan. Available at: http://www.hpa.gov.tw/BHPNet/Web/Stat/Statistics.aspx. (Accessed: February 2015).
Lee, C. H. et al. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int J Cancer 113, 475–482 (2005).
Mostert, B. et al. KRAS and BRAF mutation status in circulating colorectal tumor cells and their correlation with primary and metastatic tumor tissue. Int J Cancer 133, 130–141 (2013).
Kanwar, N. et al. Identification of genomic signatures in circulating tumor cells from breast cancer. Int J Cancer 137, 332–344 (2015).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10, 6897–6904 (2004).
Hong, B. & Zu, Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics 3, 377–394 (2013).
Kraan, J. et al. External quality assurance of circulating tumor cell enumeration using the CellSearch system: a feasibility study. Cytometry B Clin Cytom 80, 112–118 (2011).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13, 920–928 (2007).
Acknowledgements
This work was supported by the National Science Council Grants MOST 103-2314-B-182A-064- and 105-2314-B-182A-030-; Chang Gung Memorial Hospital Grants CMRPG3E1631-32, CMRPG3E0511-12 to C.H. Hsieh, NMRPD1E011 to H.M. Wang and CMRPD2D0041-42 to M.H. Wu. In addition, the authors thank all the members of the Cancer Centre, Chang Gung Memorial Hospital, for their invaluable help.
Author information
Authors and Affiliations
Contributions
Dr. P.-J.S., Dr. H.-M.W., Dr. W.-K.H., Dr. C.-E.W. and Dr. C.-H.H. designed and ran the prospective clinical trial. They enrolled patients from their clinics, monitored the safety, and ethical issues during the trial. Professor M.-H.W., Dr. T.-K.C., Ms. N.M.-J.L., Ms. S.-R.Y., Ms. J.Y.-C.L. and Dr. C.-H.H. carried out the isolation of circulating tumour cells from cancer patients, identified, and enumerated typical cells. Dr. H.-K.C., Dr. Y.-K.C. and Dr. C.-K.T. independently confirmed the clinical outcomes, such as events of progression and cancer-related death, and did the statistical analysis for clinical correlations. Finally, Dr. P.-J.S., Dr. H.-M.W., Professor M.-H.W., Dr. C.-L.L. and Dr. C.-H.H. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Su, PJ., Wu, MH., Wang, HM. et al. Circulating Tumour Cells as an Independent Prognostic Factor in Patients with Advanced Oesophageal Squamous Cell Carcinoma Undergoing Chemoradiotherapy. Sci Rep 6, 31423 (2016). https://doi.org/10.1038/srep31423
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31423
- Springer Nature Limited
This article is cited by
-
Clinical value of folate receptor-positive circulating tumor cells in patients with esophageal squamous cell carcinomas: a retrospective study
BMC Cancer (2023)
-
Karyotyping of circulating tumor cells for predicting chemotherapeutic sensitivity and efficacy in patients with esophageal cancer
BMC Cancer (2019)
-
Baseline circulating stem-like cells predict survival in patients with metastatic breast Cancer
BMC Cancer (2019)
-
The Prognostic Value of Circulating Tumor Cells in Asian Neuroendocrine Tumors
Scientific Reports (2019)