Abstract
The aim of this study was to evaluate the antifungal activity of essential oils (EOs) of Citrus sinensis (C. sinensis) and Citrus latifolia (C. latifolia) against five Candida species: Candida albicans, Candida tropicalis, Candida glabrata, Candida lusitaniae and Candida guilliermondii; and perform its genotoxic evaluation. The EOs of C. sinensis and C. latifolia were obtained from the peel by hydro-distillation. The major components determined by GC-MS were in C. sinensis, d-limonene (96%) and α-myrcene (2.79%); and in C. latifolia, d-limonene (51.64%), β-thujene (14.85%), β-pinene (12.79%) and γ-terpinene (12.8%). Antifungal properties were studied by agar diffusion method, where C. sinensis presented low activity and C. latifolia essential oil was effective to inhibit growing of C. lusitaniae and C. guilliermondii with IC50 of 6.90 and 2.92 μg respectively. The minimum inhibitory concentrations (MIC) for C. sinensis were in a range of 0.42–3.71 μg and for C. latifolia of 0.22–1.30 μg. Genotoxic evaluation was done by Ames test where none of the oils induced point mutations. Flow cytometry was used to measure toxicity in human oral epithelial cells, C. sinensis was not cytotoxic and C. latifolia was toxic at 21.8 μg. These properties might bestow different odontological applications to each essential oil.
Similar content being viewed by others
Introduction
Oral candidiasis (OC) is a mucosal illness caused by infection of Candida species, mainly by Candida albicans (C. albicans)1. These infections have been described as a secondary complication in several diseases and it frequently sprouts in immunosuppressed patients that underwent high exposition to antibiotics and corticosteroids. OC is also common on pediatric and elderly patients2,3,4.
The incoming infection with most Candida species, other than C. albicans and the growing evidence of antifungal resistance, leads necessarily to seek for new therapeutic alternatives5,6,7,8. Throughout human history there has been increasing interest in natural alternative medicine and nowadays, active principles of medical plants are the focus of scientific papers9. In this regard, several studies have shown antioxidant properties derived from flavonoids, carotenoids and vitamin C, which are present at high concentrations in the Citrus genus10,11,12,13. Essential oils (EOs) from Citrus species like C. sinensis, C. aurantium, C. deliciosa, C. paradise, C. reticulate, C. limon, C. aurantifolia, C. maxima, have also shown antibiotic and antimycotic activities14,15,16,17,18,19,20,21. Nonetheless, the biological activities of EOs from C. sinensis and C. latifolia are still unknown.
The genotoxic effects of many plant extracts and EOs in humans cannot be underestimated, despite its natural origin22,23,24,25,26,27,28. Genotoxic evaluation of drugs and any chemical compound or mixtures being considered for clinical applications must be performed accordingly to international institutions such as FDA, IARC and EPA15. The Ames test is perhaps the first selected genotoxic evaluation test, designed to detect induced DNA point mutations, it is recommended for its high carcinogenic predictive value and high specificity for detecting mutagenic activities of possible carcinogens in the subsequent rodent trials. Negative results from an Ames test are associated to safety but it is frequently required a second step of validation onto eukaryotic cells system. Additionally, it is highly recommended to have certainty that a new product with potential use in alternative medicine will not be toxic for its target cells29,30.
The aim of this study was to evaluate EOs from C. sinensis and C. latifolia as antimycotic on Candida albicans, Candida tropicalis, Candida glabrata, Candida guilliermondii and Candida lusitaniae isolated from elderly patients assisting a geriatric clinic of a third-level National Hospital (Hospital Juarez de México). Two additional test of safe usage were performed: Genotoxic evaluation by means of the Ames test and toxicity against oral epithelium cells.
Material and Methods
Reagents
EOs of C. sinensis and C. latifolia were obtained by hydro-distillation of the peel, kindly donated by Frutech International Corporation Cargee Additives, Montemorelos Nuevo León México. Picrolonic Acid (PA), Methyl-N´nitro-N-nitrosoguanidine (MNNG), 2-amino-anthracene (2AA), 4-nitro-quinone-oxide (4NQO) was purchased from Sigma Chemical Co. St. Louis Missouri, USA. Dimethyl sulfoxide (DMSO) was obtained by J.T. Baker Xalostoc, Mexico. Aroclor-1254 was obtained from Supelco Bellefonte, PA; S9 aroclor-1254 induced rat liver homogenate (S9 mix), was prepared as described by Maron and Ames31. Amphotericin B was acquired from Laboratorios Pisa S.A. de C.V. México. 7-Aminoactinomycin D (7-AAD) was obtained from Becton Dickinson, PharmingenTM USA.
Biological material
Salmonella typhimurium strains, TA98, TA100 and TA102, were kindly donated by Dr. Bruce Ames, Berkley University CA, USA. C. albicans, C. tropicalis, C. glabrata, C. guilliermondii and C. lusitaniae, were previously isolated from the oral cavity of elderly patients from 60 to 104 years old, they were attending to geriatric clinical care (unpublished results).
Ethical Considerations
Samples were taken from oral epithelium of elderly patients with clinical data of oral candidiasis. All participants provided informed consent and all experimental methods were carried out in accordance with the approved guidelines. The institutional Comittees of Research, Ethics and Biosafety from Hospital Juárez de México approved the protocol under registration number: HJM2112/12-B and in accordance with “Reglamento de la Ley General de Salud en Materia de Investigación para la Salud” (http://www.conbioetica-mexico.salud.gob.mx/descargas/pdf/normatividad/normatinacional/10._NAL._Reglamento_de_Investigacion.pdf).
GC-MS analysis
Gas chromatography coupled to a JEOL GCmate mass spectrometer operating in electron ionization (EI) mode were used for component analyses. Mass spectra were acquired scanning from m/z 20 to m/z 250. The constituents of the oils were identified by using standard reference compounds and also by matching the mass spectra fragmentation pattern with NIST Mass Spectra Library in the GC-MS database.
Antimycotic activity
Antimycotic activities of C. sinensis and C. latifolia EOs were evaluated against previously isolated strains from patients: C. albicans, C. tropicalis, C. glabrata, C. lusitaniae and C. guilliermondii. Antimycotic studies were done with the agar diffusion method; 20 μl of McFarland 0.5 dilutions (equivalent to 6 × 104 CFU)32 were prepared on sterile isotonic saline, from each evaluated Candida. These solutions were inoculated on Sabouraud agar on sterile glass Petri dishes. Then agar was perforated with a 5 mm sterile penicylinders and 50 μl of the EO containing the concentrations described in Table 1. Sterile saline and tween 20% solutions were used as negative controls, amphotericin B was evaluated as a positive control (0.16 to 80 mg/hole) accordingly to M27-A3 document from the Clinical Laboratory Standard Institute (CLSI)33. Petri dishes were incubated at 37 °C for 24 hours and inhibition zones were measured. Data were analysed by one-tailed univariant Bonferroni test using SPSS v10 software. Logarithmic regression was performed to calculate values of IC50 and Minimum Inhibitory Concentrations (MICs).
Genotoxic evaluation
Mutagenicity assays
In order to evaluate the bactericide properties of both EOs, 100 μl of overnight cultures of S. typhimurium (accordingly to Maron and Ames)31 were exposed to several dilutions (Table 2). Strains were diluted to 10−5 on sterile saline solution and poured on nutrient agar plates. Plates with no treatment were considered as 100% survival rate. Cultures with survival higher to 80% were chosen for Ames method testing.
Mutagenicity evaluation was done accordingly to Maron and Ames31. Positive controls were 2AA (10 μg/Petri dish) with S9 mix for TA98, TA100 or TA102 strains. Positive controls without S9 mix were PA (50 μg/Petri dish) for TA98, MNNG (10 μg/Petri dish) for TA100 or 4NQO (10 μg/Petri dish) for TA102 strains. EO concentrations presented on Table 2 were evaluated in each strain either with or without S9 mix. Treated cells were poured on Vogel Bonner medium plates, incubated for 48 hrs and then were counted on a semi-automatic fisher colony counter. Results were considered positive when the number of histidine+ revertants was twice the obtained on spontaneous reversion plate.
Cytotoxicity assays
Cytotoxicity assays were done using human epithelial cells obtained after scrubbing with interdental brushes the internal oral mucosa of both right and left cheeks from healthy non-smoker volunteers, as described34 and samples were transported on 1X PBS. From an original cellular suspension, seven aliquots of 100 μl were taken and separated on sterile tubes; first tube was the untreated control, other tubes were treated with 10 μl of each concentration described on Table 1. 10 μl of H202 were added to the last tube as a positive control. All tubes were incubated for 1 minute and reactions were stopped adding 1 ml of PBS 1X. Samples were centrifuged at 3000 rpm on a Clay-Adams clinical centrifuge (Dynamic) for five minutes and two washing steps were done with PBS (1X) solution to eliminate residual EO. Viability was measured by flow cytometry analysing stained epithelial cells on a BD AccuryTM C6 flow cytometer system (Becton Dickinson, San José CA, USA) with 7-Actinomicine-D (7-AAD Staining Solution, BD Pharmingen™, San Jose California USA, Catalogue number 555816), a fluorescent molecule that is internalized exclusively through damaged membranes after a necrotic process, as cell toxicity indicator.
Results
Essential Oils composition
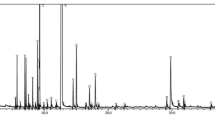
Qualitative and quantitative analysis for the composition of each essential oil are shown on Fig. 1 and Table 3. The chromatograms render different compositions for each EO (Fig. 1), being C. sinensis less complex than C. latifolia, although limonene was the major component; myrcene and α-/β-pinene were also common components on both oils. In summary, four constituents were detected in C. sinensis, d-limonene (96%) and α-myrcene (2.79%) as predominant. Thirteen components were identified on C. latifolia and the main were d-limonene (51.64%), β-thujene (14.85%), β-pinene (12.79%) and γ-terpinene (12.8%).
These results showed that there are similarities between both EOs that might contribute to the antifungal capacity, although the amounts of the corresponding compounds are very different.
Antimycotic activity
Results of EOs antimycotic activity against Candida species are presented on Fig. 2 through 6. Figure 2 show that inhibition halos of Citrus sinensis and Citrus latifolia on C. albicans cultures were statistically different (p < 0.0001) compared to control group. Calculated inhibition halos at 50% of growth were 5.51 and 9.46 mm, respectively. Both presented lower values as compared to amphotericin B (12.05 mm), meaning that growth inhibition was less evident.
Measured inhibition halos of Citrus sinensis (p = 0.0001) and C. latifolia (p = 0.047) on C. tropicalis cultures were statistically different compared to control group as it can be seen in Fig. 3. Calculated inhibition halos at 50% of growth were 4.44 and 10.87 mm, respectively. Growth inhibition was less apparent in C. sinensis meanwhile C. latifolia was similar to amphotericin B.
Statistically significant differences were found for Citrus sinensis and C. latifolia on C. glabrata cultures (p = 0.000). Inhibition halos at 50% of growth were 5.78 and 8.52 mm, respectively. This indicates that growth inhibition was less than amphotericin B, although it was clearly perceptible (Fig. 4).
Figure 5 represents the results obtained over C. lusitaniae cultures. For C. sinensis there was no growth inhibition observed (inhibition halo at 50% of 2.00 mm), with p < 0.0001 when compared with the positive control. Citrus latifolia, presented better antimycotic activity (p = 0.807, 8.06 mm) although lower to that obtained with amphotericin B (11.49 mm).
There were no inhibition halos detected on Candida guilliermondii cultures treated with C. sinensis. On the other hand, Citrus latifolia was able to diminish the growth with inhibition halos at 50% of 8.94 mm, significantly higher (p = 0.689) that amphotericin B (6.37 mm) as it can be seen on Fig. 6.
Finally, IC50 and MIC are summarized in Table 4, where C. sinensis had its best activity against C. glabrata and it has a nule effect with C. guilliermondii. Meanwhile C latifolia had effect on all species being the strongest with C. guilliermondii, surpassing the effect of amphotericin B.
Genotoxicity
Mutagenic evaluation
Neither Citrus sinensis nor Citrus latifolia induced point mutations in the presence or absence of S9 mix. Figure 7a shows that frameshift mutations were not induced on Salmonella typhimurium TA98 strain. Results were considered positive when the number of colonies was above the cutting line, as it can be observed for PA and 2AA. Figure 7b show that none of the EOs induced base pair substitution mutations, as it can be observed with the positive controls MNNG or 2AA. These EOs did not induced ROS caused mutations on the S. typhimurium TA102 strain. Only positive controls 2AA or 4NQO were able to induce a positive response as it is shown in Fig. 7c.
Cytotoxicity
Figure 8a shows that Citrus sinensis had no cytotoxic effect for human epithelial cells at the same doses used for antimycotic tests (see material and methods) since viability levels were maintained over 80% at all probed concentrations. It also can be seen on Fig. 8b that only the highest dose tested (21.8 μg) of Citrus latifolia EO was cytotoxic for these cells.
Discussion
The results presented in this work of antimycotic properties of C. sinensis and C. latifolia were performed against Candida species isolated from clinical cases of elder patients with clinical data of oral candiasis, by contrast to other reports that evaluate of other Citrus derivatives on Candida albicans and bacterial strains from ATCC13,14,15,17,19,20,21. The antimycotic evaluation of the EO of Citrus sinensis by agar diffusion method shows inhibitory effect against the studied Candida species except on C. guilliermondii. Its inhibitory effect was higher for C. glabrata (Fig. 3). Nevertheless it was significantly lower when compared with the positive control of amphotericin B. It was noticeable that the background growth in Petri dishes of C. glabrata cultures treated with C. sinensis was nonconfluent, this is to say, a diminished background was present despite they were plated from the same original 0.5 McFarland suspension, (data not shown). These data reinforce the observation of the antimycotic effect against Candida spp., specifically on C. glabrata. On the other side, antimycotic activity was demonstrated for C. latifolia on all species, being the highest against C. guilliermondii, in this case even better than amphotericin B. It is worth saying, that the antimycotic activity of these EOs is relevant since it was compared to a pure salt such as amphotericin B, a highly potent antimycotic35, meanwhile each oil is a mixture of several compounds, each component might contribute to the activity of the assembly, probably with less intensity than if they acted alone; or else, enhance/inhibit individual effects. The analysis of the individual components could be made in the future, as it was beyond the scope of this paper.
However, it could be argued, the chemical compounds like α-pinene, that have antifungal and antibacterial activities36, may contribute to the observed activity since is found in both C. sinensis and C. latifolia oils. Additionally, it has been shown that citral is an agent able to form complexes that interfere with the electron flux in mycotic cells37, which could be relevant for the inhibitory activity of C. latifolia. On the contrary, it has been demonstrated that limonene, β-pinene and myrcene are implicated on growth stimulation of other species of fungi38, which might explain the low activity observed for C. sinensis. The analysis of the components on each essential oil revealed the presence of common terpenes to the Citrus taxa39. Only one component (3,7-Nonadien-2-one,8-methyl-,(E)-), was unusual and it was present in perceptible amounts (0.93%) on the essential oil of C. latifolia and it could be further considered.
Our study presents the mutagenic evaluation of these EOs by means of Ames test, recommended as first strategy in risk-benefit evaluation of new products with possibilities to be used in humans29.
In fact, there are not previous mutagenic evaluations of Citrus derivatives in current literature, albeit Hammer et al. recommended that it should be done15. None of the evaluated EOs produced frameshift mutation, base-pair substitution or generates ROS damage when evaluated in the Ames test.
But as it has been formerly commented, not all natural occurring products and plants used in alternative medicine are innocuous. In fact it has been reported that there are some plants having mutagenic, toxic and cytotoxic effects both in vitro and in vivo, for instance, Chrysobalanus icaco22, Urtica dioica and Euphorbia rigida23, Tinctura Alchemillae, Cratagei extractum25, L. stoechas26, Myrciaria tenella, Smilax campestris, Tripodanthus acutifolius and Cassia corymbosa, among others27,28.
It is well known that some of the main components of EOs are vitamin C, flavonoids and beta-carotenes10,12,13 that may have antimutagenic properties, so it will be advisable to further evaluate the antimutagenic properties attributable to these additional components. The cytotoxic effect of C. latifolia EO should be evaluated against cancer human cell lines in order to know their possibilities as anticarcinogen.
In the present study C. sinensis was not cytotoxic for human oral epithelium cells even at higher doses probed in vitro. However, there was a toxic effect observed at the highest dose probed of C. latifolia EO (21.8 μg). Although in order to conclude, it is recommendable to perform studies on animal models where other toxicity parameters could be measured.
Consecutively Citrus sinensis EO could be used as an ingredient in oral hygiene products as prophylactic agent because it seems not mutagenic, not cytotoxic and it has moderated antimycotic activity. In case of C. latifolia it should be employed for products for external use such as elder dental prostheses and orthodontic appliances due to its toxicity. However, these oils could be a less toxic alternative to amphotericin B35, although more studies are necessary.
Conclusions
In conclusion, Citrus sinensis has antimycotic activity against C. glabrata, it was nor cytotoxic neither mutagenic.
Citrus latifolia EO has antimycotic effect against C. guilliermondii, it was not mutagenic and doses below at 20μg were not cytotoxic.
Additional Information
How to cite this article: Ruiz-Pérez, N. J. et al. Antimycotic Activity and Genotoxic Evaluation of Citrus Sinensis and Citrus Latifolia Essential Oils. Sci. Rep. 6, 25371; doi: 10.1038/srep25371 (2016).
References
Walsh, T. J. & Dixon, D. M. In Medical Microbiology, ( S. Baron, Ed chap. Chapter 75. Spectrum of Mycoses (1996).
Urizar, J. M. A. Candidiasis orales. Rev. Iberoam. Micol. 19, 17–21 (2002).
Ceballos-Salobreña, A. & Delgado-Azareño, W. Micosis Bucales. (Grupo aula médica, 1996).
Ceccotti, E. In Clínica estomatológica SIDA, cáncer y otras afecciones, ( Panamericana, Ed, pp. 161–164 (1993).
Ghannoum, M. A. & Rice, L. B. Antifungal agents: mode of action, mechanisms of resistance and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12, 501–517 (1999).
Martins, M. D. & Rex, J. H. Resistance to antifungal agents in the critical care setting: problems and perspectives. New Horiz. 4, 338–344 (1996).
Fryberg, M., Oehlschlager, A. C. & Unrau, A. M. Sterol biosynthesis in antibiotic-resistant yeast: nystatin. Arch. Biochem. Biophys. 160, 83–89 (1974).
Aguirre, J. M., Bagán, J. V. & Ceballos, A., In Terapéutica antimicrobiana en odontoestomatología, ( J. Liébana-Ureña, J. V. Bagàn-Sebastián, Eds), pp. 311–331 (IM, 1996).
Reichling, J., Schnitzler, P., Suschke, U. & Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral and cytotoxic properties–an overview. Forsch. Komplementmed. 16, 79–90 (2009).
Proteggente, A. R., Saija, A., De Pasquale, A. & Rice-Evans, C. A. The compositional characterisation and antioxidant activity of fresh juices from sicilian sweet orange (Citrus sinensis L. Osbeck) varieties. Free Radical Res. 37, 681–687 (2003).
Martínez, M. A. Aceites esenciales. J. Nat. Prod. 59, 1–34 (2003).
Rodrigo, M. J. & Zacarias, L. Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol. Tec. 43, 14–22 (2007).
Ortuño, A. et al. Citrus paradisi and Citrus sinensis flavonoids: Their influence in the defence mechanism against Penicillium digitatum. Food Chem. 98, 351–358 (2006).
Caccioni, D. R. et al. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and penicillium italicum. Int. J. Food Microbiol. 43, 73–79 (1998).
Hammer, K. A., Carson, C. F. & Riley, T. V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 86, 985–990 (1999).
Suhr, K. I. & Nielsen, P. V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 94, 665–674 (2003).
Saviuc, C. et al. The inhibitory activity of pomelo essential oil on the bacterial biofilms development on soft contact lenses. Roum. Arch. Microbiol. Immunol. 69, 145–152 (2010).
Sokovic, M. et al. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 15, 7532–7546 (2010).
Singh, P. et al. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food. Chem. Toxicol. 48, 1734–1740 (2010).
Uysal, B. et al. Essential oil composition and antibacterial activity of the grapefruit (Citrus Paradisi. L) peel essential oils obtained by solvent-free microwave extraction: comparison with hydrodistillation. Int. J. Food Sci. Tech. 46, 1455–1461 (2011).
Arriola-Guevara, E., García-Herrera, T., Guatemala-Morales, G. M. & García-Fajardo, J. A. Estudio Preliminar de las Propiedades de la Semilla de Limón Mexicano (Citrus aurantifolia swingle) para su Posible Aprovechamiento. Información tecnológica 17, 97–102 (2006).
Ferreira-Machado, S. C. et al. Genotoxic potentiality of aqueous extract prepared from Chrysobalanus icaco L. leaves. Toxicol. Lett. 151, 481–487 (2004).
Basaran, A. A., Yu, T. W., Plewa, M. J. & Anderson, D. An investigation of some Turkish herbal medicines in Salmonella typhimurium and in the COMET assay in human lymphocytes. Teratogen. Carcin. Mut. 16, 125–138 (1996).
Plewa, M. J. et al. Biochemical and mutagenic characterization of plant-activated aromatic amines. Environ. Toxicol. Chem. 12, 1353–1363 (1993).
Schimmer, O., Kruger, A., Paulini, H. & Haefele, F. An evaluation of 55 commercial plant extracts in the Ames mutagenicity test. Pharmazie 49, 448–451 (1994).
Çelik, T. & Aslantürk, Ö. Cytotoxic and genotoxic effects of Lavandula stoechas aqueous extracts. Biologia 62, 292–296 (2007).
Ames, B. N., Magaw, R. & Gold, L. S. Ranking possible carcinogenic hazards. Science 236, 271–280 (1987).
de Sa Ferreira, I. C. & Ferrao Vargas, V. M. Mutagenicity of medicinal plant extracts in Salmonella/microsome assay. Phytother. Res. 13, 397–400 (1999).
Arriaga-Alba, M., Montero-Montoya, R. & Aguirre, J. J. E. The ames test in twenty-first century. University of California, Berkeley 11, 12 (2012).
Arriaga-Alba, M. et al. Antimutagenic evaluation of vitamins B1, B6 and B12 in vitro and in vivo, with the Ames test. Food Chem. Toxicol. 53, 228–234 (2013).
Maron, D. M. & Ames, B. N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 113, 173–215 (1983).
Lum, K. Y. et al. Activity of Novel Synthetic Peptides against Candida albicans. Sci Rep. 5, 1–12; 9657 (2015).
Cantón-Lacasa, E., Martín-Mazuelos, E. & Espinel-Ingroff, A. Guía práctica de identificación y diagnóstico en micología clínica (eds Pemán, J. et al.) Ch. 15, 15a1–17 (Revista Iberoamericana de Micología, 2007).
Vázquez-Alvarado, P. et al. Genotoxic damage in oral epithelial cells induced by fluoride in drinking-water on students of Tula de Allende, Hidalgo, Mexico. J. Toxicol. Env. Health Sci. 4, 123–129 (2012).
Hamill, R. J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 73, 919–934 (2013).
Chutia, M., Bhuyan, P. D., Pathak, M. G., Sarma, T. C. & Boruah, P. Antifungal activity and chemical composition of Citrus reticulata blanco essential oil against phytopathogens from North East India. LWT-Food Sci. Tech. 42, 777–780 (2009).
Kurita, N., Miyaji, M., Kurane, R. & Takahara, Y. Antifungal activity of components of essential oils. Agric. Biol. Chem. 45, 945–952 (1981).
Filtenborg, O., Frisvad, J. C. & Thrane, U. Moulds in food spoilage. Int. J. Food Microbiol. 33, 85–102 (1996).
Lota, M. L., de Rocca Serra, D., Tomi, F., Jacquemond, C. & Casanova, J. Volatile components of ppel and leaf Ooils of Lemon and Lime species. J. Agric. Food Chem 50, 796–805 (2002).
Acknowledgements
We thank TLC María Gabriela Aguilera Hernández for her help in preparing culture medium and reagents required for this work. Also, we recognize Dr. Cornelio Barrientos Alvarado for his help on the statistical analysis. Finally, authors would like to acknowledge Dra. Guillermina Yazmín Arellano Salazar for her support on the GC-MS analysis.
Author information
Authors and Affiliations
Contributions
M.A. and N.R. made substantial contributions to conception and design, N.R. performed acquisition of data, N.R. and J.S. contributed to the analysis and interpretation of data; M.M. and T.S. contributed to experimental design of Fig. 7. M.G. and J.T. participated in drafting the article and revising it critically for important intellectual content and technical writing. All authors gave final approval of the version to be submitted and any revised version.
Mutagenic evaluation of EOs.
(a) Ames test on Salmonella typhimurium strain TA98. Spontaneous reversion: 24.67 + 1.7; 2AA: 2-amino-antraceno; PA: picrolonic acid. (b) Ames test on Salmonella typhimurium strain TA100. Spontaneous reversion: 121.44 + 8.32; 2AA: 2-amino-anthracene; MNNG: metil-N-nitro-N-nitrosoguanidine. (c) Ames test on Salmonella typhimurium strain TA102. Spontaneous reversion: 305.33 + 57.12; 2AA: 2-amino-anthracene; 4NQO: 4-nitro-quinone-oxide.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ruiz-Pérez, N., González-Ávila, M., Sánchez-Navarrete, J. et al. Antimycotic Activity and Genotoxic Evaluation of Citrus sinensis and Citrus latifolia Essential Oils. Sci Rep 6, 25371 (2016). https://doi.org/10.1038/srep25371
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25371
- Springer Nature Limited
This article is cited by
-
The antifungal potential of (Z)-ligustilide and the protective effect of eugenol demonstrated by a chemometric approach
Scientific Reports (2019)
-
Mechanisms of Nα-lauroyl arginate ethyl ester against Penicillium digitatum and Pectobacterium carotovorum subsp. carotovorum
Journal of Food Science and Technology (2018)
-
Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia
Scientific Reports (2017)