Abstract
In the field of global water purification, the issue of marine oil spills represents a significant challenge. The use of phase-selective organogelators (PSOGs) as sorbent materials in oil spill remediation is a promising solution due to their environmental adaptability and high absorption capacity. However, there are limited reports on PSOGs that can be used in powder form for rapid phase-selective gelation of crude oils. In this context, the development of innovative dicholesteryl derivatives as low-molecular-weight organogelators (LMOGs) offers a promising solution in powder form. These gelators are synthesized through a one-pot multi-component reaction as green synthesis method, which ensures high purity and eliminates the need for harsh conditions. The incorporation of cholesterol into the gelator structure demonstrate environmental adaptability. The exceptional sorption capacity was attributed to the structured 2D/3D networks observed through scanning electron microscopy (SEM). The hydrophobic properties of these gelators, as evidenced by a water contact angle of 118 degrees, enable them to efficiently gel various organic solvents at low concentrations (1% w/v) at ambient temperatures, without the need for heating–cooling cycles or co-solvents. The eco-friendly nature and efficient oil–water separation capabilities of these gelators in powder form represent a significant advancement in global water purification efforts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The pollution of aquatic ecosystems due to oil and petrochemical spills and leaks has become a global issue, with increasing accidents during various operations such as extraction, loading, and transport, posing a threat to aquatic life1. Consequently, the removal of these oil pollutants, which consist of hydrocarbons, benzene, toluene, crude oil and its derivatives (such as gasoline, diesel, and kerosene) from the aquatic environment has emerged as a crucial concern worldwide. Oil spill treatment strategies encompass in-situ burning, mechanical methods, chemical treatments, bioremediation, and adsorption2. Among these approaches, adsorption using oil sorbents is considered the most effective due to its cost-efficiency, high performance, and minimal secondary pollution. Easy regeneration and recyclability are also essential factors for an ideal sorbent3. Oil sorbents are typically classified into three main categories: inorganic mineral sorbents, synthetic organic sorbents, and natural organic sorbents4. Each type has its advantages and limitations. For instance, mineral sorbents have low oil absorption capacity and buoyancy, making them unsuitable for oil spill treatment. Synthetic organic sorbents exhibit excellent oil sorption capabilities but degrade slowly compared to mineral or natural options. Natural sorbents have limitations such as low capacity, poor hydrophobicity, selectivity, and reusability4. To overcome the drawbacks of existing materials, innovative sorbents have been developed. In recent years, low-molecular-weight organogelators have gained significant attention as phase-selective sorbents with optimal sorption properties5,6,7.
Low-molecular-weight organogelators (LMOGs) are organic molecules that form fibrillar three-dimensional networks through self-assembly, trapping organic solvent or liquid between the strands. Self-assembly results from the strong non-covalent interactions such as electrostatic, van der Waals forces, π effects and hydrophobic effects between molecules6. These supramolecular gels have gained attention for solidifying oil. They are gaining attention for solidifying oil due to their ability to rapidly and feasibly gelate without the need for co-solvents or heat-cool cycles, stability at low temperatures and against shear forces, thermal reversibility for easy oil recovery, recyclability and reusability, cost-effective synthesis, and environmental friendliness6. These supramolecular gels have been utilized by researchers to combat oil pollution effectively7,8,9, as they remain on the water surface due to their organic nature, unlike mineral-based adsorbents that sink and release absorbed materials back into the environment over time4. Organogelators effectively solidify oil spills and petrochemical products, preventing their further dispersion in liquid form. Additionally, a small quantity of organic gelator can absorb a large volume of oil, facilitating easy collection of solidified oil from water surfaces. Upon application of heat, the solidified oil becomes soluble again, releasing the contained materials. However, while organogelators do not interfere with crude oil refining processes and can burn alongside collected petrochemical products like kerosene, their organic composition may pose environmental challenges as they do not decompose naturally10. To address these environmental concerns, it is crucial to develop organogelators based on natural molecules that not only effectively mitigate pollution issues but also demonstrate compatibility with the environment.
In 1987, Weiss and colleagues highlighted the significance of the cholesteryl motif in the gelation process11. Their findings indicated that the oxygen motif in cholesteryl derivatives facilitates hydrogen bonding, while van der Waals interactions of alkyl rings and chain residues drive their assembly into a three-dimensional network, resulting in unique organogel properties12,13,14,15. Cholesteryl derivatives, as a class of Low-molecular-weight organogelators (LMOGs), show promise in encapsulating and removing oil pollutants9 from water or other environments, offering a sustainable and eco-friendly solution due to their biodegradable and renewable nature16,17. However, current gelators for treating oil spills often require a heating–cooling process or a co-solvent to address solubility issues, making them impractical on a large scale and potentially introducing additional pollutants. Therefore, there is a need to explore alternative solutions that do not rely on these methods to effectively address oil pollution15,18,19,20,21.
The use of cholesterol as a natural and non-toxic material for creating absorbents to combat oil pollution offers eco-friendly solutions for environmental cleanup and sustainable industrial practices17,22. Enhancing the synthesis methods for cholesterol-based gelators is crucial for practical and cost-effective large-scale applications. Traditional synthetic approaches have been complex, multi-step processes with low yields, limiting their feasibility7,19,20,21. Our research has successfully developed a cholesterol-based gelator using an environmentally friendly, straightforward, and single-step multicomponent method at room temperature without the need for a catalyst. Multicomponent reactions (MCRs) are considered environmentally friendly reactions as they efficiently produce complex molecules from simple starting materials in a single step, reducing waste, energy consumption, and purification steps. MCRs can be carried out under mild reaction conditions, aligning with green chemistry principles and sustainable development goals. This approach offers a promising option for environmentally friendly synthesis of complex molecules with potential wide-ranging benefits. Notably, only 1% (w/v) of the powder gelator is required to create a robust nano-sorbent at room temperature without the need for heating–cooling cycles or co-solvents.
Experimental section
Materials
All reagents needed for the synthesis of the gelators were purchased from Merck, Acros, and Alfa-Aesar companies and used without any further purification. All solvents (≥ 99% purity) were obtained from commercial sources and used without further purification.
Characterization
Differential scanning calorimetry (DSC) analysis was conducted on DSC131-SETARAM (France) device over the temperature range of 0–250 °C with a heating and cooling rate of 5 °C min−1. Fourier transform infrared spectroscopies were directed on Jasco-Japan 6300. For the preparation of KBr pellets, a small amount of each powder gelator was mixed with anhydrous KBr powder. The liquid or gel samples were examined directly as a thin film, “neat”, between two plates of NaCl. To observe the surface structure of the gel, optical microscopic images were taken on an OLYMPUS U-AN360P. For this regard, the prepared gel was put on the surface of the microscope slide and seen by the microscope with proper magnification. The xerogel surface was prepared by exposing the slide containing the gel to air for three weeks and then seen by microscope. AFM measurements were performed on a Brisk Inspect Nanoscope-Iran system. Scans were performed using silica probes with a tip radius of 8 nm in tapping mode. The force constant was approximately 40 N/m. As in the optical microscopy procedure, the prepared gel was loaded onto microscope slides and exposed to air for drying. SEM images of the xerogel of dicholesteryl gelator 5a were taken on a MIRA3TESCAN-XMU, FE-SEM instrument to study the morphology of these samples. The xerogel was prepared by freeze-drying the gel in liquid nitrogen, and then evaporating by a vacuum pump. To determine the strength of the gel against the exerted strain and/or stress, the rheological properties of the gel were obtained. These properties were measured by Physica MCR 301 (Anton Paar) instrument. For our measurements, this rheometer was equipped with two stainless steel-coated parallel plates (25 mm diameter) with a 1000 µm gap distance. All measurements were carried out at room temperature (25 °C). For investigation of the linear viscoelastic region (LVER) of the gel, stress sweep measurements at a constant frequency of 10 rad/s were conducted in the stress range of 1.0–100.0 Pa. A frequency sweep was conducted in a 0.1–500 rad s−1 angle frequency range at a definite strain of 1%. A temperature sweep was conducted at 4 to 180 °C in a 6.28 rad s−1 angle frequency under a certain strain of 1% to record the gel-sol transition temperature. The powder X-ray diffraction (XRD) data of the xerogel of the gel was collected on a D8ADVANCE diffractometer with Cu Kα X-ray created (λ = 1.540598 ºA) under a voltage of 40 kV and a current of 40 mA. The scan rate was 0.5° min−1. The xerogel was prepared by using a liquid nitrogen freeze-dryer for the gel and then evaporating by a vacuum pump. Water contact angle (CA) was obtained by the pendant drop contact angle method using CA-ES10. In this method, by introducing a water droplet via a micro syringe on the dried gel on a microscope slide, the contact angle of a water droplet is measured. The water contact angle photos were recorded with a camera equipped with a microscope.
Synthesis of the gelators
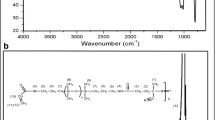
The dicholesteryl derivatives of malonoester as gelator and stabilizer were prepared through a multi-component reaction which has been reported by our group23. These compounds were synthesized via a one-pot five-component reaction by different isocyanide derivatives at room temperature in the absence of any catalyst (Fig. 1). In this way, a well-magnetically stirred solution of 2-oxo-2-aryl acetaldehyde 1 (1 mmol) as α-ketoaldehyde and Meldrum’s acid 2 (0.144 g, 1 mmol) in dichloromethane (5 mL) was prepared. A solution of cholesterol 4 (2 mmol) in dichloromethane (2 mL) was added to the reaction mixture. After 10 min, a solution of an isocyanide derivative 3 (1 mmol) in dichloromethane (2 mL) was added dropwise at 0 °C in an ice bath over 10 min. After the addition of isocyanide to the reaction mixture, the final mixture was stirred at room temperature for 24 h. The product was obtained as a concentrated solution which is extracted by adding methanol. The obtained precipitate was washed completely with methanol to remove impurities to reach a white precipitate. The synthesized products were characterized through FT-IR, 1H-NMR, and 13C-NMR spectra.
Preparation of organogel
To prepare a gel, a specific quantity of a gelling agent (5a-5d) as per Table 1 is added to a specified volume (0.2 ml) of solvent and then wait until the sample dissolves spontaneously. As the gelator dissolved, the viscosity of the oil on the water's surface increased until complete gelation occurred. For the samples that were unable to be dissolved in the used solutions by themselves, they were dissolved by the application of heat. When the sample is completely dissolved, it is left to cool to room temperature. In this case, the sample may appear as a solution, partial gel, complete gel, or as a precipitate. If the sample has gelled or partially gelled, when the sample container is inverted according to the inverse tube test method, if no fluidity is observed and the sample has taken a solid form, it is considered as a gel. If a small amount of flow is observed in the sample, it is considered partially gelled. When the sample forms a gel, determining the minimum gelling concentration (MGC) is crucial for assessing its gelling ability. Through successive gelation tests, it was determined that n-dodecane is the most effective solvent for gelation, as it completely gelled two of the samples and had the lowest MGC for all. This solvent was able to form gel with 1% (w/v) as the MGC for dicholesteryl gelator 5a and 2% (w/v) for dicholesteryl gelator 5d. According to the literature, a concentration of 1% (w/v) of the gelator in n-dodecane is considered suitable. The term (w/v) represents weight/volume, indicating the weight of the solute (in this case, the gelator) per unit volume of solution.
Results and discussion
Design of the gelators
It has been proved in our previous work that malonoester structures possessing amide groups can act as low-molecular-weight organogelators and efficiently gel organic solvents23. Cholesteryl derivatives, known for their ability to gel organic solvents including oils, are promising for environmental applications such as pollutant remediation due to their biodegradable and renewable nature24. Introducing cholesteryl units into the malonoester structure is expected to lead to robust supramolecular gel formation, leveraging multiple non-covalent interactions. This approach aligns with green chemistry principles and sustainable development. The synthesis of the cholesteryl-containing malonoester structure is described as feasible through a simple and efficient multi-component reaction, expected to result in enhanced gelation properties due to the combined effects of cholesteryl units and existing non-covalent interactions.
Gelation behavior of the compounds
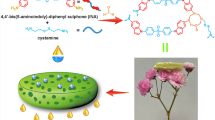
The gelation behaviors of compound dicholesteryl gelator 5a were investigated in non-polar organic solvents found in petroleum as oil pollutants, detailed in (Table 1). The compound was found to form stable gels with a lower minimum gelation concentration (MGC) value, making it the preferred gelator. At just 1% (w/v) MGC, this compound effectively gels n-dodecane, petroleum, diesel, kerosene, and silicone oil solvents, as depicted in. Additionally, a stable gel was achieved in n-dodecane at 5 °C with only 0.5% (w/v) MGC. However, the compound resulted in an unstable gel in n-octane and a partial gel in n-heptane. These findings position compound dicholesteryl gelator 5a as an excellent candidate for gelation in a range of petroleum-based solvents, with potential implications for various practical applications. Indeed, incorporating cholesterol into the gelator structure aligns with the principles of green chemistry due to its renewable and biodegradable nature. The ability to form stable gels without the need for additional solvents further supports its environmentally friendly profile. These characteristics make the gelator a promising candidate for applications such as absorbing crude oil in seawater, where minimizing environmental impact is crucial. Furthermore, the addition of an oily solvent in conjunction with water and the powder of a gelator resulted in the observation of phase-selective gelation (Fig. 2). Further observation revealed that the majority of the resultant gels exhibited thermostable characteristics, maintaining their gel state for several weeks. Additionally, as illustrated in Fig. 3a, the gels formed in oil products were found to be thermo-reversible, meaning that they could be liquefied by heating the gel above its Tgel and then solidified below Tgel (the gel-to-solution transition temperature, Tgel, is 58 °C). This transition can be repeated at least ten times (Fig. 3b). This behavior is attributed to the reversible interactions, such as hydrogen bonding and π-π stacking, which are responsible for the gelation process5. Dicholesteryl gelator 5d forms weaker gels compared to dicholesteryl gelator 5a when used with the same solvents. The similar intramolecular interactions between the amide and ester groups, van der Waals interactions between cholesteryl moieties, and π-π stacking interaction of the phenyl groups in these four structures indicate that the difference in gelation behavior is due to the van der Waals interactions of the different R groups in the isocyanide derivatives. It is concluded that the cyclohexyl group in dicholesteryl gelator 5a and the n-butyl group in dicholesteryl gelator 5d lead to networked assemblies that enclose alkyl solvents through capillary forces and interface tensions25. However, the t-butyl and the 2,4,4-trimethyl pentane groups in dicholesteryl gelator 5b and 5c do not lead to networked assemblies due to their branching. The difference in gelation ability between the cyclohexyl and n-butyl groups is related to conformational freedom. The larger and bulkier nature of the cyclohexyl group compared to the n-butyl group restricts conformational freedom, resulting in better preorganization of the gelator structures, which improves the gelation process26. The data in Table 1 indicates that as the solvent becomes less polar and heavier, gel formation increases due to the growing van der Waals interactions. The derivatives were tested in a range of polar solvents, including aprotic (DMSO, DMF, THF) and protic solvents (acetonitrile, ethyl acetate, ethanol, methanol), but none of them demonstrated the ability to form an appropriate gel (Table S 1). This suggests that these gelators are selectively able to form gels in nonpolar aliphatic solvents, such as those found in petroleum.
The phase-selective gelation behavior of dicholesteryl gelator 5a in petroleum in the presence of seawater. The gelator is in its (a) solution state, (b,c) gel state. (A video file (Video-Fig. 2) in appendix supports this Figure).
The ability of dicholesteryl gelator 5a as oil spill treatment
One of the reported applications of low-molecular-weight organogelators is in the treatment of oil spills. In this context, the organogelator needs to have the capability to solidify oil products. The gelation ability of dicholesteryl gelator 5a was investigated in non-polar organic solvents commonly found in petroleum (as shown in Table 1). The results indicated that dicholesteryl gelator 5a can effectively gel oil products at low concentrations of the gelator (1 and 2% (w/v)). To simulate the collection of oil from the water surface, the organogelator should be insoluble in water, as it is unable to enter the water and selectively immobilize oil in biphasic oil–water mixtures6,8. Some petroleum as oil phase was poured onto the surface of seawater (Fig. 4a), after which the powdered gelator was applied on top (Fig. 4b). The container was then agitated gently to simulate the sea waves, after which the dissolution of the gelator in the oil was observed at room temperature. As the gelator dissolved, the viscosity of the oil on the water's surface increased until complete gelation occurred (Fig. 4c). Accordingly, by solidifying the leaked oil and separating it from the water surface (Fig. 4d,e, the gelator can be separated and reused. This demonstrated that the organogel can be formed at the seawater surface at room temperature without heating–cooling and co-solvent and stably remain on the water surface without absorbing water due to its hydrophobic nature. The hydrophobicity was confirmed using the water contact angle method in Sect. “Poor wetting”. By distilling the solvent from the removed gel layer, the gelator was recovered and found to be reusable.
Selective gelation of petroleum in the presence of seawater (a) biphasic solution of 20 ml water and 2 ml petroleum, (b) adding gelator 5a on the water surface, (c) waiting for gelation after dissolving at room-temperature, (d) collecting the gelled oil by spatula in one side, (e) removing the gel from the water surface (cleaning the surface of the seawater from petroleum). (A video file (Video-Fig. 4) in appendix supports this Figure).
Prior to utilization of the synthesized derivative as an absorber for oil spillage remediation, it was necessary to ascertain its biocompatibility, which is defined as the lack of toxicity to organisms. For this purpose, a fish species was selected as the test organism and 12 mg of gelator was added to water to test for toxicity. The lack of mortality among the fish over the 40-day test period indicates that the gelator does not exhibit toxic effects on aquatic organisms (Figure S17).
Thermal stability of the gel
The thermal properties of gels, particularly organogel, are important for understanding their behavior and potential applications. One of the key thermal properties of gels is gelation temperature (Tgel). This is the temperature at which the gel-sol transition occurs. It indicates the thermal stability of the gel, which determines the range of temperatures over which the gel remains stable. One of the simplest and quickest techniques for determining Tgel is the 'tube inversion' method. This method involves visually observing the point at which a sample begins to flow, and is based on the principle that as the strength of the network increases, the temperature of the gel transition to solution increases, and eventually the gel becomes capable of flowing. In this study, different concentrations of dicholesteryl gelator 5a were prepared, ranging from 4 to 50 mg/ml. The Tgel was obtained by heating each sample at a rate of 2 °C per minute and using the inverted test tube technique. As depicted in Figure S18a, it was observed that as the concentration of the gel increased, the Tgel also increased. This trend continued until the concentration reached 30 mg/ml, after which the Tgel value remained constant. This behavior is consistent with previous findings in the literature for organogel, indicating a saturation point in the relationship between gel concentration and Tgel5,23,27.
Another method for investigating the transfer of gel to solution is using differential scanning calorimetry (DSC) technique. DSC thermal curves provide valuable information about phase transfer, stability, and energy storage/release properties, indicating the behavior of gels under thermal changes. In general, the peaks in the DSC curve, which are created due to temperature changes, indicate changes in the system's structure. In DSC curves, two types of peaks appear: endothermic peaks that occur due to an increase in temperature and exothermic peaks that are created during the temperature decrease stage28. Multiple endothermic peaks indicate the conversion of gel to solution or reflect the polymorphism resulting from temperature changes in the network. This phenomenon occurs when a substance exhibits different crystalline structures at different temperatures. On the other hand, multiple exothermic peaks in DSC curves indicate gel formation or crystallization. In some cases, the peaks in DSC curves become very broad. The broadening of the endothermic peaks can be interpreted by considering an organogel as a binary system. The broadening of the peak reflects the distribution of energy required for the transition from the gel phase to the solution phase, which may be due to the presence of gradient compositions in the binary system. This phenomenon results in a range of temperatures for gel melting instead of a single temperature. The multiple endothermic peaks shown in Figure S19 clearly demonstrate these aspects. The first endothermic peak observed indicates the beginning of molecular mobility in the gel network, representing the transition from a glassy state to a rubbery state29. At this stage, with the weakening of the weakest hydrogen bonds, the stable gel network begins to lose its rigidity and becomes more fluid. If the onset temperature (resulting from the corresponding peak) of the first endothermic peak is assigned to Tg, this temperature corresponds to what is observed in the “tube inversion” method. According to DSC curves, increasing the concentration of the gelator from 2 to 8% (w/v) leads to an increase in the value of Tg. This result indicates an increase in the strength of the gel network with the increase in gelator concentration up to 8% (w/v). Furthermore, based on the DSC curves shown in Figure S19–S21, with the increase in gelator concentration, the intensity of the second endothermic peak decreases, and this peak merges with the first endothermic peak. This can be attributed to a decrease in the probability of polymorphism30,31. Because as the gelator concentration increases, the space between fibrils becomes more restricted, reducing the gel's ability to reform multiple crystalline structures (so-called thermotropic polymorphism). Therefore, these two endothermic peaks appear as a very broad and extensive peak. Another key property of gels, in addition to Tg, is the temperature of the highest endothermic peak, known as Tm, where the solid phase completely dissolves. Literature studies indicate that the reported values for Tg and Tm using DSC method differ from those obtained using tube inversion method and rheological measurements. This difference is mainly attributed to different heating rates, signal-to-noise ratios, and experimental conditions (such as sample weight or volume)32. As shown in Table 2, the DSC technique shows a higher value for Tg compared to other techniques (around 10 degrees Celsius).
In addition to the mentioned properties, investigating the thermal reversibility of organogel, which is achieved by reducing the temperature of the sample in the solution phase, can also help in describing the behavior of gels. In this process, the first endothermic peak is related to the freezing process32. This temperature is called Tc, another important feature of the gel. As shown in Figure S19, the Tc temperature obtained from the endothermic peak in the cooling process is lower than both endothermic peak temperatures in the heating process. This phenomenon is called hysteresis heat and is observed in many reversible heat systems33. In gels that are thermally reversible, there are crystalline and amorphous regions. Therefore, it can be assumed that the formation of a new ordered structure with stable heat transfer zones is the reason for the existence of hysteresis heat.
Reports indicate that the temperature at which the rheology shows the transition of a gel system to a solution (Tgs) is between two temperatures, Tg and Tm31. This temperature is the same temperature at which the rheology analysis shows G' and G'' to be equal34. Figure S18b confirms this phenomenon quite well.
The interactions between the organogelator and the solvent during self-assembly
One of the powerful tools for studying the intermolecular interactions between the organogelator and the solvent in the self-assembly process is FT-IR spectroscopy35. Therefore, to achieve an internal view of the self-assembly process and investigate the intermolecular interactions in our organogelator molecule, the FT-IR spectroscopies of the original powder of dicholesteryl gelator 5a were compared with the gel state and the solution state of this compound in the n-dodecane solvent. The FT-IR spectroscopy of pure n-dodecane solvent (Fig. 5a) was also compared with other FT-IR spectrums. The FT-IR spectrum of the original powder (Fig. 5e) shows the stretching vibration of NH and C=O functions of the amide group in 3387 and 1675 cm−1, respectively. These amounts shifted to 3299 and 1639 cm−1 in the spectrum of the gel state with 10% (w/v) and 25% (w/v) concentrations of the gel (Fig. 5c,d). Furthermore, other shifts to 3299 and 1637 cm−1 in the spectrum of the solution state are observed (Fig. 5b). These observed shifts represent the participation of the NH and C=O groups of the amide structure in hydrogen bonding36. Other spectral shifts are observed for C=O groups of the ester and the ketone in the structure (Table 3), which approve the participation of these groups in the assembly process. As seen in Table 3, another piece of evidence for forming the gel state is a vibration shift of the CH2 group of n-dodecane solvent from 2958 in the solvent to 2962 in the gel state. The reason for this spectral shift is related to the vibrational freedom, that is lower energy is needed for the vibration of solvent molecules in a liquid state than in the gel state37.
The self-assembled nano-fiber structures
The microstructures of the surface of the gel network of dicholesteryl gelator 5a was studied by optical microscopic images taken on an OLYMPUS U-AN360P (Figure S22) in a petroleum and n-dodecane solvent. As shown in Figure S22a,b, the xerogels show a uniform texture.
The morphology of the gel was also characterized by atomic force microscopy (AFM). As shown in Figure S22, the presence of the entangled network inclusions of fibrous or tubular structure shown by the AFM images of the xerogel is in agreement with the SEM images.
To obtain a more detailed understanding of the microstructure of the gel 5a, xerogel was studied by SEM observation. Figure 6 illustrates SEM images of the xerogel. As depicted in Fig. 6, a three-dimensional network was created by aggregating vigorous self-assembled nano-fiber structures with fiber diameters of 32–116 nm which trapped the solvent molecules in their three-dimensional spaces. Nano-structured gels have a high surface area which leads to closer contact with the liquid phase and quick internal diffusion kinetic. Therefore, nano-structured gels are superior to micro-structured gels6. Furthermore, because of the importance of nanofiber structures in biomedical applications such as wound dressings, drug delivery systems, enzyme immobilization, and carriers for cell cultivation, a large number of studies have been dedicated to these structures38. It is interesting to note that one of the methods for producing nanofiber structures is the molecular self-assembly technique39. Referring to Table 4, having a nanofiber structure is a significant property of our gelator rather than the micro-structures reported in the literature. Further investigation of the SEM images shows that the morphology of the xerogel is characterized by thin ribbons which makes an entangled network structure. This morphology of the xerogel is consistent with the structures of organogels18. Since these gels are obtained by low-molecular-weight organic molecules with non-covalent interactions, they can be considered supramolecular gels.
XRD analysis
The structure of the xerogel was studied by PXRD analysis. According to the FT-IR and SEM results and the chemical structure of the compound, it can be said that there are interactions that are responsible for assembling the plane of the compound. These interactions are caused by the aromatic groups stacked over each other by π-π stacking interaction and the amide and ester groups of each molecule which interact with another molecule by hydrogen bonding interaction. Referring to Figure S23, the d spacing of the most intense peak in the PXRD pattern of xerogel is 32.91489 A°, which appear in 2.6820 [°2Theta]. The amorphous peak, which continues as a broad hump, indicates a decrease in the crystallinity of the interlayer distance of the xerogel29. As shown in the SEM images, the 3D entangled network which is the result of the interconnection of thin fibers in the interlayer causes to amorph peak.
The solid-like viscoelastic behavior of the gel
Having no regular stereochemical configuration rather than perfect regular spheres leads to specific rheological properties as reported in the literature21,40. Therefore, rheological properties were carried out on the gel in the n-dodecane solvent. The storage modulus G', related to the energy storage, and the loss modulus G", associated with the loss of energy, were measured as functions of stress amplitude at room temperature. Firstly, stress sweep measurements at a constant angular frequency of ω = 10 rad/s and varied shear stress τ were performed to determine the linear viscoelastic range of the organogel. As the figure shows, both G' and G" values for gel are nearly constant until a critical stress value called dynamic yield stress. After this stress, an abrupt decrease in both G' and G" occurred that represents the destruction of the gel network structure. Figure 7 also shows that the storage modulus G' is greater than the loss modulus G" below the critical stress value. This demonstrates that the samples under study mainly exhibit elastic behavior41.
To detect the tolerance performance of the gel against external forces, a frequency sweep test was conducted18. A frequency sweep was conducted in a 0.1–500 rad s−1 angle frequency range at a definite strain of 1%. The results are shown in (Fig. 7). The storage modulus G' was greater than loss modulus G'' in the entire frequency range. This observation is another confirmation that the networks of the gel show solid-like viscoelastic properties. Moreover, there is almost weak dependence on frequency, specifically, within a range of frequencies from ~ 0.1 to ~ 100 rad/s. This behavior suggests that samples have powerful physical junctions in the frequency range. Therefore, this leads to showing good endurance against external forces.
The role of the concentration of the gelator in the rheological properties was studied. For this purpose, the behavior of the G' value for different concentrations of 1 and 2% (w/v) of dicholesteryl gelator 5a versus the angular frequency was investigated (Figure S24). The used solvent was n-dodecane. It can be understood that the G' value for the gel with a 2% (w/v) concentration is higher than that of a 1% (w/v). This observation states that the gel with a 2% (w/v) concentration of the gelator is elastically stronger. This influence of the concentration of the gelator on the stability of the gel network is reported in the literature18,42. Furthermore, the storage modulus of the gel of 1% (w/v) gelator concentration shows more dependency on the frequency change, rather than that of 2% (w/v), which is nearly constant in the frequency range shown.
Poor wetting
Surface wettability is a critical property in the context of oil/water separation processes43. In this regard, the surfaces of oil absorbents utilized for oil/water separation need to be lipophilic, meaning they should attract oil while repelling water, making them hydrophobic. Soft materials like gels exhibit varying surface wettabilities due to their diverse nanostructures35. To investigate this, a gel composed of dicholesteryl gelator 5a in n-dodecane was applied onto glass and dried under laboratory conditions. Subsequently, the pendant drop contact angle method was employed to assess the wettability of the dried gel surface. The contact angle of a water droplet on a flat surface was found to be 118°, as illustrated in (Fig. 8). In accordance with the literature, a contact angle between 90 and 150° indicates a hydrophobic surface. Therefore, our xerogel surface can be classified as hydrophobic.
The superiority and efficiency of the dicholesteryl gelator 5a compared to the other reported cholesteryl-based gelators
A notable aspect of the proposed gelators is that they are synthesized through a one-pot multi-component reaction, without the need for temperature or catalyst, with high efficiency and purified through simply purification. Since complex and multi-step synthesis methods for producing gelators consume various resources such as time, cost, and energy, providing simple and high-yielding synthesis methods for these materials is crucial. By developing efficient synthesis methods, the performance and efficiency of these gelators in oil–water separation processes can be improved. This research contributes to the development of green and sustainable technologies, addressing environmental issues caused by oil pollution in water and soil. Comparing the gelling performance of the dicholesteryl gelator with other cholesteryl-based gelators in existing literature (Table 4), our gelator stands out for its ability to form a gel at room temperature without requiring a heating–cooling cycle or co-solvent. Its minimum gelation concentration (MGC) value is lower than several counterparts. The minimum gelation concentration (MGC) value of our gelator is lower than several counterparts. Scanning electron microscopy (SEM) images show the presence of nanofibers and intricate networks at the nanoscale, distinguishing our gelator from others that form gel networks at the microscale. In conclusion, the novel dicholesteryl-based gelator proposed in this research offers competitive advantages over other cholesteryl gelators. Its straightforward synthesis method and favorable properties for establishing stable gels make it a promising candidate for various applications.
Conclusions
In conclusion, to solve the environmental issues posed by oil spill, new and efficient cholesteryl-based low-molecular-weight organogelators have been designed and synthesized. A simple and one-step synthesis of these cholesteryl-based derivatives by multi-component reaction without any catalyst and harsh conditions, suggest the green and applicable process for synthesizing absorbent practical and cost-effective real-scale usage. The capacity of these gelators to self-assemble in organic solvents with only 1% (w/v) of these derivatives in powder form, without the need for heating and cooling cycles and the addition of any co-solvent, makes these derivatives an excellent choice for oil spill remediation in real-scale applications. These properties render this gelator superior to other gelators reported in the literature. The intermolecular interactions between organogelator and organic solvent in the self-assembly process leading to stable organogel are confirmed by FT-IR spectroscopy. The solid-like viscoelastic behavior and good thermo-reversibility properties of the synthesized gel confirmed by rheological studies and DSC measurements, respectively. These properties lead to easy separation of crude oil from the water surface and after separation, oil recovery and gelator reuse can be achieved. To the best of our knowledge, these cholesterol derivatives are the first LMOGs synthesized by the multi-component reaction which exhibit the unique ability to gel leaked oil simply by pouring the powder onto the surface of the sea.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Likon, M., Remškar, M., Ducman, V. & Švegl, F. Populus seed fibers as a natural source for production of oil super absorbents. J. Environ. Manag. 114, 158–167 (2013).
Liu, H., Geng, B., Chen, Y. & Wang, H. Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain. Chem. Eng. 5, 49–66 (2017).
Sabir, S. Approach of cost-effective adsorbents for oil removal from oily water. Crit. Rev. Environ. Sci. Technol. 45, 1916–1945 (2015).
Al-Majed, A. A., Adebayo, A. R. & Hossain, M. E. A sustainable approach to controlling oil spills. J. Environ. Manag. 113, 213–227 (2012).
Hoshyarmanesh, P., Mohammadbagheri, Z. & Rahmati, A. Synthesis and application of bisurea derivatives: Effect of structural differences on the gelation properties. J. Environ. Chem. Eng. 9, 105220 (2021).
Okesola, B. O. & Smith, D. K. Applying low-molecular weight supramolecular gelators in an environmental setting–self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 45, 4226–4251 (2016).
Zhang, X. et al. Reusable solid-form phase-selective organogelators for rapid and efficient remediation of crude oil spill. Langmuir 40, 2091–2101 (2024).
Doshi, B., Sillanpää, M. & Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 135, 262–277 (2018).
Saharan, Y. et al. Novel supramolecular organo-oil gelators for fast and effective oil trapping: Mechanism and applications. J. Hazard. Mater. 442, 129977 (2023).
Deschamps, G., Caruel, H., Borredon, M. E., Bonnin, C. & Vignoles, C. Oil removal from water by selective sorption on hydrophobic cotton fibers. 1. Study of sorption properties and comparison with other cotton fiber-based sorbents. Environ. Sci. Technol. 37, 1013–1015 (2003).
Lin, Y. C. & Weiss, R. G. Liquid-crystalline solvents as mechanistic probes. 24. A novel gelator of organic liquids and the properties of its gels. Macromolecules 20, 414–417 (1987).
Zhao, Y. L., Aprahamian, I., Trabolsi, A., Erina, N. & Stoddart, J. F. Organogel formation by a cholesterol-stoppered bistable [2] rotaxane and its dumbbell precursor. J. Am. Chem. Soc. 130, 6348–6350 (2008).
Ajayaghosh, A., Vijayakumar, C., Varghese, R. & George, S. J. Cholesterol-aided supramolecular control over chromophore packing: Twisted and coiled helices with distinct optical, chiroptical, and morphological features. Angew. Chem. Int. Ed. 45, 456–460 (2006).
Xue, M., Gao, D., Chen, X., Liu, K. & Fang, Y. New dimeric cholesteryl-based A (LS) 2 gelators with remarkable gelling abilities: Organogel formation at room temperature. J. Coll. Interface Sci. 361, 556–564 (2011).
Yang, H. K., Wang, X. M., Liu, L. L. & Shi, H. X. Design and gelation behaviors of cholesterol-based derivatives as organogelators: An investigation of the correlation between molecular structures and gelation behaviors. New J. Chem. 43, 3366–3373 (2019).
Peng, J. et al. New dicholesteryl-based gelators: Gelling ability and selective gelation of organic solvents from their mixtures with water at room temperature. New J. Chem. 32, 2218–2224 (2008).
Abuoudah, M., Giwa, A., Nashef, I., AlMarzooqi, F. & Taher, H. Bio-based herding and gelling agents from cholesterol powders and suspensions in organic liquids for effective oil spill clean-up. Chem. Eng. J. Adv. 12, 100357 (2022).
Peng, J. et al. New dicholesteryl-based gelators: Chirality and spacer length effect. Langmuir 24, 2992–3000 (2008).
Shimasaki, T. et al. Synthesis, structure and properties of cholesterol-based A (LS) 2-and A (LS) 3-type gelators without hydrogen bond linkers. Tetrahedron 72, 1517–1523 (2016).
Panja, A., Raza, R. & Ghosh, K. Cholesterol-coupled diazine-phenol gelator: Cyanide sensing, phase-selective gelation in oil spill recovery and dye adsorption. ChemistrySelect 5, 11874–11881 (2020).
Chen, X., Liu, K., He, P., Zhang, H. & Fang, Y. Preparation of novel W/O gel-emulsions and their application in the preparation of low-density materials. Langmuir 28, 9275–9281 (2012).
Lewis, R. A. Hawley’s Condensed Chemical Dictionary (John Wiley & Sons, 2016).
Googol, F. & Rahmati, A. Synthesis of a new series of multifunctional dialkyl 2-(1-(alkylamino)-1, 3-dioxo-3-phenylpropan-2-yl) malonates as low molecular weight supramolecular organogelators using five-component reaction. Tetrahedron 74, 240–252 (2018).
Hou, X. et al. Novel dimeric cholesteryl derivatives and their smart thixotropic gels. Langmuir 27, 12156–12163 (2011).
Žinic, M., Vögtle, F. & Fages, F. Cholesterol-based gelators. In Low Molecular Mass Gelator (eds Žinic, M. et al.) (Springer, 2005).
McNeice, P., Zhao, Y., Wang, J., Donnelly, G. F. & Marr, P. C. Low molecular weight gelators (LMWGs) for ionic liquids: the role of hydrogen bonding and sterics in the formation of stable low molecular weight ionic liquid gels. Green Chem. 19, 4690–4697 (2017).
Placin, F., Desvergne, J.-P. & Lassegues, J.-C. Organogel electrolytes based on a low molecular weight gelator: 2, 3-bis (n-decyloxy) anthracene. Chem. Mater. 13, 117–121 (2000).
Nishinari, K. Rheological and DSC study of sol-gel transition in aqueous dispersions of industrially important polymers and colloids. Coll. Polym. Sci. 275, 1093–1107 (1997).
Sravan, B., Kamalakar, K., Karuna, M. & Palanisamy, A. Studies on organogelation of self assembling bis urea type low molecular weight molecules. J. Solgel Sci. Technol. 71, 372–379 (2014).
Xu, J., Chen, S., Tang, G. & Wang, X. The synthesis of amide dendritic gelators and its self-assembly behavior in MMA. J. Macromol. Sci. A 48, 896–903 (2011).
Schwaller, D., Christ, E., Legros, M., Collin, D. & Mésini, P. J. Investigation of an organogel by micro-differential scanning calorimetry: Quantitative relationship between the shapes of the thermograms and the phase diagram. Gels 7, 93 (2021).
Toro-Vazquez, J. & Pérez-Martínez, J. Molecular Gels: Structure and dynamics (The Royal Society of Chemistry, 2018).
Lai, L. & Chao, S. A DSC study on the gel−sol transition of a starch and hsian-tsao leaf gum mixed system. J. Agric. Food Chem. 48, 3267–3274 (2000).
Tomšič, M., Prossnigg, F. & Glatter, O. A thermoreversible double gel: Characterization of a methylcellulose and κ-carrageenan mixed system in water by SAXS, DSC and rheology. J. Coll. Interface Sci. 322, 41–50 (2008).
Cao, X. et al. Regulation gel formation, hierarchical structures and surface wettability via isomeride effect in supramolecular organogel system. J. Coll. Interface Sci. 494, 170–177 (2017).
Yamada, N., Imai, T. & Koyama, E. Lyotropic aggregate of tripeptide derivatives within organic solvents: Relationship between interpeptide hydrogen bonding and packing arrangements of components. Langmuir 17, 961–963 (2001).
Slaný, M., Jankovič, Ľ & Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci 176, 11–20 (2019).
Širc, J. et al. Morphological characterization of nanofibers: Methods and application in practice. J. Nanomater. https://doi.org/10.1155/2012/327369 (2012).
Niece, K. L., Hartgerink, J. D., Donners, J. J. & Stupp, S. I. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J. Am. Chem. Soc. 125, 7146–7147 (2003).
Peng, J. et al. Water-in-oil gel emulsions from a cholesterol derivative: Structure and unusual properties. J. Coll. Interface Sci. 336, 780–785 (2009).
George, M., Funkhouser, G. P., Terech, P. & Weiss, R. G. Organogels with Fe (III) complexes of phosphorus-containing amphiphiles as two-component isothermal gelators. Langmuir 22, 7885–7893 (2006).
Laupheimer, M., Preisig, N. & Stubenrauch, C. The molecular organogel n-decane/12-hydroxyoctadecanoic acid: Sol–gel transition, rheology, and microstructure. Coll. Surf. A Physicochem. Eng. Asp. 469, 315–325 (2015).
Dang, Z., Liu, L., Li, Y., Xiang, Y. & Guo, G. In situ and ex situ pH-responsive coatings with switchable wettability for controllable oil/water separation. ACS Appl. Mater. Interfaces 8, 31281–31288 (2016).
Lu, L., Cocker, T. M., Bachman, R. E. & Weiss, R. G. Gelation of organic liquids by some 5α-cholestan-3β-yl N-(2-aryl) carbamates and 3β-cholesteryl 4-(2-anthrylamino) butanoates. How important are H-bonding interactions in the gel and neat assemblies of aza aromatic-linker-steroid gelators?. Langmuir 16, 20–34 (2000).
Xue, M., Gao, D., Liu, K., Peng, J. & Fang, Y. Cholesteryl derivatives as phase-selective gelators at room temperature. Tetrahedron 65, 3369–3377 (2009).
Yu, X. et al. Effect of water on the supramolecular assembly and functionality of a naphthalimide derivative: Tunable honeycomb structure with mechanochromic properties. J. Mater. Chem. C 5, 5910–5916 (2017).
Acknowledgements
We gratefully acknowledge financial support from the Research Council of the University of Isfahan.
Author information
Authors and Affiliations
Contributions
Faride Googol: Investigating the research and performing the experiments, writing original draft, writing review & editing, visualization, software, formal analysis Abbas Rahmati: Supervision, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Googol, F., Rahmati, A. Synthesis of dicholesteryl organogelator as a green sorbent nanomaterial for oil spill remediation. Sci Rep 14, 21111 (2024). https://doi.org/10.1038/s41598-024-72077-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72077-9
- Springer Nature Limited