Abstract
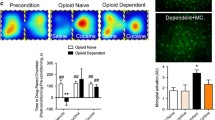
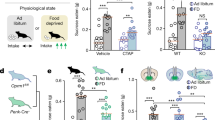
Heroin use disorder in humans and chronic opioid administration to mice result in an increase in the number and a decrease in the size of detected hypocretin (Hcrt, or orexin) neurons. Chronic morphine administration to mice increases Hcrt axonal projections to the ventral tegmental area (VTA), the level of tyrosine hydroxylase (TH) in VTA and the number of detected TH+ cells in VTA, and activates VTA and hypothalamic microglia. Co-administration of morphine with the dual Hcrt receptor antagonist suvorexant prevents morphine-induced changes in the number and size of Hcrt neurons, the increase in Hcrt projections to the VTA and microglial activation in the VTA and hypothalamus. Co-administration of suvorexant with morphine also prevents morphine anticipatory behavior and reduces opioid withdrawal symptoms. However, suvorexant does not diminish morphine analgesia. Here we show that combined administration of opioids and suvorexant may reduce the addiction potential of opioid use for pain relief in humans while maintaining the analgesic effects of opioids.






Similar content being viewed by others
Data availability
All data presented in this work have been deposited in G-Node (https://gin.g-node.org/fmfwu/Siegel_NatureMH_2024) and are available without restrictions from the corresponding author.
References
Cicero, T. J. No end in sight: the abuse of prescription narcotics. Cerebrum 2015, 1–15 (2015).
Mack, K. A., Jones, C. M. & McClure, R. J. Physician dispensing of oxycodone and other commonly used opioids, 2000–2015, United States. Pain Med. 19, 990–996 (2018).
Kelly, M. M., Reilly, E., Quinones, T., Desai, N. & Rosenheck, R. Long-acting intramuscular naltrexone for opioid use disorder: Utilization and association with multi-morbidity nationally in the Veterans Health Administration. Drug Alcohol Depend. 183, 111–117 (2018).
Parthvi, R., Agrawal, A., Khanijo, S., Tsegaye, A. & Talwar, A. Acute opiate overdose: an update on management strategies in emergency department and critical care unit. Am. J. Ther. 26, e380–e387 (2019).
Thannickal, T. C. et al. Human narcolepsy is linked to reduced number, size and synaptic bouton density in hypocretin-2 labeled neurons. Abstr. Soc. Neurosci. 26, 2061 (2000).
Thannickal, T. C. et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000).
Peyron, C. et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 6, 991–997 (2000).
Thannickal, T. C. et al. Opiates increase the number of hypocretin-producing cells in mouse and human brain, and reverse cataplexy in a mouse model of narcolepsy. Sci. Transl. Med. 10, eaao4953 (2018).
Fragale, J. E., James, M. H. & Aston-Jones, G. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addict. Biol. 26, e12946 (2021).
James, M. H. et al. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol. Psychiatry 85, 925–935 (2019).
Guilleminault, C. & Cao, M. T. in Principles and Practice of Sleep Medicine (eds Kryger, M. H. et al.) 957–968 (Elsevier, 2011).
Darke, S., Kaye, S., Duflou, J. & Lappin, J. Completed suicide among methamphetamine users: a national study. Suicide Life Threat. Behav. 49, 328–337 (2019).
Zhu, J., Spencer, T. J., Liu-Chen, L. Y., Biederman, J. & Bhide, P. G. Methylphenidate and μ opioid receptor interactions: a pharmacological target for prevention of stimulant abuse. Neuropharmacology 61, 283–292 (2011).
Ponz, A. et al. Abnormal activity in reward brain circuits in human narcolepsy with cataplexy. Ann. Neurol. 67, 190–200 (2010).
Schwartz, S. et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain 131, 514–522 (2007).
Kiyashchenko, L. I. et al. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 22, 5282–5286 (2002).
McGregor, R., Wu, M.-F., Barber, G., Ramanathan, L. & Siegel, J. M. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J. Neurosci. 31, 15455–15467 (2011).
Mileykovskiy, B. Y., Kiyashchenko, L. I. & Siegel, J. M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798 (2005).
Wu, M. F., Nienhuis, R., Maidment, N., Lam, H. A. & Siegel, J. M. Role of the hypocretin (orexin) receptor 2 (Hcrt-r2) in the regulation of hypocretin level and cataplexy. J. Neurosci. 31, 6305–6310 (2011).
Wu, M. F., Nienhuis, R., Maidment, N., Lam, H. A. & Siegel, J. M. Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch. Ital. Biol. 149, 492–498 (2011).
Blouin, A. M. et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun. 4, 1547 (2013).
Farahimanesh, S., Zarrabian, S. & Haghparast, A. Role of orexin receptors in the ventral tegmental area on acquisition and expression of morphine-induced conditioned place preference in the rats. Neuropeptides. 66, 45–51 (2017).
Meye, F. J., van Zessen, R., Smidt, M. P., Adan, R. A. H. & Ramakers, G. M. J. Morphine withdrawal enhances constitutive μ-opioid receptor activity in the ventral tegmental area. J. Neurosci. 32, 16120–16128 (2012).
Stefano, G. B. & Kream, R. M. Endogenous morphine synthetic pathway preceded and gave rise to catecholamine synthesis in evolution (review). Int. J. Mol. Med. 20, 837–841 (2007).
Baimel, C. et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br. J. Pharmacol. 172, 334–348 (2015).
Vittoz, N. M., Schmeichel, B. & Berridge, C. W. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur. J. Neurosci. 28, 1629–1640 (2008).
Narita, M. et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J. Neurosci. 26, 398–405 (2006).
Balcita-Pedecino, J. J. & Sestak, S. R. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J. Comp. Neurol. 503, 668–684 (2007).
Ji, K., Miyauchi, J. & Tsirka, S. E. Microglia: an active player in the regulation of synaptic activity. Neural Plast. 2013, 627325 (2013).
Schafer, D. P., Lehrman, E. K. & Stevens, B. The ‘quad-partite’ synapse: microglia–synapse interactions in the developing and mature CNS. Glia 61, 24–36 (2013).
Zhan, Y. et al. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Taylor, A. M. W. et al. Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41, 949–959 (2016).
Vilca, S. J., Margetts, A. V., Pollock, T. A. & Tuesta, L. M. Transcriptional and epigenetic regulation of microglia in substance use disorders. Mol. Cell. Neurosci. 125, 103838 (2023).
Hutchinson, M. R. et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav. Immun. 23, 240–250 (2009).
Hutchinson, M. R. et al. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav. Immun. 22, 1248–1256 (2008).
Maduna, T. et al. Microglia express mu opioid receptor: insights from transcriptomics and fluorescent reporter mice. Front. Psychiatry 9, 726 (2019).
Xiong, X. et al. Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke 44, 764–770 (2013).
Vickers, A. P. Naltrexone and problems in pain management. BMJ 332, 132–133 (2006).
Spitzer, N. C. Neurotransmitter switching? No surprise. Neuron 86, 1131–1144 (2015).
Liu, B., Kwok, R. P. S. & Fernstrom, J. D. Colchicine-induced increases in immunoreactive neuropeptide levels in hypothalamus: use as an index of biosynthesis. Life Sci. 49, 345–352 (1991).
McGregor, R. et al. Hypocretin/orexin interactions with norepinephrine contribute to the opiate withdrawal syndrome. J. Neurosci. 42, 255–263 (2022).
Juarez-Portilla, C. et al. Brain activity during methamphetamine anticipation in a non-invasive self-administration paradigm in mice. eNeuro 5, 1–14 (2018).
LeSauter, J., Balsam, P. D., Simpson, E. H. & Silver, R. Overexpression of striatal D2 receptors reduces motivation thereby decreasing food anticipatory activity. Eur. J. Neurosci. 51, 71–81 (2020).
Spitzer, N. C. Activity-dependent neurotransmitter respecification. Nat. Rev. Neurosci. 13, 94–106 (2012).
McGregor, R., Shan, L., Wu, M. F. & Siegel, J. M. Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: implication for understanding and treating neuronal loss. PLoS ONE 12, e0178573 (2017).
Georgescu, D. et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J. Neurosci. 23, 3106–3111 (2003).
Ma, H. et al. Excitation–transcription coupling, neuronal gene expression and synaptic plasticity. Nat. Rev. Neurosci. 24, 672–692 (2023).
Yap, E. L. & Greenberg, M. Activity-regulated transcription: bridging the gap between neural activity and behavior. Neuron 100, 330–348 (2018).
Yamanaka, A., Tabuchi, S., Tsunematsu, T., Fukazawa, Y. & Tominaga, M. Orexin directly excites orexin neurons through orexin 2 receptor. J. Neurosci. 30, 12642–12652 (2010).
Robinson, J. D. & McDonald, P. H. The orexin 1 receptor modulates kappa opioid receptor function via a JNK-dependent mechanism. Cell. Signal. 27, 1449–1456 (2015).
Keith, D. R. et al. Time of day influences the voluntary intake and behavioral response to methamphetamine and food reward. Pharmacol. Biochem. Behav. 110, 117–126 (2013).
Kosobud, A. E. K., Pecoraro, N. C., Rebec, G. V. & Timberlake, W. Circadian activity precedes daily methamphetamine injections in the rat. Neurosci. Lett. 250, 99–102 (1998).
Paqueron, X. et al. Is morphine-induced sedation synonymous with analgesia during intravenous morphine titration? Br. J. Anaesth. 89, 697–701 (2002).
Obukuro, K. et al. Nitric oxide mediates selective degeneration of hypothalamic orexin neurons through dysfunction of protein disulfide isomerase. J. Neurosci. 33, 12557–12568 (2013).
John, J. et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol. 74, 786–793 (2013).
Jalewa, J., Wong-Lin, K., McGinnity, T. M., Prasad, G. & Holscher, C. Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav. Brain Res. 272, 196–204 (2014).
Palomba, M. et al. Alterations of orexinergic and melanin-concentrating hormone neurons in experimental sleeping sickness. Neuroscience 290, 185–195 (2015).
Crocker, A. et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 65, 1184–1188 (2005).
Lopresti, N. M., Esguerra, M. & Mermelstein, P. G. Sex differences in animal models of opioid reward. Curr. Sex. Health Rep. 12, 186–194 (2020).
Ito, D. et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain. Res. 57, 1–9 (1998).
Maldonado, R. et al. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science 273, 657 (1996).
Acknowledgements
Support: DA034748 to J.M.S., DA058639 to J.M.S, HL148574 to J.M.S and the Medical Research Service of the Department of Veterans Affairs to J.M.S. A preprint of some of this work has been published (R. McGregor, M.-F. Wu, T. C. Thannickal & J. M. Siegel, Preprint at bioRxiv https://doi.org/10.1101/2023.09.22.559044 (2023). Opiate anticipation, opiate induced anatomical changes in hypocretin (Hcrt, orexin) neurons and opiate induced microglial activation are blocked by the dual Hcrt receptor antagonist suvorexant, while opiate analgesia is maintained. bioRxiv 559044v1, PMC10542511).
Author information
Authors and Affiliations
Contributions
R.M., M.-F.W., T.C.T. and J.M.S. designed the study. R.M. ran the anatomical experiments, M.-F.W. ran the behavioral experiments (wheel running and analgesia) and T.C.T. and S.L. ran the microglial studies. R.M., M.-F.W., T.C.T., S.L. and J.M.S. analyzed the results and R.M., M-F.W., T.C.T. and J.M.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks John Peever and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
McGregor, R., Wu, MF., Thannickal, T.C. et al. Opioid-induced neuroanatomical, microglial and behavioral changes are blocked by suvorexant without diminishing opioid analgesia. Nat. Mental Health 2, 1018–1031 (2024). https://doi.org/10.1038/s44220-024-00278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-024-00278-2
- Springer Nature America, Inc.
This article is cited by
-
Hypocretin receptor antagonists prevent opioid addiction
Nature Mental Health (2024)