Abstract
Silicate has been proven to be highly-effective at immobilizing soil heavy metals, but the effects of silicate stabilizers on rice grain cadmium (Cd) reduction and rice quality under field conditions are not clear. In this study, a field experiment was conducted over three consecutive years was conducted to examine the Cd reduction in rice grains and to reveal the potential effects of silicate stabilizers on rice grain nutrients, by setting different amounts of bentonite (B), silica‒calcium fertilizer (SC) and zeolite powder (ZP). The results revealed that the application of the B, SC and ZP significantly decreased the soil CaCl2‒Cd concentration (> 39%) and significantly reduced the grain Cd concentration in both early rice (> 70%) and late rice (> 18%) under field conditions; the silicate stabilizers reduced the soil available iron (Fe) but did not limit rice grain Fe nutrition. Additionally, the three silicates promoted rice yield and improved the rice grain Ca and Mg contents; and the application of B increased the amylose concentration of the late rice grains. In conclusion, high amounts of silicate stabilizers did not adversely influence the soil conventional nutrient indices, rice minerals or rice taste, but changes in rice selenium content need attention. Overall, in comparison with lime, silicate stabilizers can improve not only the safety of rice but also the nutritional and taste qualities of rice and are more eco-friendly for long-term use in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
In recent years, the in situ immobilization technique for heavy metals has been widely used to reduce the risk of heavy metal pollution in farmlands by decreasing the mobility and bioavailability of heavy metal ions in soil via the application of highly efficient stabilizers1,2,3. Inorganic salt-based soil heavy metal immobilization materials are the most widely used soil conditioning agents for heavy metal pollution control in acidic soils in southern China; the more common heavy metal immobilization materials are carbonate, phosphate and silicate materials4, owing to the advantages of a wide source of raw materials, low cost, high mechanical stability, and quick effects on the immobilization of heavy metals in the soil5,6. Additionally, the application of silicate stabilizers can effectively supplement soil silicate fertilizer, which is commonly deficient in Chinese cropland soils7,8. Therefore, silicate stabilizers have high potential for the in situ immobilization remediation of extensive low–medium cadmium (Cd)-contaminated paddy soils in China9,10.
Calcium silicate, bentonite and zeolite are three commonly used silicate stabilizers9,10,11. The main methods of immobilizing heavy metals in soil are (i) to increase the pH of acidic soil to reduce the mobility and availability of heavy metal cations in soil5,6, (ii) to adsorb heavy metal cations through the surface area and ion exchange of silicate minerals12,13, and (iii) to form complex precipitates with heavy metal ions by grafting organic functional groups to the clay minerals of silicates14,15. However, most reports related to the silicate immobilization of soil heavy metals have been based on pot or laboratory simulation experiments rather than field conditions5,6,7,8,9,10,11, which often seem to be not robust enough to guide agricultural practices. In addition, due to the differences in application amount, crop type and soil type, great variations were found in the effects of the silicate stabilizers on the reduction in soil available Cd and plant Cd16.
In the past, the long-term use of lime achieved great success in the preventing and controlling heavy metal pollution in acidic soils in southern China17; however, long time application of lime to soil not only significantly raises soil pH18, but also substantially increases soil Ca2+ and Mg2+19,20, and these changes resulted in the loss of exchangeable potassium21 and soil organic matter18 in the soil, and secondly reduced the mobility of phosphorus and some microelements in the soil (such as Cu and Zn)22,23,24, additionally, the utilization of liming materials caused the rapid accumulation of NO3− in soil, posing a threat to agricultural surface pollution25. Therefore, as an alkaline inorganic salt-based soil heavy metal stabilizer, whether the long-term application of silicate stabilizers in soil deteriorates the soil quality and affects the nutrient uptake of rice needs to be determined under field conditions.
Herein, different amounts of bentonite, silica–calcium fertilizer and zeolite powder were applied in a typical Cd-contaminated paddy field in southeast China for 3 years to investigate the effects of the silicate stabilizers on the rice Cd reduction in the field, especially to reveal the potential adverse effects of long-term silicate application on soil quality and rice quality through high-amount treatment, and finally, provide a scientific reference for the long-term safe application of silicate stabilizers in Cd-contaminated rice fields in southern China.
Materials and methods
Study area and experimental materials
The field experiment was conducted in Yushui District, Xinyu City, Jiangxi Province, China (27.77458079° N, 114.97818202° E), a typical double-cropping rice growing area. The local popular early rice variety Zhuliangyou 2013 and the late rice variety Yongyou 1538 were selected for field experiments. The field in the study area has been contaminated by wastewater irrigation and atmospheric deposition from an iron and steel plant since the 1990s26. The area of Cd contamination was over 120 ha, with Cd levels ranging from 0.4 to 0.9 mg kg−1, exceeding the risk screening value (0.3 mg kg−1, pH ≤ 5.5) of soil contamination on agricultural land (GB 15618-2018)27. Prior to the field experiment, a total of 20 surface samples were collected at different locations in the experimental field for background soil investigation via the checkerboard method. Statistical analysis revealed that the soil pH was 5.15 ± 0.19, the organic matter content was 31.24 ± 4.98 g kg−1, and the total Cd content was 0.52 ± 0.05 mg kg−1 in the test fields. In the study area, the commonly used local tiller was selected for tilling, and the general tilling depth was 12 cm. Before tilling, the basal fertilizer was applied with compound fertilizer (N:P2O5:K2O = 17%:17%:17%) at a dosage of 675 kg ha−1, and the same type of compound fertilizer was applied retroactively during the period of pregnancy and spiked at a dosage of 150 kg ha−1.
The chemical composition, partial properties and manufacturers of bentonite (B), silica–calcium fertilizer (SC) and zeolite powder (ZP) are shown in Table 1.
Experimental design
The three silicate stabilizers (B, SC and ZP) were each set with 5 gradients CK(0.0%), T1(0.1%), T2(0.2%), T3(0.5%) and T4(1.0%) according to the mass fraction of the topsoil. Three parallel plots were set up for each silicate treatment, and the experimental plots for each treatment were randomly separated, each with an area of 6 m × 5 m, for a total of 39 test plots. Before the plots were divided, the test plots were tilled several times to reduce heterogeneity among the plots. The plots were then separated by separate field ridges, which were covered with polyethylene film to isolate the influences between different plots. The cropping system and field management practices were the same among the treatment plots. The field application amounts of each gradient were 0.00, 1.56, 3.12, 7.80 and 15.60 t ha−1, which were calculated according to a soil ploughed-layer depth of 12 cm and a soil bulk weight of 1.30 g cm−3. According to the design of each experimental plot, B, SC and ZP were applied for three consecutive years before early rice planting, i.e., in March 2020, 2021 and 2022.
Sampling and determination
Topsoil (0–12 cm) and rice samples were collected postharvest from early and late rice in 2022. Soil samples from each plot were collected via a soil drill; specifically, one parallel sample was taken from each of the four corners (1 m from the ridge) and the centre of each field plot and was uniformly mixed as the sample for that treatment plot. Rice yields were collected and counted by an onsite thresher. Rice samples were collected from the rice grains harvested in each plot. All the soil samples were air-dried and crushed, after which visible roots were removed, and then the soil samples were passed through a 2 mm sieve and stored for subsequent analyses. The rice samples were dried, husked and powdered and then stored for analysis.
The determination of the soil physicochemical properties followed the methods recommended by Lu28. The total SOC of each sample was determined via the oxidation method of potassium dichromate combined with concentrated sulfuric acid; the soil pH was determined electrometrically via a pH electrode; the soil available nitrogen (AN) content was determined via the alkali-hydrolysed diffusion method; the soil available phosphorus (AP) content was measured via NH4F-HCl extraction combined with the phosphomolybdate blue spectrophotometry method (UV/VIS-4802, UNICO, Shanghai, China); the soil available potassium (AK) content was determined via the NH4OAc extraction method combined with the flame photometric method (FP6431, Shanghai, China); the ammonium acetate exchange method combined with EDTA complexometric titration was used to determine the soil exchangeable calcium (exch-Ca) and exchangeable magnesium (exch-Mg); the soil available iron (AFe) content was determined via DTPA extraction combined with the atomic absorption spectrophotometry method (AA-3300, Shanghai, China), and the soil available silicon (ASi) content was measured via citric acid extraction combined with the spectrophotometry method. The Cd concentration in the soil exchangeable fraction (CaCl2–Cd) was extracted via 0.01 M CaCl2 and analysed via inductively coupled plasma‒mass spectrometry (ICP-MS, X2, Thermo Fisher, USA).
The concentrations of mineral elements in rice (Cd, Ca, Mg, Fe, Cu, Mn and Zn) were determined via an inductively coupled plasma‒mass spectrometry method (ICP-MS, X2, Thermo Fisher, USA) with HNO3–HClO3 digestion as a pretreatment. The Se content in rice was quantified via atomic fluorescence spectrometry (AFS-6801, Shanghai, China) after digestion with HNO3–HCl. The amylose content of the rice was determined via acetic acid‒iodine chromogenic spectrophotometry (UV/VIS-4802, UNICO, Shanghai, China) after boiling with boiling water, ethanol, and sodium hydroxide. Rice crude protein was determined via the Kjeldahl method with an automatic Kjeldahl azotometer (K9860, Hanon, Shandong Province, China).
The method of parallel double-sample determination was adopted during the whole testing process for quality control, i.e., a parallel double assay was performed for every 20 samples, and the relative standard deviation of each element determination result in the laboratory should be < 35%. To determine the total content of elements in the soil, two kinds of standard substances (GBW07405 and GBW07407) were adopted to perform the recovery test for each batch of samples, and GBW10010 was adopted to perform the recovery test to control the accuracy of the test for each batch of plant samples. The recoveries of these standard substances were within the permissible error range of 85–115%.
Data processing and analysis
The Shapiro‒Wilk normality test was performed for each group of samples. One‐way ANOVA (LSD–Tamhane T2) was used to analyse the differences in the soil properties, rice yield, rice grain Cd content, and rice mineral content under the different treatments. Correlation analyses (Spearman’s tests) and two-way ANOVA (Bonferroni) were used to determine the effects of the application amount and silicate type on the rice grain Cd and soil CaCl2–Cd concentrations at a significance level of p < 0.05. All the statistical analyses were performed via the SPSS 26.0 package (IBM Corp., Armonk, NY, USA).
Results
Effect of the silicate stabilizer on the soil properties
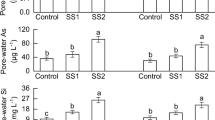
The properties of the soils treated with various silicate stabilizers were analysed after late rice harvest, and the results revealed that B, SC and ZP significantly reduced the concentrations of CaCl2-extractable Cd in the surface paddy soil, compared with those in the CK (p < 0.05), and the corresponding decreases were 56.06–62.07%, 46.35–85.46% and 39.08–46.40%, respectively (Fig. 1). However, the reduction in the CaCl2-extractable Cd in the soil did not increase with increasing application amount.
CaCl2–Cd concentrations in late rice paddy soil under different application amounts of silicate stabilizers. B bentonite, SC silica‒calcium fertilizer, ZP zeolite powder, CK T1, T2, T3 and T4 represent silicate application amounts of 0.0%, 0.1%, 0.2%, 0.5% and 1.0%, respectively, CaCl2–Cd CaCl2-extractable Cd in soil. The means of the columns subjected to different treatments with the same silicate stabilizer followed by different letters are significantly different at p < 0.05. The error bar represents the standard deviation of the mean.
The soil pH under different treatments of the B, SC and ZP were increased by 0.52–1.87, 0.40–1.61 and 0.53–1.69, respectively (Fig. 2a), while no significant changes were found in the concentrations of SOC, AN, AP and AK (p > 0.05), compared to the CK (Fig. 2b–e); the application of the B and SC significantly increased the concentrations of soil exch-Ca by 57.38–76.88% and 76.88–132.59% (p < 0.05), respectively, with different application amounts (Fig. 2f); the high amount treatments of B (T4) and SC (T3 and T4) significantly promoted the concentrations of soil exch-Mg (p < 0.05) (Fig. 2g); the soil ASi concentrations under the application of the B, SC and ZP were increased by 13.07–55.22%, 27.45–74.68%, and 17.02–40.98%, respectively, with the increase of the application amount (Fig. 2i); however, the AFe concentrations in the surface paddy soil were reduced by 21.39–27.35% (B), 11.30–25.27% (SC) and 3.54–13.46% (ZP), respectively, and significant variations in the concentrations of the soil AFe were observed under all treatments of the B and the T4 treatment of the SC, in comparison with the CK (p < 0.05) (Fig. 2h).
Soil properties in late rice paddy fields under different application amounts of silicate stabilizers. B bentonite, SC silica‒calcium fertilizer, ZP zeolite powder, CK T1, T2, T3 and T4 represent silicate application amounts of 0.0%, 0.1%, 0.2%, 0.5% and 1.0%, respectively, SOC soil organic carbon; AN soil available nitrogen, AP soil available phosphorus, AK soil available potassium, Exch-Ca soil exchangeable Ca2+, Exch-Mg soil exchangeable Mg2+, AFe soil available iron; ASi soil available silicon. Means in the columns under different dose treatments from the same silicate stabilizer followed by different letters were significantly different at p < 0.05. The error bar represents the standard deviation of the mean.
Effects of silicate stabilizers on rice yield and grain Cd content
With increasing of application amount, both the 1000-grain weight and rice yield first increased but then decreased. The 1000-grain weight and rice yield under the B, SC and ZP treatments were greater than those under the CK treatment, in which the increases in the 1000-grain weight and rice yield under the T2 treatment of the three silicates were greatest (Fig. 3).
Effects of silicate stabilizers on the 100-grain weight and yield of rice. B bentonite, SC silica‒calcium fertilizer, ZP zeolite powder, CK T1, T2, T3 and T4 represent silicate application amounts of 0.0%, 0.1%, 0.2%, 0.5% and 1.0%, respectively. Means in the columns under different dose treatments from the same silicate stabilizer followed by different letters were significantly different at p < 0.05. The error bar represents the standard deviation of the mean.
Compared with the CK, the application of B, SC and ZP significantly reduced the grain Cd concentrations in early rice (p < 0.05), with decreases of 73.85–85.37%, 73.68–84.69% and 70.60–82.38%, respectively, with different application amounts (Fig. 4a–c), and the decreases in the late rice grain Cd were 32.89–69.81%, 27.23–45.09% and 18.84–86.36% (p < 0.05), respectively, except for those in the T1 treatment of SC (Fig. 4d–f). However, there was no obvious dose effect of the silicate stabilizer on grain Cd reduction.
Grain Cd concentrations of early and late rice under different application amounts of silicate stabilizers. B bentonite, SC silica‒calcium fertilizer, ZP zeolite powder, CK T1, T2, T3 and T4 represent silicate application amounts of 0.0%, 0.1%, 0.2%, 0.5% and 1.0%, respectively. Means in the columns under different dose treatments from the same silicate stabilizer followed by different letters were significantly different at p < 0.05. The error bar represents the standard deviation of the mean.
Effects of silicate stabilizers on rice quality
With different application amounts, B, SC and ZP increased the grain Ca concentrations of early rice by 7.04–89.04%, 3.58–52.68%, and 24.35–47.80%, respectively, and increased the grain Mg concentrations of early rice by 14.53–53.86%, 11.49–77.01%, and 0.59–53.04%, respectively, in comparison with those of the CK (Fig. 5a,b). In total, no significant variations were observed in the early rice grain Fe contents of the three silicates. The grain Se concentrations in early rice under the different treatments fluctuated (Fig. 5g). Additionally, no significant changes were detected in the concentrations of Cu, Mn, Zn, amylose or crude protein in the early rice grains under the three silicate treatments compared with those under the CK treatment (p > 0.05) (Fig. 5d–f,h,i).
Effects of silicate stabilizers on the nutrients of early rice grains. B bentonite, SC silica‒calcium fertilizer, ZP zeolite powder; CK T1, T2, T3 and T4 represent silicate application amounts of 0.0%, 0.1%, 0.2%, 0.5% and 1.0%, respectively, Ca calcium concentration, Mg magnesium concentration, Fe iron concentration, Cu copper concentration, Mn manganese concentration, Zn zinc concentration, Se selenium concentration. Means in the columns under different dose treatments from the same silicate stabilizer followed by different letters were significantly different at p < 0.05. The error bar represents the standard deviation of the mean.
There were no significant changes in the grain Ca concentrations of late rice under the silicate treatments (Fig. 6a), whereas there were several treatments significantly increased the grain Mg concentrations of the late rice, including B(T2), SC(T2 and T3) and ZP(T2, T3 and T4), compared with those under the CK treatment (Fig. 6b); the concentrations of the grain Fe, Cu, Mn, Zn and crude protein of the late rice treated with the silicates were not significantly different from those under the CK treatment (Fig. 6c–f,i); the grain Se concentrations in late rice under different treatments also exhibited complex changes (Fig. 6g). Compared with the CK treatment, the T3 and T4 treatments (B) significantly increased the amylose content in the late rice grains, which increased by 19.39% (T3) and 22.08% (T4), respectively (Fig. 6h).
Effects of silicate stabilizers on the nutrients of late rice grains. B bentonite, SC silica‒calcium fertilizer, ZP zeolite powder, CK T1, T2, T3 and T4 represent silicate application amounts of 0.0%, 0.1%, 0.2%, 0.5% and 1.0%, respectively. Means in the columns under different dose treatments from the same silicate stabilizer followed by different letters were significantly different at p < 0.05. The error bar represents the standard deviation of the mean.
Effects of different silicate treatments on rice grain Cd and soil CaCl2–Cd contents
Spearman’s correlation analysis (Table 2) revealed that the silicate application amount was significantly negatively correlated with the Cdearly, Cdlate and CaCl2–Cdsoil. The silicate type was significantly positively correlated with only Cdearly, but was poorly correlated with Cdlate and CaCl2–Cdsoil.
All three factors (application amount, silicate type and their interaction) had significant main effects on the Cdearly, Cdlate and CaCl2–Cdsoil. The spatial η2 value suggested that the amount had a greater main effect on the Cdearly and Cdlate than did the silicate type, and the interaction between the amount and the silicate had a greater main effect on the Cdearly than the Cdlate through comparing the patial η2 value (Table 3).
Above all, the application amount of the silicate stabilizer made a greater contribution to the reduction in the number of rice grains and the amount of available Cd in the soil than did the type of silicate stabilizer. There was a larger effect of the amount × silicate on the Cdearly than the Cdlate.
Discussion
Effects of silicate stabilizers on soil quality
Soil acidification has been a major obstacle to improving soil quality and reducing heavy metal safety risk in croplands in southern China23,29. As with lime30, silicate stabilizer applied in large quantities to soil can rapidly raise soil pH and significantly reduce the soil CaCl2–Cd (Figs. 1, 3), whereas the difference21,25 was that the silicate stabilizer did not lead to a decrease in soil exchange K or an enrichment of NO3− in the surface soil with the increase of Ca2+ and Mg2+ in the soil (Fig. 2). In addition, the SOC and the AP likewise did not change significantly with the silicates application (Fig. 2). In contrast, the application of silicate stabilizers significantly increased soil available Si (Fig. 2). On the other hand, the passivation effect of lime on soil Cd was maintained for a short period of time, due to its single immobilization mechanism, and long-term application of lime tends to lead to soil compaction and nutrient loss19,20,31. In contrast, the silicate stabilizer had a more diverse Cd passivation mechanism, and the passivation effect was more stable. In addition, the reaction between the silicate stabilizer and the soil was gentler. The present study did not find that the silicate stabilizer had a negative impact on the soil fertility and that it could increase the amount of soil silica and promote crop growth32. Therefore, as long term stabilizers for heavy metals in soil, silicate materials are more eco-friendly than liming materials are.
Effects of silicate stabilizers on the soil CaCl2–Cd and rice Cd contents
Generally, silicate stabilizers fix Cd2+ in the soil in two main ways32,33: the first is to produce a large amount of OH− and SiO3− within the soil, which results in precipitation with soil Cd2+; the second is to utilize the loose and porous surface area of silicate stabilizers to facilitate their adsorption and fixation with Cd2+. As a result, the application of B, SC and ZP significantly decreased the soil CaCl2–Cd concentration and hence the grain Cd concentration of both early and late rice compared with that of the CK, in which the concentration of the rice grain Cd under all the silicate stabilizer treatments was lower than the limit value (0.20 mg kg−1) of rice grain Cd in China’s national food safety standard (GB2762-2022). Additionally, the grain Cd reduction in the early rice was obviously greater than that in the late rice under silicate treatments. This finding could be related to the results of the statistical analysis that grain Cd reduction in early rice was more sensitive to silicate type and it was more strongly affected by the amount × silicate than that in the late rice.
Effects of silicate stabilizers on rice yield and quality
Silicon is one of the most important nutrients required by rice, and its role is second only to that of soil N, P and K34. In general, both increases in soil pH and soil silicon fertilizer can not only promote the root growth of rice35, but also increase the photosynthesis of rice, resulting in an increase in one or more yield components, such as the effective panicle number, seed setting rate and 1000-grain weight36. Here, the silicate stabilizer increased the soil ASi and soil pH, which may be the main factors responsible for the increase in the 1000-grain weight and yield of rice. However, the excessive application of silicate stabilizers did not linearly increase rice yield, which was related to the soil available silicon grade as well as the limited plant demand for silicon37.
Compared with the CK, the application of silicate stabilizer did not affect the concentrations of Fe, Cu, Mn or Zn in either the early or late rice grains in the study area, whereas the medium silicate stabilizer increased the Ca and Mg contents of the early rice grains and the Mg contents of the late rice grains, which was likely caused by the effects of silicate stabilizers on the soil available Ca and Mg contents. Amylose and crude protein contents are closely related to the taste of the rice38. In this study, except for the high B treatment, which increased the concentration of late rice amylose, the other treatments did not affect the flavour quality of the rice. However, the effects of silicon fertilizer application on rice amylose and crude protein contents vary among different reports because the influence of silicon on the content of rice amylose and crude protein varies with rice variety and application period38. It should be noted that the complex changes of rice grain Se under the silicate treatments (Figs. 5g, 6g), which of transfer from the soil to the rice grains was affected by the interaction of multiple soil properties, and current reports related to the effects of different soil properties on the soil Se bioavailability were controversial39,40, need to be further research.
Thus, compared with liming materials, silicate stabilizers not only increase the yield of both crop rice and the nutritional quality of rice.
Conclusions
The application of B, SC and ZP significantly decreased the soil CaCl2–Cd content by > 39% and significantly reduced the grain Cd content in both the early (> 70%) and late rice (> 18%) stages under field conditions, indicating the excellent ability of rice to reduce Cd. The application of high amounts of silicate stabilizers did not affect the soil conventional nutrient indexes. The three silicate stabilizers could increase the uptake of Ca and Mg in rice grains by improving soil available Ca and Mg, and B could increase the amylose content in the late rice grains. In addition, the application of high amounts of silicate stabilizers did not significantly affect the rice mineral nutrients or rice taste, but changes in rice Se content need to be emphasized. Taken together, compared to the liming materials, silicate stabilizers are not only beneficial to rice yield and rice quality, but also environmentally friendly.
Data availability
All data generated or analysed during this study are included in this published article.
References
Liu, K. et al. Sustainability assessment and carbon budget of chemical stabilization based multi-objective remediation of Cd contaminated paddy field. Sci. Total Environ. 819, 152022 (2022).
Ou, J. et al. In situ immobilisation of toxic metals in soil using Maifan stone and illite/smectite clay. Sci. Rep. 8, 4618 (2018).
Irfan, M. et al. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 11, 18416 (2021).
Liang, Y., Wang, X. C. & Cao, X. D. Immobilization of heavy metals in contaminated soils with phosphate-, carbonate-, and silicate-based amendments: A review. Environ. Chem. 31(1), 16–25 (2012).
Ma, J. et al. Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 182, 776–782 (2018).
Yuan, X. et al. Immobilization of heavy metals in two contaminated soils using a modified magnesium silicate stabilizer. Environ. Sci. Pollut. Res. 25, 32562–32571 (2018).
Zhang, C. C., Wang, L. J., Nie, Q., Zhang, W. X. & Zhang, F. S. Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environ. Exp. Bot. 62(3), 300–307 (2008).
Chen, Z. J. et al. Alleviating effects of silicate, selenium, and microorganism fertilization on lead toxicity in ginger (Zingiber officinale Roscoe). Plant Physiol. Biochem. 145, 153–163 (2019).
Ning, D., Liang, Y., Song, A., Duan, A. & Liu, Z. In situ stabilization of heavy metals in multiple-metal contaminated paddy soil using different steel slag-based silicon fertilizer. Environ. Sci. Pollut. Res. Int. 23(23), 23638–23647 (2016).
Wang, H. Y. et al. Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ. Sci. Pollut. Res. 23, 3781–3788 (2016).
Hamid, Y. et al. Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci. Rep. 8, 17839 (2018).
Wu, J. W., Shi, Y., Zhu, Y. X., Wang, Y. C. & Gong, H. J. Mechanisms of enhanced heavy metal tolerance in plants by silicon: A review. Pedosphere 23(6), 815–825 (2013).
Zhang, G. Y., Lin, Y. Q. & Wang, M. K. Remediation of copper polluted red soils with clay materials. J. Environ. Sci. 23(3), 461–467 (2011).
Shi, W. Y., Shao, H. B., Li, H., Shao, M. A. & Du, S. Co-remediation of the lead-polluted garden soil by exogenous natural zeolite and humic acids. J. Hazard. Mater. 167, 136–140 (2009).
Ciesielczyk, F. et al. Adsorption of Ni(II) from model solutions using corecipitated inorganic oxides. Adsorption 19, 1–12 (2013).
Cheng, S. F. & Hseu, Z. Y. In-situ immobilization of cadmium and lead by different amendments in two contaminated soils. Water Air Soil Pollut. 140, 73–84 (2002).
Mahar, A., Wang, P., Li, R. H. & Zhang, Z. Q. Immobilization of lead and cadmium in contaminated soil using amendments: A review. Pedosphere 25(4), 555–568 (2015).
Hao, X., Cho, C., Racz, G. J. & Chang, C. Chemical retardation of phosphate diffusion in an acid soil as affected by liming. Nutr. Cycl. Agroecosyst. 64, 213–224 (2002).
Lucchini, P., Quilliam, R., DeLuca, T., Vamerali, T. & Jones, D. L. Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ. Sci. Pollut. Res. 21, 3230–3240 (2014).
Cui, J. et al. Effects of simulated Cd deposition on soil Cd availability, microbial response, and crop Cd uptake in the passivation-remediation process of Cd-contaminated purple soil. Sci. Total Environ. 683, 782–792 (2019).
Brennan, R. F., Bolland, M. D. A. & Bell, R. W. Increased risk of zinc deficiency in wheat on soils limed to correct soil acidity. Aust. J. Soil Res. 43(5), 647–657 (2005).
Zhang, Y. et al. Effect of a novel Ca–Si composite mineral on Cd bioavailability, transport and accumulation in paddy soil-rice system. J. Environ. Manag. 233, 802–811 (2019).
Suo, L. N., Ma, J., Liu, B. C., Sun, X. Y. & Chen, G. F. Soil conditioner application status and application of risk research. J. Agro. Environ. Sci. 40(6), 1141–1149 (2021).
Xiang, Z. L. et al. Study on the treatment of nickel-contaminated soil using calcium oxide. Water Air Soil Pollut. 231, 188 (2020).
Ren, Z. W., Kopittke, P. M., Zhao, F. J. & Wang, P. Nutrient accumulation and transcriptome patterns during grain development in rice. J. Exp. Bot. 74(3), 909–930 (2023).
Lin, X. B. et al. Screening of rice varieties with low cadmium accumulation based on multiple indicators. Acta Agric. Zhejiangen. 35(11), 2507–2515 (2023).
Standard of the People’s Republic of China. Soil Environmental Quality; Risk Control Standard for Soil Contamination of Agricultural Land (Standard of the People’s Republic of China, 2018).
Lu, R. K. Analytical Methods for Soil and Agro-chemistry (China Agricultural Science and Technology Press, 2000).
Zhao, X. Q. et al. Scientific issues and strategies of acid soil use in China. Acta Pedol. Sin. 60(5), 1248–1263 (2023).
Abad-Valle, P., Álvarez-Ayuso, E., Murciego, A. & Pellitero, E. Assessment of the use of sepiolite amendment to restore heavy metal polluted mine soil. Geoderma 280, 57–66 (2016).
Kuang, X. L., Si, K. Y., Song, H. J., Peng, L. & Chen, A. W. Lime-phosphorus fertilizer efficiently reduces the Cd content of rice: Physicochemical property and biological community structure in Cd-polluted paddy soil. Front. Microbiol. 12, 749946 (2021).
Kumar, A., Nayak, A. K., Pani, D. R. & Das, B. S. Application of phosphorus, iron, and silicon reduces yield loss in rice exposed to water deficit stress. Agron. J. 111(3), 1488–1497 (2019).
Xu, Y. et al. Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere 27(2), 193–204 (2017).
Wang, Y. W., Zhang, B. B., Jiang, D. X. & Chen, G. X. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 158, 117–124 (2019).
Chen, Z. et al. Mechanisms of Fe biofortification and mitigation of Cd accumulation in rice (Oryza sativa L.) grown hydroponically with Fe chelate fertilization. Chemosphere 175, 275–285 (2017).
Liu, H. J. et al. Effects of long-term fertilization on iron fraction and availability in brown soil. J. Plant Nutr. Fertil. 23(1), 36–43 (2017).
Hossain, M. T. et al. Growth promotion and an increase in cell wall extensibility by silicon in rice and some other Poaceae seedlings. J. Plant Res. 115(1117), 23–27 (2002).
Wei, X. D., Zhang, Y. D., Song, X. M. & Wang, C. L. Research progress on rice quality improvement by applying silicon-magnesium-zinc fertilizer. Jiangsu J. Agric. Sci. 37(3), 783–788 (2021).
Shao, Y. et al. Controlling factors of soil selenium distribution in a watershed in Se-enriched and longevity region of South China. Environ. Sci. Pollut. Res. 25, 20048–20056 (2018).
Wang, D. et al. Selenate redistribution during aging in different Chinese soils and the dominant influential factors. Chemosphere 182, 284–292 (2017).
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangxi Province (20232BAB213081, 20224BAB205009), the Young Talents Training Project of Jiangxi Province (20204BCJL23040), and the Key Research and Development Project of Jiangxi Academy of Sciences (2022YSBG21008, 2022YSBG22009, 2022YSBG21009).
Author information
Authors and Affiliations
Contributions
Conceptualization, F.M. and X.W.; methodology, F.M.; software, L.L.; validation, X.W., L.L. and Z.X.; formal analysis, F.M.; investigation, X.W. and F.M.; resources, X.W.; data curation, X.L.; writing—original draft preparation, F.M.; writing—review and editing, X.W. and L.L.; visualization, L.L.; supervision, X.W. and X.S.; project administration, T.Z.; funding acquisition, X.W. L.L. and H.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Min, F., Wang, X., Li, L. et al. Effects of silicate stabilizers on cadmium reduction and the quality of rice grains in acidic paddy soil. Sci Rep 14, 20551 (2024). https://doi.org/10.1038/s41598-024-71741-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71741-4
- Springer Nature Limited