Abstract
The recent investigation targets to use adapted carbon paste (CP) with copper nanoparticles (CuNs) operating in a phosphate buffer (PBS) medium with a pH range of 5.0–8.0, to synthesize a novel, susceptible, and simple electrochemical sensor for the detection of one of the most important drugs, vitamin B6. Copper (Cu) is one of the most three common essential trace elements found in the bodies of both humans and animals, along with iron and zinc for all crucial physiological and biochemical functions. Its properties, which are assessed using a variety of methods including scanning electron microscopy (SEM), cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS), have also drawn a lot of attention recently. We considered the effects of pH, buffer, scan rate, interference, and calibration curve. The susceptible electrode's linear calibration curve encompassed concentration values between 8.88 and 1000.0 µM. The calculated limits of detection and quantification were 32.12 and 107.0 µM, respectively. Furthermore, this method was established in real human urine samples and drug validation which have been shown satisfactory results for vitamin B6 detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
There are two primary categories of nutrients: macronutrients and micronutrients. The body needs high quantities of macronutrients like proteins, carbohydrates, and fats since they give the human body molecules for metabolic and structural processes. In contrast, the body requires trace levels of micronutrients, which include vitamins and minerals, for optimal operation. An individual's metabolic activities and life cycle determine the need for micronutrients. Although our body needs only a small amount of micronutrients (in mg or μg), they are considered as significant as macronutrients. They are necessary for the body to develop. Since our bodies are unable to synthesize micronutrients, they must be obtained at appropriate levels from the diet. If their supply is insufficient, this can lead to a host of diseases. Vitamins are essential for the body's normal functioning and development. Two main categories can be used to classify vitamins: fat-soluble and water-soluble. While vitamins A, D, E, and K are fat-soluble, vitamins B1, B2, B3 (niacin), B5 (pantothenic acid), B6 (pyridoxine), B7 (biotin), B9 (folate), B12 (cyanocobalamin), and C (ascorbic acid) are water-soluble. Our study will be concerned with vitamin B6 (Pyridoxine), which belongs to the B-complex group of vitamins; it is a crucial co-factor for various biochemical processes that control fundamental cellular metabolism1. Vitamin B6 is a necessary food component for a well-balanced diet, which is also relatively abundant in many foods2. It proceeds as a coenzyme in several metabolic transformations in biological systems, including amino acid metabolism, transculturation, and glycogen phosphorylation3. It acts as an antioxidant, red blood cell formation, and haemoglobin production4. It may help in treating PMS symptoms5 and nausea during pregnancy6, may boost mood by alleviating depression symptoms7,8,9,10, may be used to treat rheumatoid arthritis-related inflammation11,12,13,14, promote eye health for avoiding eye diseases15,16,17,18,19, It also lowers the risk of Alzheimer's20,21,22, heart disease23,24,25,26 and cancer risks27,28. From the up mentioned we found that Vitamin B6 deficiency is associated with microcytic anemia, electroencephalographic abnormalities, dermatitis with cheilosis (scaling on the lips and cracks at the corners of the mouth), glossitis (swollen tongue), depression, confusion, and weakened immune function, while its increase may lead to lack of muscle control or painful, skin lesions, heartburn and nausea.
Chickpeas, turkey, fish (tuna, salmon), bananas, potatoes, organ meats, whole grains, fortified cereals, and vegetables are vitamin B6-rich foods. Currently, adult males and females up to the age of fifty should consume 1.3 mg of vitamin B6 per day from meals or supplements.
The recommended daily allowance (RDA) is 1.7 mg for males and 1.5 mg for females over fifty4,29,30.
Many searches investigated various analytical techniques for vitamin B6 (Scheme1) detection, such as spectrophotometric, fluorometric, electrophoresis, and HPLC. All these methods are time-consuming and need more flexibility and portability. Because of high sensitivity, simple procedure, good accuracy and fast response, electro-catalytic methods are currently receiving a lot of interest. Utilizing vanadium (III) Schiff base complex amended GCE for vitamin B6 determination was one of the electrochemical methods for vitamin B6 detection that had been the subject of several prior research31, using a copper (II) hexacyanoferrate (III) modified CPE to measure vitamin B6 in the production of medicinal chemicals32, voltametric sensor for ZrO2 nanoparticle/ionic liquids CPE in food samples for the immediate measurement of vitamin B6 and vitamin C33. As a result, the electrochemical sensors are perfect for monitoring the incorporation of biological compounds utilizing portable devices, mainly when this study uses inexpensive carbon paste electrodes loaded with simply prepared (electrodeposition), available, stable, highly reactive, low cost and well conducting nanoparticles such as copper nanoparticles (CuNs), which made its usage in numerous applications more easier, like catalysis, cooling fluid or conductive inks34,35,36,37,38.
This work aims to create a simple, highly sensitive, and inexpensive sensor for vitamin B6 detection which has been achieved compared to our previously published work that utilized iron nanoparticles39. A novel B6 sensor was created by simple electrodeposition of CuNPs onto the CPE surface compared to the complicated multistep preparation of iron nanoparticles in order to achieve the required modified electrode. Although both of the electrodes gave comparable detection limits values but our new CuNCP sensor shown much higher response toward Vitamin B6 as will be discussed upcoming. The utility of the CuNCP sensor was examined in real human urine samples and with drugs.
Experimental
Chemicals and reagents
Pure vitamin B6 (≥ 99.0%) from Pharaonia Pharmaceuticals (Egypt) was utilized to make the stock solution. Sigma-Aldrich's copper sulphate. To prepare 0.1 M supporting electrolytes, Sigma-Aldrich supplies ammonium hydroxide, sodium phosphate dibasic, sodium phosphate monobasic, and (sulphuric, phosphoric, and hydrochloric) acids. CPE was prepared using graphite microparticles (less than 50 μm) from Merck, Germany, and paraffin oil from Aldrich in the USA. The interference studies used caffeine powder from Alpha Chemika (Mumbai, India) and ascorbic acid from Merck. The following materials were acquired from MISR-Scientific Company: starch, glucose, urea, sucrose, and uric acid. l(+)-Ascorbic acid is obtained from Alpha-Chem, an Indian company.
Apparatus
The electrochemical studies were conducted at room temperature utilizing a standard 25 mL three-electrode setup. The working electrode (WE) was either the bare electrode or the CuNCPE, while a saturated calomel electrode served as the reference electrode (RE), and a platinum rod functioned as the counter electrode (CE).
The electrochemical cell was linked to a computer-operated EC-Lab® electrochemical software and a Bio-logic SAS model SP-150 potentiostat. Different electrochemical measurements, such as CV, EIS, CA, and DPV, could be done accurately with this setup. Using a sinusoidal voltage amplitude of 10 mV, electrochemical impedance spectroscopy (EIS) was performed between 100 mHz and 100 kHz in the frequency range. The EC-Lab® program was utilized to perform the fitting and analysis of the data, employing the most optimal equivalent circuit model. Measurements were done at least three times to achieve a repeatable result.
The Adwa 1030 digital pH meter (Romania) was connected to measure the pH solution. SEM (Model Quanta 250 Field Emission Gun) was used to examine the morphology by attaching with an energy dispersive X-ray (EDX) Unit (FEI Company, Japan). Transmission electron microscopy (TEM) analysis was made using a JEM-1400 Electron Microscope (JEOL, Japan). The PAN-analytical X-Ray Diffraction equipment model X׳Pert PRO with secondary monochromator, operating at 45 kV and 35 mA with Cu-radiation (λ = 1.542 Å) and scanning speed of 0.04° s−1, was used for structural investigations. The diffraction lines located at 2θ values between 2° and 60°, the corresponding spacing (d, Å), and relative intensities (I/Io), were obtained. The diffraction charts and relative intensities are obtained and compared with ICDD files.
CuNCPE preparations
The CPE could be synthesized by first achieving a homogenous paste and manually combining 5.0 g of graphite powder and 3.0 mL of paraffin oil in a clean mortar for approximately 10 min. Then, fill a 3 mm-diameter hole at the end of a Teflon tube with this mixture. After that, CuNs were precipitated over the carbon paste electrode by immersing the electrode in the solution of 10–4 M CuSO4 and 0.25 M H2SO4 using a current density of 0.24 mA cm−1 for 15 min to achieve the CuNCPE as represented in Scheme 240,41.
Applications of sensor and preparing of real sample
Vitamin B6 level is properly measured using two samples (urine and multivitamin). First, add 0.25 mL of urine sample to 100 mL of 0.1 M PBS (pH 6.0) to create a ratio of 1:400 to eliminate any matrix effect. Then, take 22.5 mL of this mixture and add it gradually to the electrochemical cell using vitamin B6 until we reach 25 mL using the standard addition method. Second, one tablet was dissolved in 250 mL of distilled water to make the pharmacological samples. Next, 2.5 mL from the drug solution was mixed with 20 mL of 0.1 M PBS (pH 6.0) and assembled with the electrochemical cell utilizing vitamin B6 to make additions until reaching 25 mL.
Results and discussion
Characterizations of the surface
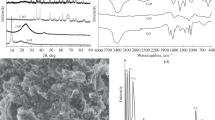
Using SEM and EDX analysis techniques, the surface morphology of the amended electrode was examined, and the result was displayed in Fig. 1. Study of Fig. 1A, represented by CP, that looks like an opaque compact surface, which agrees with the high graphite powder compatibility in the paste formation with the mineral oil. The modified paste with CuNs was represented in Fig. 1B–D using different magnifications as shown, and it seems to be an aggregate of spherical nano-sized shapes like a cluster with large particle sizes as recorded on image Fig. 1D. Successful formation of CuNs onto the electrode surface, with spherical shape (and sizes of 54–70 nm) was evaluated from TEM examination as seen in Fig. 1E. The presence of copper, carbon, oxygen, and sulphur peaks is represented and confirmed using the EDX data of the CuNCPE displayed in Fig. 1F, which is a good representative of the paste formation.
The XRD pattern of the CuNCPE shows sharp diffraction lines at 26.6°, 42.5°, 50.2° and 54.5° (Fig. 1G). The two sharp diffraction lines located at 2θ = 42.5° (with d-spacing of 2.1 Å) and 50.2° (with d-spacing of 2.0 Å) correspond to the CuNs, while the other two peaks located at 2θ = 26.6° (with d spacing of 3.36 Å) and 54.5° (with d-spacing of 1.0 Å) are attributed to carbon, the other main constituent of the modified electrode. No additional lines originating from any other crystalline elements can be noticed, which indicates the high purity of the deposited CuNs and that their good crystallinity is supported by the sharpness of diffraction lines. The definite line broadening of the peaks suggests that CuNs are in the nanometer size range.
Electrochemistry of vitamin B6 at CuNCPE modified sensor
Various kinds of buffer solutions like 0.1 M of each HCl, H2SO4, Britton-Robinson buffer (B-R), and phosphate buffer (PBS) were tested for use as supporting electrolytes, and that was because of enhancing the conditions of measurements. Figure 2A illustrates almost no peaks when using HCl and B-R buffer solutions. On the other hand, a wide and broad peak appears in the case of the H2SO4 buffer solution. However, PBS revealed a sharp peak, which makes it the appropriate operational solution. A voltammogram in the supporting electrolyte without vitamin B6 was also completed but it was nearly blank one with no peaks and coincided on the curve concerning B_R buffer.
To achieve a developed highly performed electrochemical sensor, how it performs towards the substance you want to measure compared with CPE is required. This substance was vitamin B6, and the measurements were taken. The sensitivity and validity of CuNCPE were checked in the detection of vitamin B6 using the CV technique. Figure 2B demonstrates CVs of CPE and CuNCPE in 0.1 M of PBS at pH = 5.0 employed as supporting electrolytes, including 1.0 mM vitamin B6, using a scan rate of 0.05 V s−1. Currently, the vitamin B6 oxidation on CuNCPE is an irreversible process. A distinct oxidation peak can be seen at a peak potential of about 0.99 V, and the peak current is about 243.661 µA, approximately 1.266 times higher than the bare electrode's 192.4 µA.
The study of EIS was done at the peak potential (1.0 V), using Bode and Nyquist plots displayed in Fig. 2C and D, correspondingly and the results give a good confirmation for the results obtained from CVs, which declare that CuNCPE reaches an advanced electrocatalytic activity than bare CPE when it comes to the oxidation of 1.0 mM vitamin B6. The semi-circle-shaped Nyquist plot illustrates that the process depends on charge transfer resistance. With a 1% average error, the corresponding circuit's best attempt was to fit the data shown in Fig. 2D inset. Q2, the constant phase element CPE, is connected in parallel to R2, the outer layer resistance, while R1 denotes the solution resistance. The inner layer resistance, R3, and the double layer capacitance, C3, are connected in parallel42,43. Surface roughness causes an empirical exponent (α = 0 to 1) to be projected to inspect the departure from a capacitive idealist behaviour. The optimal capacitor is associated with α = 1, and the CPE in the Warburg component emerges at α = 0.544,45. There is no straight line observed in the obtained curves which refer no diffusion behaviour is present. As well the empirical exponent values (α) wasn’t equal to 0.5, which is the value that may refer to the presence of Warburg component and diffusion. The fitting is completed applying EC-Lab® software. A larger bare semicircle diameter than CuNCPE, implying a greater conductivity. These outcomes verify the maximum oxidation Ip acquired from CVs for CuNCPE electrode. The solution's resistance value, R1, is about a constant for both electrodes within the experimental error's limits. CuNCPE exhibits comparatively more significant capacitance values or lesser impedance values than BCPE, demonstrating a more conducting behaviour and confirming the uppermost oxidation peak current that was attained from CV's findings.
Effect of pH
The impact of altering the solution's pH on the electro-oxidation of vitamin B6 was investigated at a concentration of 1.0 mM vitamin B6 in 0.1 M PBS (pH 4.0–6.0) at CuNCPE (Fig. 3A). The complicated scattering of species resultant from hydration and acid–base equilibria makes it clear that the pH affects vitamin B6 electro-oxidation. In an acidic medium (pH = 4), the expansion and eventual disappearance of the current peak can be accredited to vitamin B6 molecule protonation, which is electroactive only in its un-protonated form. An optimal peak appears as an increasing pH; functioning pH has been set to 5.0 for this work. The influence of pH on the current peak (Fig. 3A inset) demonstrates that the current raises when pH increases from 4.0 to 5.0 and then falls as pH upsurges, ensuring that vitamin B6 protonation processes in an acidic media and becomes electro-inactive. Additionally, when the pH solution increased, the peak current decreased and reached its virtual maximum at pH = 5.0. This is predictable as the vitamin is only in an anionic form in robust basic solutions (pH > 8.0) because of the deprotonation of the −OH group attached to the pyridinic ring. The peak current might decrease because of this. This decrease in anodic peak current in high basic pH could be ascribed to variations in the pace of reactivity of the electrode and the electrostatic interaction between CuNs and vitamin B6. Moreover, Nyquist and Bode plots were utilized to examine the effects of altering the pH of PBS (Fig. 3C and D). It tends to behave similarly to CVS. It demonstrates that pH = 5.0 maximum conductivity and minimum impedance are obtained with the lowest semicircle diameter. The anodic peak potential Ep change, as seen in Fig. 3B, illustrates how vitamin B6 oxidizes in response to pH. An increasing pH value was found to cause a negative shift in anodic Ep, which corresponded to a linear relationship between pH and the potential peak in terms of the given equation:
The proton-transfer process in vitamin B6 electro-oxidation was used to illustrate the anodic peak potential reliance on pH. Because of the complexity of the oxidation process, the slope (0.006326 V/pH) changes from the ideal Nernstian slope (= 0.059 V/pH) at 25 °C. This indicates the number of transported protons and electrons is not equal.
Effect of scan rate
The CV technique Fig. 4A illustrates the scan rate influence on the anodic peak current of 1.0 mM vitamin B6 in 0.1 M PBS. In a wide range, there was an increase in the scan rate (from 0.01 to 2.0 V s−1) followed by a rise in peak current; this caused the peak potential shifts to higher positive values, confirming the irreversible electrochemical oxidation process46. Figure 4B shows a linearity amongst the anodic peak current and the square root of the scan rate, which confirms the existence of a diffusion-controlled mechanism that could be signified by:
Figure 4C illustrates a linear relation between log current against log scan rate with a slope of 0.296657, which designates the presence of a controlled mechanism; this means that through the electrode reaction, the active surface of CPEs has proven that they are an excellent environment for adsorbing particle and that was because of their surface passivation. After all, a mixed diffusion-adsorption mechanism was dominated.
Scheme 3 shows that the pyridoxine electro-oxidation mechanism can form the aldehyde pyridoxal and then oxidize it to pyridoxic acid. Some cases show that detecting pyridoxal in its hemiacetal form was limited to an overall process. Alternatively, we might exclude the electroactive free aldehyde; however, this would necessitate a prior chemical change at the electrode within the voltammetric experiment's time frame47.
The diffusion coefficients of CPE and CuNCPE were assessed to be 0.613 and 0.57798 cm2 s−1, respectively, by inserting the electrode area, and the slope in Fig. 4B equals (2.69 × 105) n3/2ACD1/2, based on the Randles–Sevcik equation. It is clear that adding CuN as a modifier has increased the active surface area and allowed vitamin B6 molecules to diffuse through the electrolyte.
Calibration curve study
To ensure the validity and sensitivity of CuNCPE for the electrochemical determination of vitamin B6, Fig. 5 shows a linearity among the anodic current peak and different concentrations of vitamin B6 as follows:
Figure 5 inset displays the equivalent differential pulse patterns for vitamin B6. The samples were scanned at a rate of 0.01 V s−1, applying CuNCPE in 0.1 M of PBS (pH 5.0) with concentrations varying from 8.88 to 1000.0 µM. The limit of quantification (LOQ) and the limit of detection (LOD) were computed using the respecting formulas48:
The determined values of 107.0 and 32.12 µM validate the electrode's sensitivity. The reproducibility was well-ordered five times under the same standings as the suggested electrode by repeated measurements, producing a relative standard deviation (RSD) of 2.1%.
Table S1 compares the several electrochemical sensors that have already been discussed for vitamin B6 detection. In contrast to the modified electrode suggested in this work, those approaches involve the use of costly or hazardous chemicals in addition to being more complex to fabricate. Thus, with a comparatively low detection limit and strong selectivity, this approach has demonstrated its dependability and sensitivity for vitamin B6 detection.
Samples analysis
To determine the utility of the new sensor by DPV, vitamin B6 was identified in real samples, including urine and drugs, by spiking the samples with standard vitamin B6 concentrations utilizing the standard addition method.
The calibration curve for the CuNCP electrode using baby urine samples is displayed in Fig. 6 and results in a straight line in the concentration range of 8.0–1000.0 µM. The calibration curve equation was utilized to estimate the amount of vitamin B6 in urine samples: Ipa (µA) = 0.012C (M) + 3.241. The LOD was 25.0 µM, the LOQ was 83.3 µM, and the correlation value was r2 = 0.845. Table S2 demonstrates the recommended method of detecting vitamin B6 for four different concentrations on the curve with accuracy and precision; each was performed five times to ensure that the recommended methodology was validated in urine samples.
Table S2 displays the results, demonstrating that vitamin B6 can be estimated in pharmaceutical samples using the modified electrode, with satisfactory recoveries for each sample falling between 98.1 and 103.4% with RSDs of 1.2% to 3.9%. The amount of vitamin B6 in the real samples was determined by averaging five replicate measurements for each measurement of the oxidation current peak.
HPLC analysis was performed for Vitamin B6 sample and the results compared with that obtained from our proposed sensor to ensure excellent electrode response. The retention time was 4.43 min.
HPLC analysis was made on a chromatographic system model waters (Austria) equipped with UV 275 nm detector, 4.6-mm × 15-cm; packing L1 column and injection size equal to 10 µL.
For the analysis, the mobile phase consisted of Acetonitrile, tetrahydrofuran, and Buffer (25:20:955), adjusted with glacial acetic acid to a pH of 4.5 was used, at a flow rate of 1 mL/min.
Typical chromatogram for Vitamin B6 obtained as illustrated in Fig. 7 inset. The mean value of peak area for each concentration was taken for the calibration curve at three different concentrations of Vitamin B6 with satisfied correlation coefficient factor of 0.995 and the slope of the curve used to calculate the LOD value which found to be 43.56 µM which is a comparable and little higher value to that is found in case of using our proposed sensor.
Interfering materials, sensor selectivity, reproducibility and long-term stability
Table S3 displays the results of studies on the voltammetric response with vitamin B6. The influence of various interfering substances on the determination of vitamin B6 by CuNCPE was evaluated by spiking a constant concentration of vitamin B6 (500 µM) with the same and doubled concentrations of different compounds, such as sucrose, starch, urea, glucose, uric acid, and vitamins, like ascorbic acid (vitamin C), thiamine hydrochloride (B1), riboflavin (B2), nicotinic acid (B3), pantothenic acid (B5), biotin (B8), folic acid (B9), and cyanocobalamin (B12). The results showed that, in the stated system (within the investigated potential range and in the selected supporting electrolyte), B1, B2, B3, B5, and B8 were not electroactive. Also, additional vitamins like ascorbic acid (vitamin C) were tested to choose the recommended method for vitamin B6. CV measurements were carried out under the same experimental settings, and without affecting the response of the vitamin B6 sensor, the sensor was capable of identifying both at different peak potentials of 1.12 V for vitamin B6 and 0.48 V for vitamin C (Fig. 8). This indicates that the sensors are highly selective.
To ensure the reproducibility of CuNCPE validation in terms of RSD, five consecutive voltammetric readings for 50 μM of vitamin B6 were recorded. The results showed no discernible fluctuation in vitamin B6 peak currents, with an RSD value of 1.9%. This guarantees the electrode being investigated is accurate.
The suggested procedure's long-term stability was evaluated by refrigerating CuNCPE at 4 ◦C for one week. After that, a voltammetric measurement for vitamin B6 was performed, and the results showed that the electrode had excellent storage stability, with a current response at 99.0% of the value achieved after fresh fabrication.
Conclusions
Herein, we electrodeposited Cu nanoparticles onto a CPE surface to produce a new vitamin B6 sensor. Thanks to the increase in the electro-active area produced by Cu NPs, as established by the CV and EIS examinations, this sensor exhibited a brilliant vitamin B6 sensing routine. The electrochemical vitamin B6 response revealed a limit of detection of 32.12 × 10–6 M and a broad linear detection range (8.88 × 10–6–1.0 × 10–3 M). For determining vitamin B6, the sensor has good selectivity, sensitivity, and stability. Moreover, it has been shown that the sensor can precisely detect vitamin B6 in urine samples and Centrum multivitamins. We trust that CuNCPE could be helpful in the electrochemical detection of vitamin B6.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Muhammad, A. et al. Vitamins and minerals: Types, sources and their functions. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations 149–172 (2020).
Schmidt, A., Schreiner, M. G. & Mayer, H. K. Rapid determination of the various native forms of vitamin B6 and B2 in cow’s milk using ultra-high performance liquid chromatography. J. Chromatogr. A 1500, 89–95 (2017).
Lee, J. H., Lauw, S. J. & Webster, R. D. The electrochemical reduction of carbon dioxide (CO2) to methanol in the presence of pyridoxine (vitamin B6). Electrochem. Commun. 64, 69–73 (2016).
Skarupski, K. A. et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am. J. Clin. Nutr. 92(2), 330–335 (2010).
Doll, H., Brown, S. U. S. A. N., Thurston, A. M. A. N. D. A. & Vessey, M. A. R. T. I. N. Pyridoxine (vitamin B6) and the premenstrual syndrome: A randomized crossover trial. J. R. Coll. Gen. Pract. 39(326), 364–368 (1989).
Vutyavanich, T., Wongtra-ngan, S. & Ruangsri, R. Pyridoxine for nausea and vomiting of pregnancy: A randomized, double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 173(3), 881–884 (1995).
Clayton, P. T. B 6-responsive disorders: A model of vitamin dependency. J. Inherit. Metab. Dis. 29, 317–326 (2006).
Lenze, E. J. et al. Onset of depression in elderly persons after hip fracture: Implications for prevention and early intervention of late-life depression. J. Am. Geriatr. Soc. 55(1), 81–86 (2007).
Hvas, A.-M., Juul, S., Bech, P. & Nexø, E. Vitamin B6 level is associated with symptoms of depression. Psychother. Psychosom. 73(6), 340–343 (2004).
Merete, C., Falcon, L. M. & Tucker, K. L. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J. Am. Coll. Nutr. 27(3), 421–427 (2008).
Chiang, E.-P.I., Bagley, P. J., Selhub, J., Nadeau, M. & Roubenoff, R. Abnormal vitamin B6 status is associated with severity of symptoms in patients with rheumatoid arthritis. Am. J. Med. 114(4), 283–287 (2003).
Chiang, E.-P.I., Selhub, J., Bagley, P. J., Dallal, G. & Roubenoff, R. Pyridoxine supplementation corrects vitamin B6 deficiency but does not improve inflammation in patients with rheumatoid arthritis. Arthritis Res. Ther. 7, 1–8 (2005).
Woolf, K. & Manore, M. M. Elevated plasma homocysteine and low vitamin B-6 status in nonsupplementing older women with rheumatoid arthritis. J. Am. Diet. Assoc. 108(3), 443–453 (2008).
Huang, S. C., Wei, J. C. C., Wu, D. J. & Huang, Y. C. Vitamin B6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur. J. Clin. Nutr. 64(9), 1007–1013 (2010).
Christen, W. G., Glynn, R. J., Chew, E. Y., Albert, C. M. & Manson, J. E. Folic acid, vitamin B6, and vitamin B12 in combination and age-related macular degeneration in a randomized trial of women. Arch. Intern. Med. 169(4), 335 (2009).
Sofi, F. et al. Low vitamin B6 and folic acid levels are associated with retinal vein occlusion independently of homocysteine levels. Atherosclerosis 198(1), 223–227 (2008).
Axer-Siegel, R. et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am. J. Ophthalmol. 137(1), 84–89 (2004).
Seddon, J. M., Gensler, G., Klein, M. L. & Milton, R. C. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am. J. Ophthalmol. 141(1), 201–203 (2006).
Seddon, J. M., Gensler, G., Klein, M. L. & Milton, R. C. C-reactive protein and homocysteine are associated with dietary and behavioral risk factors for age-related macular degeneration. Nutrition 22(4), 441–443 (2006).
Malouf, R. & Sastre, A. A. Vitamin [B. sub. 12] for cognition. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004394 (2003).
Seshadri, S. et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 346(7), 476–483 (2002).
Oulhaj, A. et al. Homocysteine as a predictor of cognitive decline in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 25(1), 82–90 (2010).
Lin, P.-T. et al. Low pyridoxal 5′-phosphate is associated with increased risk of coronary artery disease. Nutrition 22(11–12), 1146–1151 (2006).
Vermeulen, E. G. J. et al. Effect of homocysteine-lowering treatment with folic acid plus vitamin B6 on cerebrovascular atherosclerosis and white matter abnormalities as determined by MRA and MRI: A placebo-controlled, randomized trial. Eur. J. Clin. Investig. 34(4), 256–261 (2004).
Vermeulen, E. G. et al. Effect of homocysteine-lowering treatment with folic acid plus vitamin B6 on progression of subclinical atherosclerosis: a randomised, placebo-controlled trial. Lancet 355(9203), 517–522 (2000).
Endo, N. et al. Antioxidant activity of vitamin B6 delays homocysteine-induced atherosclerosis in rats. Br. J. Nutr. 95(6), 1088–1093 (2006).
Bird, R. P. The emerging role of vitamin B6 in inflammation and carcinogenesis. Adv. Food Nutr. Res. 83, 151–194 (2018).
Ueland, P. M., McCann, A., Midttun, Ø. & Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 53, 10–27 (2017).
Katan, M. How much vitamin B6 is toxic?. Nederlands tijdschrift voor geneeskunde. 46, 2545–2546 (2005).
Spinneker, A. et al. Vitamin B6 status, deficiency and its consequences—An overview. Nutricion hospitalaria 22(1), 7–24 (2007).
Prasad, P. S., Kumar, S. P., Bharathi, K. & Narayanan, V. Determination of Vitamin-B6 by Vanadium (III) Schiff base complex modified GCE. Mater. Today Proc. 5(2), 9026–9032 (2018).
Mekonnen, A., Saini, R. C., Tadese, A. & Pal, R. Square wave voltammetric determination of pyridoxine in pharmaceutical preparations using cobalthexacyanoferrate modified carbon paste electrode. J. Chem. Pharm. Res. 6(1), 544–551 (2014).
Baghizadeh, A., Karimi-Maleh, H., Khoshnama, Z., Hassankhani, A. & Abbasghorbani, M. A voltammetric sensor for simultaneous determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode. Food Anal. Methods 8, 549–557 (2015).
Dinesh, K. & Geetha, K. Synthesis and characterization of copper and copper oxide nanoparticles by thermal decomposition method. 321–327 (2014).
Grouchko, M., Kamyshny, A., Ben-Ami, K. & Magdassi, S. Synthesis of copper nanoparticles catalyzed by pre-formed silver nanoparticles. J. Nanoparticle Res. 11(3), 713–716 (2009).
Usman, M. S. et al. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 4467–4479 (2013).
Tomaszewska, E. et al. Comparison of the effect of dietary copper nanoparticles with copper (II) salt on bone geometric and structural parameters as well as material characteristics in a rat model. J. Trace Elem. Med. Biol. 42, 103–110 (2017).
Yoon, K. Y., Byeon, J. H., Park, J. H. & Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 373(2–3), 572–575 (2007).
Moustafa, A., El-Kamel, R. S., Abdelgawad, S., Fekry, A. M. & Shehata, M. Electrochemical determination of vitamin B6 (pyridoxine) by reformed carbon paste electrode with iron oxide nanoparticles. Ionics 28(9), 4471–4484 (2022).
Hosseini, J.-B., Raoof, S. & Ghasemi, Z. Gholami, Synthesis of Pt–Cu/poly (o-Anisidine) nanocomposite onto carbon paste electrode and its application for methanol oxidation. Int. J. Hydrogen Energy 40, 292–302 (2015).
Atta, N. F., Galal, A. & Azab, S. M. Electrochemical morphine sensing using gold nanoparticles modified carbon paste electrode. Int. J. Electrochem. Sci 6, 5066–5081 (2011).
Fekry, A. Impedance and hydrogen evolution studies on magnesium alloy in oxalic acid solution containing different anions. Int. J. Hydrogen Energy 23, 12945–12951 (2010).
Fekry, A. M. Electrochemical behavior of a novel nano-composite coat on Ti alloy in phosphate buffer solution for biomedical applications. RSC Adv. 24, 20276–20285 (2016).
Abdelrahman, E. & Essa, K. Three least-squares minimization approaches to interpret gravity data due to dipping faults. Pure Appl. Geophys. 172(2), 427–438 (2015).
Essa, K. S. & Elhussein, M. A new approach for the interpretation of magnetic data by a 2-D dipping dike. J. Appl. Geophys. 136, 431–443 (2017).
Bard, A. J. & Faulkner, L. R. Fundamentals and applications. Electrochem. Methods 2, 482 (2001).
Brunetti, B. & Desimoni, E. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J. Food Compos. Anal. 2, 155–160 (2014).
Shehata, M., Fekry, A. M. & Walcarius, A. Moxifloxacin hydrochloride electrochemical detection at gold nanoparticles modified screen-printed electrode. Sensors 20(10), 2797 (2020).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A. moustafa: experimental data, preparation of results, data analysis, and writing original draft preparation. S. A. Abdel-Gawad: conceptualization, validation, supervision, and writing review and editing. M. Shehata: conceptualization, validation, writing—review and editing. R. S. El-Kamel: preparation of results, data analysis, and writing original draft preparation. A.M. Fekry: conceptualization, validation, supervision, and writing review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
moustafa, A., Abdel-Gawad, S.A., Shehata, M. et al. Electrochemical sensing of vitamin B6 (pyridoxine) by adapted carbon paste electrode. Sci Rep 14, 21972 (2024). https://doi.org/10.1038/s41598-024-71341-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71341-2
- Springer Nature Limited