Abstract
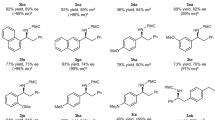
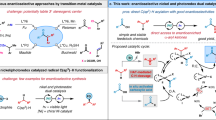
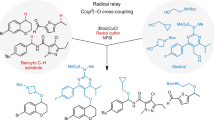
Functionalization of inert C–H bonds has received tremendous attention due to the inherent atom economy and efficiency of the transformations of the starting materials. As compared to transition-metal-catalysed C–H activation, organocatalysis is much less commonly applied for direct functionalization of inert C–H bonds. The α C(sp3)–H bonds of NH2-unprotected benzylamines usually are inert in most reactions due to the extremely low Brønsted acidity. Here we utilize a chiral pyridoxal bearing a quaramide side chain as a bifunctional carbonyl catalyst to activate the α C(sp3)–H bond of NH2-unprotected benzylamines, making it acidic enough to be deprotonated under mild conditions. Based on the carbonyl catalysis strategy, we develop a direct asymmetric α C–H addition of benzylamines to aldehydes, providing one of the most straightforward methods for the synthesis of chiral β-aminoalcohols with excellent diastereo- and enantioselectivities.





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information file, or from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2121718 ((R,R)-1a) and CCDC 2121753 ((R,R)-4ae). These data can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/structures/.
References
Campos, K. R. Direct sp3 C–H bond activation adjacent to nitrogen in heterocycles. Chem. Soc. Rev. 36, 1069–1084 (2007).
Mitchell, E. A., Peschiulli, A., Lefevre, N., Meerpoel, L. & Maes, B. U. W. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 18, 10092–10142 (2012).
Seidel, D. The azomethine ylide route to amine C–H functionalization: redox-versions of classic reactions and a pathway to new transformations. Acc. Chem. Res. 48, 317–328 (2015).
Gonnard, L., Guérinot, A. & Cossy, J. Transition metal-catalyzed α-alkylation of amines by C(sp3)‒H bond activation. Tetrahedron 75, 145–163 (2019).
Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications (Wiley-VCH, 2010).
Gonzalez, A. Z. et al. Selective and potent morpholinone inhibitors of the MDM2–p53 protein–protein interaction. J. Med. Chem. 57, 2472–2488 (2014).
Huang, H. et al. Oxazolidinone-based allosteric modulators of mGluR5: defining molecular switches to create a pharmacological tool box. Bioorg. Med. Chem. Lett. 26, 4165–4169 (2016).
Degnan, A. P. E. A. Oxazolidinones as modulators of mGluR5. Patent WO 2012064603 (2012).
Desimoni, G., Faita, G. & Jørgensen, K. A. Update 1 of: C2-symmetric chiral bis(oxazoline) ligands in asymmetric catalysis. Chem. Rev. 111, PR284–PR437 (2011).
Zhang, Z.-H., Dong, X.-Q., Chen, D. & Wang, C.-J. Fine-tunable organocatalysts bearing multiple hydrogen-bonding donors for construction of adjacent quaternary and tertiary stereocenters via a Michael reaction. Chem. Eur. J. 14, 8780–8783 (2008).
Park, Y. S., Boys, M. L. & Beak, P. (−)-Sparteine-mediated α-lithiation of N-Boc-N-(p-methoxyphenyl)benzylamine: enantioselective syntheses of (S) and (R) mono- and disubstituted N-Boc-benzylamines. J. Am. Chem. Soc. 118, 3757–3758 (1996).
Niwa, T., Yorimitsu, H. & Oshima, K. Palladium-catalyzed benzylic arylation of N-benzylxanthone imine. Org. Lett. 10, 4689–4691 (2008).
Chen, Y.-J., Seki, K., Yamashita, Y. & Kobayashi, S. Catalytic carbon–carbon bond-forming reactions of aminoalkane derivatives with imines. J. Am. Chem. Soc. 132, 3244–3245 (2010).
Shibahara, F., Kobayashi, S.-i, Maruyama, T. & Murai, T. Diastereo- and regioselective addition of thioamide dianions to imines and aziridines: synthesis of N-thioacyl-1,2-diamines and N-thioacyl-1,3-diamines. Chem. Eur. J. 19, 304–313 (2013).
Li, M. et al. Transition-metal-free chemo- and regioselective vinylation of azaallyls. Nat. Chem. 9, 997–1004 (2017).
Deng, G. et al. Transition-metal-free allylation of 2-azaallyls with allyl ethers through polar and radical mechanisms. Nat. Commun. 12, 3860 (2021).
Jun, C.-H. Chelation-assisted alkylation of benzylamine derivatives by Ru0 catalyst. Chem. Commun. 13, 1405–1406 (1998).
Dastbaravardeh, N., Schnürch, M. & Mihovilovic, M. D. Ruthenium(II)-catalyzed sp3 C–H bond arylation of benzylic amines using aryl halides. Org. Lett. 14, 3792–3795 (2012).
Chen, X., Engle, K. M., Wang, D.-H. & Yu, J.-Q. Palladium(II)-catalyzed C−H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 48, 5094–5115 (2009).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Wencel-Delord, J., Dröge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C−H bond activation. Chem. Soc. Rev. 40, 4740–4761 (2011).
Bordwell, F. G. & Liu, W.-Z. Effects of sulfenyl, sulfinyl and sulfonyl groups on acidities and homolytic bond dissociation energies of adjacent C–H and N–H bonds. J. Phys. Org. Chem. 11, 397–406 (1998).
Crugeiras, J., Rios, A., Riveiros, E. & Richard, J. P. Substituent effects on electrophilic catalysis by the carbonyl group: anatomy of the rate acceleration for PLP-catalyzed deprotonation of glycine. J. Am. Chem. Soc. 133, 3173–3183 (2011).
Chen, J. et al. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction. Science 360, 1438–1442 (2018).
di Salvo, M. L. et al. On the catalytic mechanism and stereospecificity of Escherichia coli l-threonine aldolase. FEBS J 281, 129–145 (2014).
Wang, Q., Gu, Q. & You, S.-L. Enantioselective carbonyl catalysis enabled by chiral aldehydes. Angew. Chem. Int. Ed. 58, 6818–6825 (2019).
Li, S., Chen, X.-Y. & Enders, D. Aldehyde catalysis: new options for asymmetric organocatalytic reactions. Chem 4, 2026–2028 (2018).
Yin, Q., Shi, Y., Wang, J. & Zhang, X. Direct catalytic asymmetric synthesis of α-chiral primary amines. Chem. Soc. Rev. 49, 6141–6153 (2020).
Cheng, A. et al. Efficient asymmetric biomimetic aldol reaction of glycinates and trifluoromethyl ketones by carbonyl catalysis. Angew. Chem. Int. Ed. 60, 20166–20172 (2021).
Ma, J. et al. Enantioselective synthesis of pyroglutamic acid esters from glycinate via carbonyl catalysis. Angew. Chem. Int. Ed. 60, 10588–10592 (2021).
Wen, W. et al. Diastereodivergent chiral aldehyde catalysis for asymmetric 1,6-conjugated addition and Mannich reactions. Nat. Commun. 11, 5372 (2020).
Wen, W. et al. Chiral aldehyde catalysis for the catalytic asymmetric activation of glycine esters. J. Am. Chem. Soc. 140, 9774–9780 (2018).
Zhu, F. et al. Direct catalytic asymmetric α-allylic alkylation of aza-aryl methylamines by chiral-aldehyde-involved ternary catalysis system. Org. Lett. 23, 1463–1467 (2021).
Ma, J. et al. Asymmetric α-allylation of glycinate with switched chemoselectivity enabled by customized bifunctional pyridoxal catalysts. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202200850 (2022).
Xu, B. et al. Catalytic asymmetric direct α-alkylation of amino esters by aldehydes via imine activation. Chem. Sci. 5, 1988–1991 (2014).
Zhong, X. et al. Chiral Lewis acid-bonded picolinaldehyde enables enantiodivergent carbonyl catalysis in the Mannich/condensation reaction of glycine ester. Chem. Sci. 12, 4353–4360 (2021).
Shi, L. et al. Chiral pyridoxal-catalyzed asymmetric biomimetic transamination of α-keto acids. Org. Lett. 17, 5784–5787 (2015).
Liu, Y. E. et al. Enzyme-inspired axially chiral pyridoxamines armed with a cooperative lateral amine chain for enantioselective biomimetic transamination. J. Am. Chem. Soc. 138, 10730–10733 (2016).
Cai, W. et al. Asymmetric biomimetic transamination of α-keto amides to peptides. Nat. Commun. 12, 5174 (2021).
Alemán, J., Parra, A., Jiang, H. & Jørgensen, K. A. Squaramides: bridging from molecular recognition to bifunctional organocatalysis. Chem. Eur. J. 17, 6890–6899 (2011).
Tang, S., Zhang, X., Sun, J., Niu, D. & Chruma, J. J. 2-Azaallyl anions, 2-azaallyl cations, 2-azaallyl radicals, and azomethine ylides. Chem. Rev. 118, 10393–10457 (2018).
Crugeiras, J., Rios, A., Riveiros, E., Amyes, T. L. & Richard, J. P. Glycine enolates: the effect of formation of iminium ions to simple ketones on α-amino carbon acidity and a comparison with pyridoxal iminium ions. J. Am. Chem. Soc. 130, 2041–2050 (2008).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Lu, L.-Q., An, X.-L., Chen, J.-R. & Xiao, W.-J. Dual activation in organocatalysis: design of tunable and bifunctional organocatalysts and their applications in enantioselective reactions. Synlett 2012, 490–508 (2012).
Breslow, R. Biomimetic chemistry and artificial enzymes: catalysis by design. Acc. Chem. Res. 28, 146–153 (1995).
Chen, J., Liu, Y. E., Gong, X., Shi, L. & Zhao, B. Biomimetic chiral pyridoxal and pyridoxamine catalysts. Chin. J. Chem. 37, 103–112 (2019).
Dalko, P. I. & Moisan, L. Enantioselective organocatalysis. Angew. Chem. Int. Ed. 40, 3726–3748 (2001).
List, B. Introduction: organocatalysis. Chem. Rev. 107, 5413–5415 (2007).
MacMillan, D. W. C. The advent and development of organocatalysis. Nature 455, 304–308 (2008).
Acknowledgements
We are grateful for the generous financial support from the National Natural Science Foundation of China (21871181, 22271192), the Shanghai Municipal Education Commission (2019-01-07-00-02-E00029), the Shanghai Municipal Committee of Science and Technology (20JC1416800) and Shanghai Engineering Research Center of Green Energy Chemical Engineering (18DZ2254200).
Author information
Authors and Affiliations
Contributions
B.Z. conceived and directed the project and wrote the paper. C.H. conducted most of the experiments including the synthesis of the chiral pyridoxamine catalysts and the development of the reaction. B.P., S.Y. and Z.Y. performed pyridoxal catalyst development. J.C. performed MS analysis for the project. X.X. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Weiwei Zi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, References, Figs. 1–26, Tables 1–12 and Equations (1)–(7).
Supplementary Data 1
The .cif file of compound (R,R)-1a (CCDC 2121718).
Supplementary Data 2
The .cif file of compound (R,R)-4ae (CCDC 2121753).
Supplementary Data 3
The computational data for pKa determination.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, C., Peng, B., Ye, S. et al. Catalytic asymmetric α C(sp3)–H addition of benzylamines to aldehydes. Nat Catal 5, 1061–1068 (2022). https://doi.org/10.1038/s41929-022-00875-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00875-3
- Springer Nature Limited
This article is cited by
-
Asymmetric α-C(sp3)−H allylic alkylation of primary alkylamines by synergistic Ir/ketone catalysis
Nature Communications (2024)
-
Enantioconvergent synthesis of axially chiral amides enabled by Pd-catalyzed dynamic kinetic asymmetric aminocarbonylation
Nature Communications (2024)
-
Biomimetic asymmetric catalysis
Science China Chemistry (2023)