Abstract
A sex change phenomenon was reported in some free-living, non-sessile coral species of the Family Fungiidae. However, there are no reports describing sex change in sessile colonial species. Timing and cellular processes of sex change are also unclear in corals. Here, we report sex change of the colonial coral, Fimbriaphyllia ancora, and its cellular process. Of 26 colonies monitored at Nanwan Bay, southern Taiwan, about 70% changed their sex every year after annual spawning for least 3-4 consecutive years, i.e., colonies that were male two years ago became female last year, and male again this year. The remaining 30% were permanently male or female. Sex-change and non-sex-change colonies grew in close proximity or even side-by-side. No significant differences were found in colony size between sex-change and non-sex-change colonies. Histological analysis showed that, in female-to-male sex change, small oocytes were present up to 3 months in some gonads after spawning and disappeared by 5 months. This suggests that sex change occurred 4-5 months after spawning. In contrast, in male-to-female sex change, oocytes appeared weeks after sperm release and in most gonads by 3 months, suggesting that male-to-female sex change occurred 0–3 months after sperm release.
Similar content being viewed by others
Introduction
Scleractinian corals (hereafter, corals) are the keystone species of coral reef ecosystems, creating complex 3D structures that support the highest marine biodiversity on the planet1,2. Reproduction of corals is foundational for colony formation, coral growth, and coral reef development3. Asexual reproduction amplifies the number of individuals in a relatively short period of time, while sexual reproduction produces genetically diverse individuals and expands their distribution to wide areas by releasing gametes or larvae3. A better understanding of coral reproductive biology will not only enable more accurate predictions of anthropogenic impacts on corals and on coral reef development, but also will help establish methods to preserve and restore existing coral populations.
Coral mass spawning events4,5 have captured the attention of researchers and the general public for decades. To date, sexual reproduction of more than 400 coral species, accounting for about one-third of existing species, has already been investigated6. Approximately 30% of these species are gonochoristic, while about 70% are hermaphroditic6. Hermaphroditic corals include species possessing both male and female germ cells at the same time (simultaneous hermaphroditism, e.g., most Acropora) and species reproducing as one functional sex during a given reproductive season or event, and as the other sex subsequently (sequential hermaphroditism or sex change; e.g., Fungia repanda)6,7,8. Corals have two reproductive modes, i.e., spawning and brooding6. Spawners, which constitute approximately three-quarters of corals, release gametes into the ocean via the mouth for external fertilization. The remaining corals are brooders that release planula larvae rather than gametes. These planula larvae are generally produced by internal fertilization within polyps6. Planulae can be produced internally by both sexual and asexual reproduction9,10,11,12.
Sex change/sequential hermaphroditism is a reproductive strategy to increase odds of successful propagation7. It is classified as protandrous (from male to female), protogynous (from female to male), or serial/bidirectional13,14,15,16. To date, sex change has been observed in some coral species belonging to the Family Fungiidae, which comprises free-living, non-sessile species8,17. These species are males when they are small and become females after reaching a certain size, i.e., protandrous sex change8,17. Bidirectional sex change has also been observed in some fungiid corals8,17,18. However, sex change in other families of corals, especially sessile colonial corals, had not been reported. Additionally, it is still unclear how sex-change occurs at cellular level in any coral species.
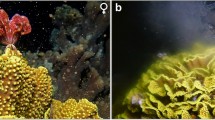
Fimbriaphyllia ancora (Family Euphyllidae) is a reef-building coral widely distributed in Indo-Pacific tropical and subtropical waters19,20. This is a colonial species forming a dome or cushion-shaped colony, and is typified by its flabello-meandroid skeleton and tentacles with anchor-like (or hammer-like) tips (Fig. 1a, b). Multiple colour variants, including green, purple, and orange, are present, making this species one of the most popular species in the aquarium ornamental industry21. Fimbriaphyllia ancora is gonochoristic and an annual spawner22,23. Development of ovaries and testes can be readily observed in female and male colonies, respectively, as the reproductive season approaches (Fig. 1c, d). In Taiwan, F. ancora forms many large colonies (1–6 m in diameter) at depth of approximately 10 m in Nanwan Bay, the southernmost part of the main island of Taiwan (Fig. 1e1–e3). The spawning season of F. ancora in the Nanwan Bay occurs in late April or May22,23.
a An F. ancora colony from Nanwan Bay, Taiwan. b Tentacles of the F. ancora colony. The flabello-meandroid skeleton and anchor-like tentacles typify F. ancora. c Representative micrograph of an ovary with a mesenterial filament (mf). d Representative micrograph of a testis with a mesenterial filament. e1–e3. Maps of survey/sampling locations in Nanwan Bay, southern Taiwan. The map e3 shows the approximate location of Sites 1 and 2, where F. ancora colonies were collected. Illustrations showing the approximate locations of F. ancora colonies in Site 1 (f) and Site 2 (g). Numbers (#1-26) indicate colony IDs. Approximate distances of sex-change colonies (letters in black), non-sex-change female colonies (letters in red), and non-sex-change male colonies (letters in blue) are shown.
During our preceding, long-term investigation of gametogenesis in F. ancora in Nanwan Bay, we observed that the sex ratio of F. ancora colonies varied from year to year. This finding led us to hypothesize that F. ancora changes its sex at the colony level, prompting us to verify this hypothesis by monitoring the sexes of labeled colonies over consecutive reproductive seasons. Here we report annual sex change of F. ancora. Additionally, we present histological evidence of the timing and processes of sex change.
Results
Observation of annual sex change in F. ancora
In Nanwan Bay, there are two sites with many F. ancora colonies (Fig. 1e1–e3, f, g). To understand reproductive characteristics of F. ancora, we labeled 11F. ancora colonies at one site (Site 1) in 2010, and sampled them several times, commencing a few months before spawning. We determined sex and gametogenesis status histologically (Fig. 2a).
Sexes of colonies at Site 1 (a) and Site 2 (b). At Sites 1 and 2, surveys were performed during 2010–2013 and 2015–2018, respectively. In each survey year, fragments of each colony were sampled at different months and sexes were histologically determined. Each circle indicates the result of histological analysis. Blue denotes males and pink denotes females. In cases in which sampling was not possible due to colony mortality, inclement weather, etc., circles are indicated in grey. c Sizes (area, m2) of colonies analyzed in this study. Sex-change colonies are shown in grey, non-sex-change male colonies are shown in blue, and non-sex-change female colonies are shown in pink. d Comparison of colony size between sex-change colonies and non-sex-change colonies. Numbers in parentheses indicate the number of colonies. Data are expressed as the mean ± SEs.
In February 2011, about a year later, we resampled and analyzed the labeled colonies, and found that 9 of 11 labeled colonies had switched sex (Fig. 2a). In March and April 2011, not only to re-confirm the sex change phenomenon, but also to rule out the possibility of sampling error, we conducted another two rounds of sampling. As of February 2011, the sex of 9 of 11 colonies was different from that in 2010 (Fig. 2a). In the other two colonies (#6 and 7), the sex remained female (Fig. 2a).
In February 2012, we resampled the labeled colonies. Three of the labeled colonies (# 1, 9, and 11) were dead and could not be sampled. However, 9 labeled colonies (8 colonies labeled in 2010, 1 colony newly labeled in 2011) were resampled three times in 2012. The analysis showed that the sex of colonies # 2, 3, 4, 5, 8, and 11 had switched back to the original sex in 2010 (Fig. 2a). Two colonies #6 and 7 remained female.
The following year, from December to May 2013, we resampled the labeled colonies 3 times and performed the same analysis, which again confirmed the sex change phenomenon in 7 of 9 colonies, with 2 colonies remaining female (Fig. 2a).
Subsequently, we conducted similar surveys from 2015–2018 for a total of 14 new colonies at Site 2. As at Site 1, there were both annual sex-change colonies and non-sex-change colonies (Fig. 2b). Five non-sex-change male colonies were identified and non-sex-change female colonies were not found at Site 2.
During 8 years of observations, we examined 26 colonies at Sites 1 and 2 (Supplementary Table 1). Among them, 19 colonies (73.1%) changed their sex every year, while the remaining 7 colonies (26.9%) were fixed as male or female. Numbers of non-sex-change female and non-sex-change male colonies were 2 and 5 (16.7% and 35.7% of the total), respectively.
Location and size of sex-change and non-sex-change colonies
Next, we investigated whether there were any differences in the location and colony size between sex-change and non-sex-change colonies. At both Sites 1 and 2, sex-change and non-sex-change colonies were located within 1–10 m (Fig. 1f, g). No significant differences were observed in colony size (Fig. 2c, d). There were also no prominent differences in colony color, as seen underwater. Most were either green or light green.
Timing and processes of sex change
Subsequently, we sought to discover the process and timing of sex change by histological analysis. Samples collected during two reproductive seasons, from several months before spawning, through a few weeks after spawning, to the next spawning season were examined.
First, the process of female-to-male sex change was investigated (Table 1). During the female phase (December to May, Fig. 3a), all gonads collected from December to April were ovaries filled with developing oocytes. Mature oocytes were observed in gonads collected in May. No testicular tissues, such as male germ-cell clusters, were observed in the ovaries during the female phase (n = 136 gonads, Fig. 3a, Table 1). Of gonads collected 0–3 months after spawning (May-August, n = 52), about 80% were sexually undifferentiated gonads with undifferentiated germ cells (Fig. 3 a and b). The remaining ~20% were gonads with residual or newly developed small oocytes (Fig. 3a, c, Table 1). However, further analysis revealed that oocyte numbers tended to decrease with time after spawning (Fig. 3d, P = 0.94; paired Student’s t-test). From one to three months after spawning (July-August), about 5 oocytes were found in gonads (Fig. 3d). But five months post-spawning (October), no oocytes were observed in gonads, and initiation of spermatogenesis was observed (n = 147 gonads, Fig. 3d, Table 1).
a–d Sex change from female to male. a Observed types of gonads and their frequencies in each month. b Representative immunohistological section of an undifferentiated gonad. Undifferentiated (early-stage) germ cells are labeled brown by immunostaining with an anti-F. ancora vasa antibody (arrows). c Representative immunohistological section of an ovary. Oocytes are labeled brown with an anti-F. ancora vasa antibody. d Average number of oocytes per gonad. Data are expressed as means ± SEs (n = 3 colonies for each group). n.d., not detected. e–g Sex change from male to female. e Observed types of gonads and their frequencies each month. f Representative immunohistological section of a gonad (mix) with an oocyte and residual sperm (spermary with sperm). Oocytes and sperm are labeled brown with an anti-F. ancora vasa antibody and an anti-F. ancora rGC antibody, respectively. g Average numbers of oocytes per gonad. Data are expressed as means ± SEs (n = 3 colonies for each group). Groups with different letters are significantly different (P < 0.05).
Next, the process of male-to-female sex change was investigated. During the male phase (December to May, Fig. 3e), all gonads collected from December to April were testes filled with developing male germ cells. Sperm were observed in gonads collected in May. No oocytes were observed in testes during the male phase (n = 167 gonads, Fig. 3e, Table 2). Analysis of gonads collected a few weeks after sperm release (May-June, n = 56 gonads) revealed that about 60% were sexually undifferentiated gonads with undifferentiated germ cells, and that about 30% were gonads having small numbers of both newly developed small oocytes and residual sperm (Fig. 3f, Table 2). Three months after sperm release (August), more oocytes were observed in about 90% of the gonads (Fig. 3e, g, n = 67 gonads, Table 2). Further analysis revealed that oocyte numbers increased as time passed from the month of sperm release (Fig. 3g, P < 0.05; one way ANOVA). Active oogenesis with apparent oocyte growth was also observed.
Discussion
The present study revealed a reproductive strategy of F. ancora, which is a sessile colonial coral species. Labelling of 26 colonies in Nanwan Bay of southern Taiwan and examining their sexes over several years primarily through histological analysis revealed that about 70% changed their sex annually, while the remaining 30% did not. Although sex change has been reported in various taxa7,13,14,15,16,17,18,24,25,26, there have been no reports of species in which colonies change sex annually, interspersed with colonies that do not change sex. Currently, cellular and molecular mechanisms underlying sexual differentiation and sexual plasticity in corals are not well understood. F. ancora may serve as a valuable model for studying these processes.
The present study revealed histologically when and how sex-change occurs in F. ancora (Fig. 4a, b). In female-to-male sex change, small oocytes were still observed in some gonads up to about 3 months after spawning (late May to August), but none were observed 5 months thereafter (October). This suggests that corals were in an intermediate phase or female phase for 0–3 months after spawning, and that female-to-male sex change occurred 4-5 months after spawning. In contrast, in male-to-female sex change, the earliest oocytes appeared several weeks after sperm release (late May-June). Oocytes appeared in most gonads 3 months after sperm release (August). This suggests that male-to-female sex change occurs 0–3 months after sperm release.
a Female-to-male sex change. From 0–3 months after spawning (late May-August), colonies have not changed completely into males, and small oocytes and undifferentiated germ cells are present in gonads (intermediate phase or female phase). Five months after spawning (October), oocytes are no longer present and the male phase (spermatogenesis) was initiated. b Male-to-female sex change. The earliest oocytes appeared a few weeks after sperm release (late May-June), and residual sperm, undifferentiated germ cells, and small oocytes were observed in some gonads (intermediate phase). Oocytes appeared in most gonads 2-3 months after sperm release (July-August) and the female phase (oogenesis) was initiated.
Sex change phenomena have been well studied in fish14,16,27. Based on differences in biological characteristics, organisms that change sex can be further classified into two types: (i) organisms that have both testes and ovaries simultaneously. Testes or ovaries are selectively developed according to their sexual phase. For example, in the bi-directional sex-changing gobiid fish, Trimma okinawae, individuals in the female phase have well-developed ovaries, but inactive testes. Conversely, individuals in the male phase have mature testes, but immature ovaries16,28; and (ii) organisms that produce eggs or sperm in the same gonad according to their sexual phase. For example, during female-to-male sex change in the grouper, Epinephelus coioides, ovarian tissue degenerates and disappears completely, while testicular tissue appears and develops, i.e., gonads completely transform from ovary to testis29,30. In F. ancora, we found that residual sperm and oocytes coexisted transiently in gonads shortly after sperm release. Then, gonads eventually developed into ovaries in a few months. This finding suggests that F. ancora is more like the second type.
In Hydra oligactis, which belongs to the same phylum as corals, females can emerge from male clones, particularly when animals are cultured at high temperatures31. In F. ancora, however, sex-change and non-sex-change colonies are found as little as 1 m apart. Thus, it is unlikely that the growing environment of these colonies largely differs, suggesting that sex change in F. ancora may be unrelated to environmental factors. Further detailed assessment of environmental factors such as temperature, depth, currents, light, proximity to the coast, etc, would be needed to verify this hypothesis.
Factors triggering sex change, such as environmental stressors, size, age, and social conditions (disappearances of males or females from a group) differ among species16,32. Previous reports of sex change in corals have linked individual/colony size and sex change. For example, in the stony coral, Stylophora pistillata, small colonies are male, but once they exceed a threshold size, they become simultaneous hermaphrodites33. Protandrous sex change has been reported in some non-sessile corals belonging to the Family Fungiidae, and there is a relationship between individual/colony size and sex change8,17. In the case of F. ancora, on the other hand, no differences in colony size were observed between sex-change and non-sex-change colonies. There were also no noticeable differences in appearance, e.g., colour or tentacle morphology, among them.
In Hydra, it has been demonstrated that the sex of an individual polyp is determined by sex-specific germline stem cells (GSCs) in the polyp34. If a polyp has male GSCs, which can only produce male germ cells, the polyp becomes male. The same applies to females. If we apply this Hydra sex-determination system to F. ancora, the following hypothesis can be considered. Non-sex-change male or female F. ancora colonies may contain only male-GSCs or female-GSCs. On the other hand, in the case of sex-change colonies, GSCs of both sex may be present in every gonad. In the male phase, gonads are functionally testes. However, a small number of female GSCs may also be present, and they undergo self-renewal to increase in number. Soon after, when most sperm are released during spawning, female GSCs, which are predominant in the gonad after sperm release, differentiate into oocytes and eventually, the gonad becomes an ovary. Proliferation of male GSCs in the testis during spermatogenesis may be repressed by some undetermined mechanism. The same applies to the change from female to male. In Hydra, molecular markers for female GSCs and male GSCs have been identified35,36. In our previous study, we have identified GCS-like cells in gonads of F. ancora37. Identification of molecular markers for female GSCs and male GSCs in F. ancora will allow us to test this hypothesis.
Fimbriaphyllia ancora was functionally gonochoric at each reproductive season. However, long-term studies across multiple reproductive seasons in this study demonstrated that many colonies can produce gametes of both sexes, i.e., that they are sequential hermaphrodites. In 2012, some colonies of the stony coral, Diploastrea heliopora, were reported to change sex after the reproductive season38. However, since only 5 colonies were labeled and studied for 14 months spanning two reproductive seasons, it is unknown whether those colonies change their sex annually. Nevertheless, our findings and this previous report suggest that corals described as gonochoric may include species undergoing sex change. Determining the reproductive mode of corals will require labeling colonies and studying them through multiple reproductive cycles.
Many corals such as acroporids are hermaphrodites, creating both sperm and eggs which are released at specific times each year39,40. The gametes drift through the ocean, they encounter gametes from other colonies, producing genetically diverse offspring through fertilization3. This biological feature is considered an adaptive evolutionary trait to overcome the disadvantage of sessility, i.e., the inability to move to find mating partners41. In F. ancora, what advantages are there in having both annual sex-change colonies (sequential hermaphroditism) and non-sex-change colonies (gonochorism) in a population? One possible advantage would be that the presence of both increases the probability of successful sexual reproduction. The presence of an annual sex-change colony near a non-sex-change male or female colony would give them a chance to reproduce sexually at least once every two years with a high probability. Another possible advantage would be that sex change increases the combination of mating partners and genetic diversity of offspring.
F. ancora is one of the most popular species in the aquarium ornamental industry21. In places where this species is now cultured, such as Indonesia, fragments are mainly collected from the wild and asexually propagated, and those that reach a certain size are shipped to the market. To simultaneously achieve conservation and restoration of the wild population of this species, as well as development of the aquaculture industry, it will be important to establish full-life cycle aquaculture with sexual propagation. Findings from this study will facilitate development of such techniques.
Summary
We discovered that many colonies in the stony coral, F. ancora, change sex annually. We also showed that female-to-male sex change occurs 4-5 months after spawning, whereas male-to-female sex change occurs 0–3 months after sperm release.
Methods
Collection of F. ancora
Species identification of F. ancora was performed based on two external characteristics, flabello-meandroid skeletons and anchor-like tentacles42. F. ancora was collected by scuba divers from 2 sites in Nanwan Bay, southern Taiwan (Site 1, 21°57'205 N, 120°46'116E; Site 2, 21°57'221 N, 120°46'092E). These two sites were chosen because of accessibility and ease of anchoring. The distance between the two sites was approximately 100 meters. This distance was established to avoid the collection of genetically identical colonies produced by asexual reproduction. Considering long-term repeated sampling in the 2 sites, all colonies of F. ancora with diameters >1 m was labeled, and the shortest distances between colonies were determined with a measuring tape. For sampling, portions of labeled colonies (approximately 5–8 cm/fragments) were collected at different times in 2010–2013 for site 1 and 2015–2018 for site 2. To avoid collecting young polyps that lack gonads, central parts of colonies were selected and sampled. Furthermore, when resampling was performed within a month or two, a region at least 10 cm away from the previously sampled location was selected to avoid collecting polyps that might have gonads physically damaged by sampling. To reveal the process and timing of sex change, sampling was performed at Sites 1 and 2 during 16 months spanning two reproductive seasons. Samples were collected 1 ~ 5 months before spawning (in December, February, March, and April; samples with developing germ cells), a few weeks before spawning (in May; samples with mature germ cells), a few weeks after spawning (in May and June; samples with residual mature germ cells, undifferentiated germ cells, and newly developed oocytes), and 1–11 months after spawning (in July, August, October, December, February, March, and April; samples with newly developed germ cells). Collecting was approved by the administration of Kenting National Park (Permit Number: 1010006545). All collected F. ancora fragments were fixed for 16 h at 4 °C in Zinc Formal-Fixx (Thermo Shandon, Pittsburgh, PA), diluted 1:5 with 0.22 μm-filtered artificial seawater. After decalcification with 5% formic acid, samples were preserved in 70% ethanol until use.
Histological analysis
Fixed samples were embedded in paraplast (Thermo Scientific), and serial 4-µm sections were prepared with a microtome (Thermo Shandon, Pittsburgh, USA). Since approximately 100–600 serial sections constitute an entire gonad, all gonadal sections were collected. Then, sections were de-waxed, hydrated, and stained with Hematoxylin and Eosin Y (H & E staining, Thermo Shandon). The number of oocytes in a gonad was determined by observing all sections of each ovary under a microscope (BX51; Olympus). All analyses were performed with Image J software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA; https:// imagej.nih.gov/ij).
Immunohistochemistry
Sample fixation, decalcification, embedding, and sectioning were performed according to methods described above. Hydrated sections were incubated for 30 min with HistoVT ONE (Nacalai Tesque, Inc, Kyoto, Japan) for antigen retrieval, and then incubated for 10 min in 3% H2O2, and blocking for 1 h in 5% skim milk. Sections were then incubated for 16 h at 4 °C in anti-F. ancora vasa antibody (vasa: germ cell marker43 diluted 1:4,000 in phosphate-buffed saline containing 0.1% Tween 20 [PBT] with 1% skim milk. To detect sperm, sections were incubated with anti-F. ancora receptor guanylate cyclase (rGC) antibody (rGC: sperm marker)44 diluted 1:1,000 in PBT with 1% skim milk for 16 h at 4 °C. After washing three times with PBT, sections were incubated with a biotinylated goat anti-rabbit IgG antibody (Vector Laboratories, Burlingame, USA; diluted 1: 4,000 in PBT with 2% skim milk) for 30 min. Sections were then incubated in avidin-biotin-peroxidase complex (ABC) solution (Vector Laboratories), and the immunoreactivity (brown colour) was visualized using 3, 3’-diaminobenzidine (DAB, Sigma-Aldrich) system. Immunostained sections were then counter-stained with Haematoxylin (blue-purple colour), and photographed under a microscope (BX51; Olympus).
Statistics and reproducibility
All data are presented as means ± standard errors (SEs). Statistical differences between two groups were determined using Student’s t-test with a statistical significance level of P < 0.05. For comparisons among more than three groups, statistical significance was determined using one-way ANOVA followed by Tukey’s test with a statistical significance level of P < 0.05. All analyses were performed using Statistical Package for the Social Sciences (SPSS) software. The numbers of colonies and gonads investigated each month ranged from 2 to 10 and 6 to 67, respectively, as shown in Tables 1 and 2.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. All source data underlying the graphs presented in the Figures were provided as Supplementary Data.
References
Odum, H. T. & Odum, E. P. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol. Monogr. 25, 291–320 (1955).
Fisher, R. et al. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 25, 500–505 (2015).
Harrison, P. L., & Wallace, C. C. Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z. editor. Ecosystems of the world 25, Coral reefs. Amsterdam: Elsevier, 133–207 (1990).
Harrison, P. L. et al. Mass spawning in tropical reef corals. Science 223, 1186–1189 (1984).
Babcock, R. C. et al. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394 (1986).
Harrison, P. L. Sexual reproduction in scleractinian corals in Coral Reefs: Ecosystem in Transition (eds. Dubinsky, Z., & Stambler, N.), 59-85 (Springer, 2011).
Policansky, D. Sex change in plants and animals. Ann. Rev. Ecol. Syst. 13, 471–495 (1982).
Loya, Y. & Sakai, K. Bidirectional sex change in mushroom stony corals. Proc. R. Soc. B: Biol. Sci. 275, 2335–2343 (2008).
Yeoh, S. R. & Dai, C. F. The production of sexual and asexual larvae within single broods of the scleractinian coral, Pocillopora damicornis. Mar. Biol. 157, 351–359 (2010).
Goffredo, S. et al. Unusual pattern of embryogenesis of Caryophyllia inornata (Scleractinia, Caryophylliidae) in the Mediterranean sea: maybe agamic reproduction? J. Morphol. 273, 943–956 (2012).
Combosch, D. J. & Vollmer, S. V. Mixed asexual and sexual reproduction in the Indo-Pacific reef coral Pocillopora damicornis. Ecol. Evol. 3, 3379–3387 (2013).
Marchini, C. et al. Annual reproductive cycle and unusual embryogenesis of a temperate coral in the Mediterranean Sea. PloS ONE 10, e0141162 (2015).
Nakashima, Y., Kuwamura, T. & Yogo, Y. Why be a both‐ways sex changer? Ethology 101, 301–307 (1995).
Nakamura, M., Kobayashi, Y., Miura, S., Alam, M. A. & Bhandari, R. K. Sex change in coral reef fish. Fish. Physiol. Biochem 31, 117–122 (2005).
Munday, P. L., Buston, P. M. & Warner, R. R. Diversity and flexibility of sex-change strategies in animals. Trends Ecol. Evol. 21, 89–95 (2006).
Kobayashi, Y., Nagahama, Y. & Nakamura, M. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Dev. 7, 115–125 (2013).
Loya, Y., Sakai, K. & Heyward, A. Reproductive patterns of fungiid corals in Okinawa, Japan. Galaxea. J. Coral Reef. Stud. 11, 119–129 (2009).
Eyal-Shaham, L. et al. Repetitive sex change in the stony coral Herpolitha limax across a wide geographic range. Sci. Rep. 9, 2936 (2019).
Luzon, K. S., Lin, M. C., Ablan-Lagman, M. C., Licuanan, W. R. Y. & Chen, C. A. Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphyllidae; clade V). Peer J. 5, e4074 (2017).
Hoeksema, B. W., & Cairns, S. World List of Scleractinia. Fimbriaphyllia ancora (Veron & Pichon, 1980). 2023. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1048073 on 2023-12-18
Bruckner, A. W. Tracking the trade in ornamental coral reef organisms: The importance of CITES and its limitations. Aquar. Sci. Conserv. 3, 79–94 (2001).
Twan, W., Hwang, J. & Chang, C. Sex steroids in scleractinian coral, Euphyllia ancora: implication in mass spawning. Biol. Reprod. 68, 2255–2260 (2003).
Twan, W. et al. The presence and ancestral role of gonadotropin-releasing hormone in the reproduction of scleractinian coral, Euphyllia ancora. Endocrinology 147, 397–406 (2006).
Allsop, D. & West, S. A. Sex-ratio evolution in sex changing animals. Evolution 58, 1019–1027 (2004).
Freeman, D. C., Harper, K. T. & Charnov, E. L. Sex change in plants: Old and new observations and new hypotheses. Oecologia 47, 222–232 (1980).
Korpelainen, H. Labile sex expression in plants. Biol. Rev. 73, 157–180 (1998).
Shapiro, D. Y. Differentiation and evolution of sex change in fishes. BioScience 37, 490–497 (1987).
Kobayashi, Y., Sunobe, T., Kobayashi, T., Nagahama, Y. & Nakamura, M. Gonadal structure of the serial-sex changing gobiid fish Trimma okinawae. Dev. Growth Differ. 47, 7–13 (2005).
Bhandari, R. K., Komuro, H., Nakamura, S., Higa, M. & Nakamura, M. Gonadal restructuring and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper (Epinephelus merra). Zool. Sci. 20, 1399–1404 (2003).
Murata, R. et al. Testicular inducing steroidogenic cells trigger sex change in groupers. Sci. Rep. 11, 11117 (2021).
Littlefield, C. L. Sex determination in Hydra: Control by a subpopulation of interstitial cells in Hydra oligactis males. Dev. Biol. 117, 428–434 (1986).
Warner, R. R. Sex change and the size-advantage model. Trends Ecol. Evol. 3, 133–136 (1988).
Rinkevich, B. & Loya, Y. Variability in the pattern of sexual reproduction of the coral Stylophora pistillata at Eilat, Red Sea: a long-term study. Biol. Bull. 173, 335–344 (1987).
Nishimiya-Fujisawa, C. & Kobayashi, S. Germline stem cells and sex determination in Hydra. Int J. Dev. Biol. 56, 499–508 (2012).
Siebert, S. et al. Stem cell differentiation trajectories in Hydra resolved at single-cell resolution. Science 365, eaav9314 (2019).
Nishimiya-Fujisawa, C., et al An ancient split of germline and somatic stem cell lineages in Hydra. bioRxiv. 07.04.546637 (2023).
Shikina, S. et al. Localization of early germ cells in a stony coral, Euphyllia ancora: potential implications for a germline stem cell system in coral gametogenesis. Coral Reefs 34, 639–653 (2015).
Guest, J. R., Baird, A. H., Goh, B. P. L. & Chou, L. M. Sexual systems in scleractinian corals: an unusual pattern in the reef-building species Diploastrea heliopora. Coral Reefs 31, 705–713 (2012).
Vargas-Angel, B., Colley, B. S., Hoke, S. M. & Thomas, D. J. The reproductive seasonality and gametogenic cycle of Acropora cervicornis off Broward county, Florida, USA. Coral Reefs 25, 110–122 (2006).
Jamodiong, E. A., Maboloc, E. A., Villanueva, R. D. & Cabaitan, P. C. Gametogenesis and inter-annual variability in the spawning pattern of Acropora hyacinthus in Northwestern Philippines. Zool. Stud. 57, e46 (2018).
Sarà, M. Sessile macrofauna and marine ecosystem. Ital. J. Zool. 53, 329–337 (1986).
Veron, J. E. N. Corals of the world. Australian Institute of Marine Science. Townsville MC, Qld, Australia (2000).
Shikina, S. et al. Germ cell development in the scleractinian coral Euphyllia ancora (Cnidaria, Anthozoa). PloS ONE 7, e41569 (2012).
Zhang, Y. et al. Discovery of a receptor guanylate cyclase expressed in the sperm flagella of stony corals. Sci. Rep. 9, 14652 (2019).
Acknowledgements
We are grateful to all lab members and divers who assisted us in collecting samples and measuring the colonies and distances between colonies. We also thank Dr. Steven Aird for his great help in preparing the manuscript. This research was supported by a grant from the Ministry of Science and Technology, Taiwan (107-2311-B-019-001) (to S.S.).
Author information
Authors and Affiliations
Contributions
S.S. conceived and designed the experiments. Y.L.C., P.H.T., and S.S. performed the experiments. Y.L.C., P.H.T., and S.S. analyzed the data. S.S. wrote the manuscript. C.F.C. modified the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Experiments were carried out in accordance with principles and procedures approved by the Institutional Animal Care and Use Committee, National Taiwan Ocean University.
Peer review
Peer review information
Communications Biology thanks Robert H. Richmond, Andrew Baird and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editors: Linn Hoffmann and David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shikina, S., Tsai, PH., Chiu, YL. et al. The stony coral Fimbriaphyllia (Euphyllia) ancora’s reproductive strategy involves a sex change every year. Commun Biol 7, 1093 (2024). https://doi.org/10.1038/s42003-024-06799-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06799-x
- Springer Nature Limited