Abstract
Large-scale production of cultured meat requires bulk culture medium containing growth-promoting proteins from animal serum. However, animal serum for mammalian cell culture is associated with high costs, ethical concerns, and contamination risks. Owing to its growth factor content, conditioned medium from rat liver epithelial RL34 cells can replace animal serum for myoblast proliferation. More seeded cells and longer culture periods are thought to yield higher growth factor levels, resulting in more effective muscle cell proliferation. However, RL34 cells can deplete nutrients and release harmful metabolites into the culture medium over time, potentially causing growth inhibition and apoptosis. This issue highlights the need for waste clearance during condition medium production. To address this issue, we introduced a lactate permease gene (lldP) and an l-lactate-to-pyruvate conversion enzyme gene (lldD) to generate a recombinant l-lactate-assimilating cyanobacterium Synechococcus sp. KC0110 strain. Transwell co-culture of this strain with RL34 cells exhibited a marked reduction in the levels of harmful metabolites, lactate and ammonium, while maintaining higher concentrations of glucose, pyruvate, and pyruvate-derived amino acids than those seen with RL34 cell monocultures. The co-culture medium supported myoblast proliferation without medium dilution or additional nutrients, which was attributed to the waste clearance and nutrient replenishment effects of the KC0110 strain. This culture system holds potential for the production of low-cost, and animal-free cultured meat.
Similar content being viewed by others
Introduction
Global food demand is expected to surge by up to 50–60% by 2050, with a 67% predicted increase in meat and fish consumption, as the global population is projected to exceed 9 billion1,2. However, conventional meat production using livestock farming is associated with adverse environmental impacts such as greenhouse gas emissions from animal digestion, deforestation, and land degradation together with the use of chemical fertilizers and agricultural chemicals to produce animal feed3,4. These environmental impacts contribute to climate change and affect global food production5. Hence, developing environmentally sustainable meat production technologies to reduce environmental burden and meet the increasing demand is required.
Cultured meat technology is attracting global attention as a potential solution to mitigate the environmental impacts of conventional meat production. According to the 2021 Life Cycle Assessment by Delft6, cultured meat production is projected to have a significantly lower environmental impact than cattle farming under conventional energy system scenarios. Cultured meat production relies on cell culture techniques, wherein the culture medium plays a crucial role in supporting the growth and proliferation of muscle cells, which are the main constituents of cultured meat. Typically, the medium consists of a basal medium containing essential nutrients, such as glucose and amino acids, supplemented with animal serum-containing proteins, such as growth factors and hormones, to enhance cell adhesion and growth. Affordable large-scale cultured meat production requires cost reductions associated with medium ingredients, particularly growth factors, which are currently obtained via supplementation with animal sera7. Furthermore, the use of animal serum poses challenges related to animal welfare and the risk of pathogenic contamination8. That is, the use of animal serum contradicts the idea of cultured meat, which aims to generate a new source of proteins independently from the livestock for low-cost, animal-free, and safety reasons. Altogether, developing an alternative to animal serum is essential for sustainable meat production.
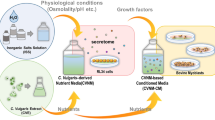
We previously suggested using the culture medium of RL34 cells (rat liver epithelial cells) as a potential alternative to serum. RL34 cells secrete growth factors such as insulin-like growth factor 2 (IGF-2) and platelet-derived growth factor (PDGF), and their conditioned medium promotes bovine myoblast proliferation9. A greater number of RL34 cells and a longer culture period may yield greater growth factor levels, resulting in more effective muscle cell proliferation. However, seeding many cells and culturing for an extended period results in the consumption of nutrients, such as glucose and pyruvate, and the buildup of harmful metabolites, such as lactate and ammonium, in the culture medium that can hinder cell growth10,11,12,13, offsetting the beneficial effects of higher growth factor levels. This challenge can be resolved through the efficient supply of nutrients and waste product removal, which would optimize RL34 culture medium as a viable serum alternative for muscle cell culture. Many cyanobacteria synthesize amino acids from ammonium14. Using Synechococcus sp. PCC 7002 as the host strain, we previously developed l-lactate-assimilating recombinant cyanobacteria (KC0110 strain) that synthesize pyruvate from l-lactate10,11. Synechococcus is appropriate for the development of l-lactate-assimilating cyanobacteria for genetic tractability and biocompatibility15,16,17.
In the present study, we co-cultivated RL34 cells with Synechococcus sp. KC0110 strain to remove l-lactate and ammonium from the culture medium. In this manner, we sought to mitigate nutrient depletion and waste accumulation in the cell culture medium, ultimately promoting cell proliferation. The objective of this study was to establish a transwell co-culture system between cyanobacteria and growth factor-secreting cells for the production of conditioned medium as an alternative to serum in mammalian cell culture. This novel culture system that is independent of animal sera will greatly contribute to a sustainable and low-cost cultured meat production, leading to an expansion of the cultured meat.
Results
Characterization of RL34 cell culture medium
Initially, we evaluated the proliferation of the murine myoblast cell line, C2C12 cells, cultured in RL34 cell conditioned medium for 2 days. The C2C12 cell line is commonly used as an in vitro experimental model for skeletal muscle cell research. We analyzed conditioned medium from RL34 seeded at different numbers and for different culture periods. The main objective was to assess whether more seeded cells and an extended culture period led to a higher production of growth factors.
RL34 cells were continuously cultured in Dulbecco's modified Eagle's medium (DMEM) without fetal bovine serum (FBS) for 1–4 days at seeding densities of 0.25 × 106, 1.0 × 106, and 4.0 × 106 cells/well in 6-well plates. The medium was then directly used for C2C12 cell culture or fresh DMEM was added in an amount equal to culture medium (50:50 v/v%) in order to supply the nutrients consumed and dilute the waste products produced by RL34 cells (Fig. 1). The proliferation of C2C12 cells in RL34 cell conditioned medium was evaluated by calculating the fold change in viable C2C12 cell numbers from the initial cell densities (4.5 ± 0.70 × 104 (Fig. 1a); 4.8 ± 0.84 (Fig. 1f) cells/well in 12-well plates) prior to treatment. The fold change of C2C12 cells cultured in DMEM without FBS was used as a control. The proliferation-promoting properties were compared among the various RL34 cell conditioned media.
Evaluation of C2C12 cell proliferation following the addition of RL34 cell culture medium: (a–e) or 50:50 v/v% of DMEM-diluted RL34 cell culture medium: (f–j). (a,f) Fold change of C2C12 cell density relative to the initial cell density (a: 4.5 ± 0.70 × 104 cells/well; f: 4.8 ± 0.84 × 104 cells/well in 12-well plates) after culture with 1 mL of DMEM or serum-free RL34 medium for 2 days (a: n = 4; f: n = 3). The darker color of columns indicates higher seeding density of RL34 cells; 0.25 × 106 (light pink), 1.0 × 106 (pink), and 4.0 × 106 (crimson) cells/well in 6-well plate. (b–e), (g–j) Time-dependent changes in (b,g) glucose; (c,h) lactate; (d,i) ammonium; (e,j) LDH content of RL34 culture medium and 50:50 v/v% of DMEM-diluted RL34 cell culture medium (4.0 × 106 cells/well in 6-well plate, (b–e): n = 4, (g–j): n = 3). Data represent the mean ± SD; ***p < 0.001; **p < 0.01; *p < 0.05.
C2C12 cells did not proliferate in DMEM without FBS after 3 days of cultivation. However, their proliferation was enhanced in all RL34 cell conditioned media tested (Fig. 1a). Myoblast proliferation was more pronounced when the conditioned medium was diluted with fresh medium (Fig. 1f). Furthermore, the proliferation rate tended to increase as the culture period and the number of seeded RL34 cells increased (Fig. 1f). This finding suggests that diluting with fresh medium replenished consumed nutrients and diluted waste products, attributing C2C12 proliferation. Even though the concentration of growth factor was diluted in RL34 conditioned medium.
We previously showed that IGF-2 was present at high levels in RL34 cell culture medium and that this was related to the conducive effect of conditioned medium on muscle cell proliferation9. While greater proliferative activity was tended to be observed in conditioned medium collected after extended culture periods, statistically significant differences among conditioned media were not detected (Fig. 1f). This finding suggests that the enhanced proliferation may be attributed to multiple factors rather than solely on the concentration of growth factors in mammalian cell culture. In fact, the proliferation-promoting effect of RL34 conditioned medium was significantly diminished without DMEM dilution (Fig. 1a compared to 1f), possibly owing to the accumulation of metabolic waste and the depletion of nutrients during cell culture (Fig. 1b–e). In particular, the elevated lactate (> 20 mM) and ammonium (> 2 mM) levels observed in RL34 cell culture medium on days 3 and 4 may impede the growth of C2C12 cells. Furthermore, the levels of cytotoxicity marker lactate dehydrogenase (LDH) in RL34 cell culture medium increased during the culture period (Fig. 1e). The same trend persisted in RL34 conditioned medium diluted with DMEM (Fig. 1g–j), highlighting the need for new waste clearance and nutrient replenishment strategies to improve RL34 cell viability and serum replacement capability of the RL34 conditioned medium. We pursued this optimization using the following optimal culture conditions for RL34 cells: 4.0 × 106 cells/well in 6-well plates, and 3-day cultivation.
Validating the transwell co-culture system combining RL34 cells and KC0110 strains
While growth factors in the RL34 cell culture medium increased with culture period, so did lactate levels, and those of glucose decreased (Fig. 1b,c). As a result, minimal muscle cell proliferation was noted even after the addition of fresh medium was RL34 cell conditioned medium (Fig. 1a,f). This is because lactate accumulation adversely affects animal cell survival at concentrations greater than 20–30 mM, and ammonium inhibits cell growth at concentrations greater than 2–3 mM12,13. Our previous report showed that in C2C12 cell culture, cytotoxicity was observed at a lactate concentration of 20 mM; however, cytotoxicity was hardly observed at 4 mM10. Therefore, we hypothesized that preventing the accumulation of waste products in the culture medium would promote RL34 cell viability and, thus, enhance the proliferation-promoting properties of RL34 cell conditioned medium.
Therefore, we had developed an l-lactate-assimilating cyanobacterium (Synechococcus sp. KC0110 strain) in the previous work, which converts lactate into pyruvate, through metabolic engineering of Synechococcus sp. PCC700210,11. The KC0110 strain was then applied to the RL34 cell culture system to reduce accumulated waste and generate nutrients.
We compared RL34 cell numbers between monoculture and transwell co-culture with the KC0110 strain to assess the validity of the co-culture system. We also analyzed the levels of lactate, ammonium, glucose, pyruvate, and LDH (Fig. 2c–g) in the culture media of RL34 cell monoculture and the RL34 cell/KC0110 strain transwell co-culture.
Comparing RL34 monoculture vs. RL34 and KC0110 strain transwell co-culture. The gray columns stand for RL34 monoculture (seeding density of 4.0 × 106 cells/well in 6-well plate for 3 days), and the green columns stand for RL34 and KC0110 strain transwell co-culture. The darker the green color of the column stands for the higher seeding density of KC0110; optical density at 750 nm (OD750) 1.0 (light green), 2.0 (green), 4.0 (dark green) cultured with 4.0 × 106 RL34 cells/well in 6-well plate for 3 days. (a) Fold change of RL34 cell density relative to the initial cell density (4.5 ± 0.50 × 106 cells/well in 6-well plates) co-cultured with KC0110 for 3 days (n = 4). (b) Change of OD750 to the initial cell density (Low: 0.97 ± 0.058; Mid: 2.0 ± 0.017; High: 4.0 ± 0.015) of KC0110 co-cultured with RL34 for 3 days (n = 3). (c–g) Metabolite content of co-culture medium (seeding density of 4.0 × 106 RL34 cells/well with OD750 2.0 KC0110 in 6-well plate for 3 days) compared to monoculture medium (seeding density of 4.0 × 106 RL34 cells/well without KC0110 in the 6-well plate for 3 days); (c) lactate; (d) ammonium; (e) glucose; (f) pyruvate and (g) LDH (n = 6). (h) 20 amino acids composition (n = 5); (i) total amount of 20 amino acids (n = 5). Data represent the mean ± SD; ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant.
In the transwell co-culture system of RL34 and KC0110 cells, RL34 cells were seeded at a high cell density (4.0 × 106 cells/well), and KC0110 strains were seeded at different cell densities of OD750 (optical density at 750 nm) = 1.0, 2.0, and 4.0 in 6-well plates. The co-culture was maintained in DMEM without FBS for 3 days. RL34 and KC0110 cells proliferated after 3 days of cultivation (Fig. 2a,b). RL34 cells in transwell co-culture exhibited proliferation equivalent to that in monoculture, indicating that the transwell cyanobacterial co-culture had neither suppressed nor promoted RL34 cell growth.
Subsequently, we performed metabolite composition analyses of conditioned media. The KC0110 strain significantly reduced cytotoxic metabolites such as lactate (38% decrease at OD750 2.0, Fig. 2c) and ammonium (93% decrease at OD750 2.0, Fig. 2d), to an extent dependent on the number of cyanobacterial cells. Meanwhile, the concentration of nutrients like glucose (3.2 mM increase at OD750 2.0, Fig. 2e) and pyruvate (0.12 mM increase at OD750 2.0, Fig. 2f) increased, also depending on the number of cyanobacterial cells. To analyze the reason for the increase in nutrients in the transwell co-culture, we evaluated the amount of glucose and pyruvate generated by KC0110 cultured in RL34 cell conditioned medium. Without KC0110, the levels of glucose and pyruvate in RL34 conditioned medium were 3.5 ± 1.7 mM or 0.73 ± 0.036 mM, respectively, while with KC0110, the levels of glucose and pyruvate were 3.2 ± 1.8 mM or 2.4 ± 0.22 mM, respectively (n = 4), showing that pyruvate increased more than three times in the culture of KC0110, whereas 11% decrease in glucose was observed. Therefore, this finding suggested that the increase in glucose in the transwell co-culture was not due to glucose supply from KC0110 strain but rather due to the excretion of pyruvate from the strain. We assumed that an increase in pyruvate in the culture medium led to an increase in pyruvate consumption by RL34 cells; therefore, the amount of glucose required by the cells decreased, resulting in an increase in the remaining amount of glucose.
However, we also observed an increase at higher KC0110 concentrations (OD750 4.0) in LDH levels compared to those in the monoculture system (Fig. 2g). Hence, we optimized the concentration of KC0110 (OD750 2.0) for the transwell co-culture system.
A quantitative comparative analysis of the amino acids present in RL34 monoculture and co-culture media revealed elevated levels of alanine and valine in the co-culture medium compared to those in the monoculture medium (Fig. 2h). The level of serine was also slightly elevated. Furthermore, the total amount of 20 proteinogenic amino acids was higher in the co-culture medium than in the monoculture medium (Fig. 2i). This finding suggests that the KC0110 strain can convert lactate and ammonium to pyruvate and some amino acids in the transwell co-culture system.
Evaluating the proliferation-promoting effects of co-culture medium
We assessed the proliferation-promoting activity of culture medium from RL34 monoculture or RL34 and KC0110 transwell co-culture using C2C12 cells. The main objective was to determine whether the reduction of waste products and production of nutrients by KC0110 would enhance proliferation.
C2C12 cells showed significantly greater proliferation in the co-culture medium compared to that in monoculture. The number of C2C12 cells increased by over threefold from the initial seeding density (4.1 ± 0.62 × 104 cells/well in 12-well plates) in condition medium from RL34 cells indirectly co-cultured with OD750 2.0 of KC0110, while C2C12 cells did not proliferate in the serum-free DMEM or RL34 monoculture medium without DMEM dilution (Fig. 3a). This was confirmed via immunofluorescence staining (Fig. 3b). Thus, the enhanced C2C12 cell proliferation in the co-culture medium compared to that in the monoculture medium was likely attributed to waste clearance and nutrient supplementation by the KC0110 strain (Fig. 2). On the other hand, the reason why it reached a plateau at a high concentration (OD750 4.0) of the KC0110 strain may be due to adverse effect of co-cultivation shown in Fig. 2g, showing that OD750 2.0 was optimal in the transwell co-culture system.
Evaluation of the proliferation of C2C12 cells in RL34 and KC0110 co-culture medium (sup.). (a) Fold change of C2C12 cell density relative to the initial cell density (4.1 ± 0.62 × 104 cells/well in 12-well plates) following culture in different media (1 mL per well, respectively) for 2 days (n = 3); DMEM (white); RL34 monoculture medium (gray, seeding density of 4.0 × 106 cells/well in 6-well plate for 3 days); RL34 and KC0110 co-culture medium (light green, OD750 1.0; green, OD750 2.0; dark green, OD750 4.0 KC0110 with 4.0 × 106 RL34 cells/well in 6-well plate for 3 days). **p < 0.01; ns not significant. (b) Immunofluorescence staining for phalloidin (red) together with nucleus (blue) and MyoD (green) in C2C12 cells cultured in DMEM, RL34 monoculture medium, or RL34 and KC0110 (OD750 2.0) co-culture medium (scale bar: 200 µm). Data represent the mean ± SD; **p < 0.01; ns not significant.
Discussion
In this paper, we developed a transwell co-culture system of l-lactate-assimilating cyanobacteria KC0110 strain and growth factor-secreting RL34 cells via a porous membrane for the development of an animal-free cell culture system and cultured meat production. Co-cultivation of the growth factor-secreting RL34 cells with the l-lactate-assimilating KC0110 strain allows for waste clearance and nutrient replenishment, which yield conditioned medium that holds potential as an alternative to animal serum. A higher seeding cell density of RL34 monocultures enhanced the proliferative activity of conditioned medium (Fig. 1a,f), in parallel to the greater accumulation of harmful metabolic waste (Fig. 1b–d). Lactate, the major waste product of energy metabolism, almost reached cytotoxic levels (20 mM) at the early stage (day 2) in confluent RL34 cell culture (4.0 × 106 cells/well in a 6-well plate). The addition of 20 mM lactate inhibits C2C12 cell proliferation13. Therefore, fresh dilution of the RL34 cell culture medium was required for efficient C2C12 cell proliferation (Fig. 1f). Here, the KC0110 strain was applied to RL34 cell culture medium at an OD750 2.0 based on the optimal density determined in our experiments assessing the proliferation-promoting capacity of the co-culture medium. Medium from the optimized transwell co-culture conditions (4.0 × 106 RL34 cells/well with OD750 2.0 KC0110 in a 6-well plate for 3 days of transwell co-culture) promoted C2C12 cell proliferation by more than threefold from the initial cell number without serum or fresh medium supplementation. Metabolite composition analysis revealed that KC0110 significantly reduced lactate to below cytotoxic levels in the co-culture medium (Fig. 2c). In addition, more nutrients such as glucose, pyruvate, and pyruvate-derived amino acids (alanine and valine)18 were present in the co-culture medium than in the monoculture medium (Fig. 2e,f,h). This observation suggests that KC0110 consumed lactate and ammonium in the co-culture medium, converted them into pyruvate and pyruvate-derived amino acids, and then released them. Furthermore, pyruvate production by the KC0110 strain might contribute to preserving glucose levels in the culture medium, which may have promoted muscle cell proliferation.
A previous study proposed a circular cell culture system11,19 that involves the independent cultivation of photosynthetic microorganisms and animal cells, exchanging medium and extracting nutrients from photosynthetic microorganisms to enable waste treatment and nutrient supplementation for animal cells. In the transwell co-culture system reported herein, photosynthetic microorganisms and animal cells share the medium through a membrane filter. These can simplify the culture system, attributing reduction of cell culture apparatus and medium use, and time saving in cell culture, resulting in low-cost and low-environmental-load cultured meat production.
Nevertheless, we used DMEM supplemented with 10% FBS in the initial stage of RL34 and C2C12 cell culture, which was thoroughly washed after the cells had adhered to the culture plates. To eliminate the use of animal serum, further experiments will explore the use of extracellular matrix components, such as collagen and laminin, to facilitate cell attachment or suspension culture techniques. Also, the use of cyanobacteria in mammalian cell culture has both positive effects that promote cell survival and proliferation owing to waste metabolite reduction and proliferation-promoting protein10,11,20 and negative effects that cause cell damage and growth inhibition16,21,22. This report also showed the cell damage of RL34 cells within the high cell density of cyanobacteria (Fig. 2g). However, the factor that was negative for the cells was not identified in this study. The identification of the positive and negative factors will improve the survival environment of RL34 cells or C2C12 cells, allowing them to secrete high levels of growth factors or increasing their proliferative potential.
We plan to extend this transwell co-culture system into a suspension culture of myoblasts by combining growth factor-secreting cells and l-lactate-assimilating photosynthetic microorganisms. Moreover, further tests are needed to determine if the cultured meat produced via this method is safe for consumption and if its production is cost-effective.
In conclusion, we believe that this co-culture approach will contribute to sustainable cultured meat production and other fields relying on cell culture technology.
Methods
Experimental protocols have been schematically illustrated in Fig. 4.
Schematic diagram of the experiments (a) Evaluation of C2C12 cell proliferation following the addition of RL34 cell culture medium. (b) Evaluation of C2C12 cell proliferation following the addition of 50:50 v/v% DMEM-diluted RL34 cell culture medium. (c) Evaluation of C2C12 cell proliferation in RL34 and Synechococcus sp. KC0110 strain co-culture medium.
Algal culture
Detailed methodology on developing the recombinant l-lactate-assimilating cyanobacterium Synechococcus sp. KC0110 can be found in our previous publication10. Briefly, KC0110 strain was established by introducing the l-lactate permease (lldP) and l-lactate-to-pyruvate conversion enzyme gene (lldD) from Escherichia coli into the unicellular euryhaline cyanobacterium Synechococcus sp. PCC 7002 (Pasteur Institute, Paris, France). KC0110 were cultured in Medium A2 (310 mM NaCl, 20 mM Mg2SO4·7H2O, 17.3 mM NaNO3, 8.3 mM Tris(hydroxymethyl)aminomethane, 8.1 mM KCl, 2.5 mM CaCl2·2H2O, 0.37 mM KH2PO4·3H2O, 550 µM H3BO3, 89 µM Na2EDTA·2H2O, 31 µM citric acid, 30 µM FeCl3·6H2O, 22 µM MnCl2·4H2O, 12 µM CuSO4·5H2O, 2.3 µM ZnCl2, 0.21 µM Na2MoO4·2H2O, 51 nM CoCl2·6H2O, and 3 nM vitamin B12) supplemented with 40 µg/mL gentamicin (Fujifilm Wako Pure Chemical, Osaka, Japan) and 4 µg/mL carbenicillin (Fujifilm Wako Pure Chemical) in a chamber for plant growth (Biotron LH-241PFDT-SC; Nippon Medical and Chemical Instruments, Osaka, Japan) under continuous light [temperature: 25 °C; CO2 concentration: 1%; photosynthetic photon flux density: 75.8 ± 8.3 µmol/m2/s (n = 5); agitation rate: 60 rpm].
Mammalian cell culture
C2C12 mouse myoblasts (ATCC® CRL-1772™) and RL34 rat liver epithelial cells (JCRB0247) were cultured in DMEM containing 25 mM glucose (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% FBS (Nichirei Biosciences, Tokyo, Japan) and 1% penicillin–streptomycin (P/S, Invitrogen, Carlsbad, CA, USA) at 37 °C in a humid atmosphere with 5% CO2. At 85–95% confluence, C2C12 and RL34 cells were washed with phosphate-buffered saline (PBS, Fujifilm Wako Pure Chemical), sub-cultured by treatment with 0.025% trypsin/0.1 mM ethylenediaminetetraacetic acid (EDTA; Fujifilm Wako Pure Chemical) for 5–10 min, and centrifuged at 300×g for 5 min at room temperature (20–25 °C). The pallets of cells were resuspended in growth medium, filtered using a 70 µm cell strainer, and divided into the experimental setup.
Monoculture of RL34 cells
RL34 cells were seeded at a density of 0.25 × 106, 1.0 × 106, and 4.0 × 106 cells/well with DMEM containing 10% FBS and 1% P/S in 6-well plates (catalog no. 353502, Corning, NY, USA) under standard cell culture conditions for 17 h to facilitate attachment to the plate. After 17 h, the cells were washed twice with PBS, and the medium was replaced with DMEM without FBS (2.5 mL). Subsequently, the RL34 cells were cultured under standard cell culture conditions for 4 days. RL34 cell monoculture medium was collected daily during cultivation and stored at − 80 °C before use.
Transwell co-culture of RL34 cells and KC0110
RL34 and KC0110 cells were co-cultured in cell culture inserts placed in 6-well plates (Corning). RL34 cells suspended in DMEM with 10% FBS and 1% P/S were seeded into 6-well plates at 4.0 × 106 cells/well and incubated under standard cell culture conditions for 17 h to allow attachment to the plate. Following cell attachment, the RL34 cells were washed twice with PBS, and the medium was replaced with DMEM without FBS (1.5 mL). At the same time, cell culture inserts (diameter: 24 mm, pore size: 0.4 μm) (catalog no. 353493, Corning) were placed in the 6-well plates, and 1 mL of KC0110, suspended in DMEM to optical densities at 750 nm (OD750) representing algal concentrations of 1.0, 2.0, and 4.0, were seeded into the cell culture insert. KC0110 cells were centrifuged at 15,000×g for 5 min and washed twice with DMEM before being seeded into the transwell co-culture system to exclude the Medium A2. The pore size of the membrane filter within the cell culture insert was 0.4 μm, preventing bacterial transport through the membrane but allowing metabolite transport. In the control group, 1 mL DMEM was added to the cell culture insert. Cells were co-cultured at 35 °C in a humidified atmosphere with 5% CO2 under continuous light (12.6 ± 1.5 µmol/m2/s, n = 5) in the CO2 incubator with the LED lights for 3 days.
Additionally, we determined the amount of pyruvate generated by KC0110 cells (OD750 2) cultured in RL34 cell culture medium, which was collected upon the RL34 cells reaching confluence in a 100-mm cell culture dish (catalog no. 664160-013, Greiner Bio-one, Kremsmuenter, Austria) under standard cell culture conditions. KC0110 cells were then washed with the RL34 cell culture medium, as previously described. A volume of 1.5 mL RL34 cell culture medium was transferred into a 6-well plate, thereafter 1 mL of KC0110 cells suspended in the RL34 cell culture medium was seeded into cell culture inserts. Subsequently, the KC0110 cells were placed in a CO2 incubator (Astec, Fukuoka, Japan) with LED lights (LED submersible light SL-30, Tetra, Tokyo, Japan) and incubated at 35 °C in a humidified atmosphere with 5% CO2 under continuous light (12.6 ± 1.5 µmol/m2/s, n = 5) for 3 days. Medium of RL34 and KC0110 cell transwell co-culture or KC0110 cell monoculture medium were collected after a 3-day cultivation period and stored at − 80 °C before use.
Metabolic characterization of conditioned media
After mono- or transwell co-culture, the cell culture medium was centrifuged twice at 15,000×g for 5 min at room temperature to remove cells and cellular debris. The media were subjected to enzymatic assays to quantify the levels of glucose, lactate, ammonium, pyruvate, and LDH using an analyzer (Cedex Bio Analyzer, Roche Diagnostics, Tokyo, Japan). Measurements were conducted according to the manufacturer’s instructions to perform a comprehensive metabolic characterization of the cell culture system. Additionally, amino acids in the media were analyzed using an amino acid analysis reagent (APDSTAG, FUJIFILM Wako Pure Chemical Corporation, Ltd, Osaka, Japan) and liquid chromatography-mass spectrometry (Automated amino acid analysis system by Shimadzu, Kyoto, Japan).
Evaluating cell proliferation
The proliferative activity of the medium was assessed by evaluating C2C12 proliferative capacity. C2C12 cells suspended in DMEM with FBS were seeded into the 12-well plates (catalog no. 353503, Corning) at a density of 6 × 104 cells/well and incubated under standard cell culture conditions for 4 h to facilitate cell adhesion. Following cell adhesion, the cells were washed twice with PBS, the medium was replaced with medium from RL34 monoculture, RL34 and KC0110 strain transwell co-culture, or serum-free DMEM (1 mL per well, respectively), and the cells were cultured for 2 days. RL34 monoculture medium was directly applied or twofold diluted with DMEM, and the co-culture medium was directly transferred to the C2C12 cell culture. Cell counting and optical microscopy were conducted to assess cell proliferation after 2 days of cultivation.
Cell counting
The number of RL34 or C2C12 cells was determined using a trypan blue exclusion assay before cell seeding, after cell adhesion, and after cell culture. The cell density of the KC0110 strain was determined by measuring the OD750 using a SpectraMax M2e cuvette module (Molecular Devices, CA, USA) before cell seeding and after cell culture.
Immunofluorescence staining
C2C12 cells grown in various conditioned media were washed three times with PBS and fixed with 4% paraformaldehyde (PFA, Muto Pure Chemicals, Tokyo, Japan) for 30 min at room temperature. After fixing, PFA was removed, and the cells were washed with PBS and treated with 0.5% Triton X-100 (Sigma-Aldrich) in PBS for 5 min at room temperature to permeabilize cell membranes. Subsequently, Triton X-100 was discarded, and the cells were washed with PBS and shaken for 30 min at 50 rpm with 2% bovine serum albumin (BSA) in PBS to block nonspecific binding. The cells were subsequently incubated with a mouse anti-MyoD primary antibody (Santa Cruz Biotechnology, Inc., Dallas, Texas, diluted 1:500 in 2% BSA solution) in a shaker at 4 °C overnight. Subsequently, the secondary antibody (Alexa Fluor 488-labelled anti-mouse IgG antibody, Invitrogen, diluted 1:500 in 2% BSA solution) was allowed to incubate with the cells for 1 h at room temperature with phalloidin (Alexa Fluor 594 phalloidin, diluted 1:500 in 2% BSA solution) and Hoechst stain (Hoechst 33258, diluted 1:1000 in 2% BSA solution) at the same time. Finally, fluorescent images were captured using a fluorescence microscope (ECLIPSE Ti2, Nikon, Tokyo, Japan) equipped with the required software (NIS-Elements BR, Nikon).
Statistical analysis
Each experiment was performed in duplicate or triplicate, and all data are presented as the means ± SD of at least three independent experiments. Statistical analyses were performed using GraphPad Prism software version 8.4.3 (GraphPad Software, Inc., San Diego, CA, USA). The Mann–Whitney U test and multiple t-tests were used to compare data between the two groups. Multiple comparisons were conducted using analysis of variance, followed by Tukey’s test for every mean or Dunnett’s test for the control mean, as appropriate. Differences were considered statistically significant at p < 0.05.
Data availability
The original data in this article will be shared upon reasonable request to the corresponding author.
References
Falcon, W. P., Naylor, R. L. & Shankar, N. D. Rethinking global food demand for 2050. Popul. Dev. Rev. 48, 921–957 (2022).
Van Dijk, M., Morley, T., Rau, M. L. & Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2, 494–501 (2021).
FAO. The Future of Food and Agriculture—Trends and Challenges (FAO, 2017).
Stoll-Kleemann, S. & O’Riordan, T. The sustainability challenges of our meat and dairy diets. Environ. Sci. Policy Sustain. Dev. 57, 34–48 (2015).
Wheeler, T. & Von Braun, J. Climate change impacts on global food security. Science 341, 508–513 (2013).
CE Delft. Life Cycle Assessment (LCA) of Cultivated Meat: Future Projections for Different Scenarios (CE Delft, 2021).
CE Delft. TEA of Cultivated Meat: Future Projections of Different Scenarios (CE Delft, 2021).
Van der Valk, J. et al. Fetal bovine serum (FBS): Past–present–future. ALTEX 35, 99–118 (2018).
Yamanaka, K., Haraguchi, Y., Takahashi, H., Kawashima, I. & Shimizu, T. Development of serum-free and grain-derived-nutrient-free medium using microalga-derived nutrients and mammalian cell-secreted growth factors for sustainable cultured meat production. Sci. Rep. 13, 498 (2023).
Kato, Y. et al. l-lactate treatment by photosynthetic cyanobacteria expressing heterogeneous l-lactate dehydrogenase. Sci. Rep. 13, 7249 (2023).
Haraguchi, Y. et al. Circular cell culture for sustainable food production using recombinant lactate-assimilating cyanobacteria that supplies pyruvate and amino acids. Arch. Microbiol. 205, 266 (2023).
Schneider, M., Marison, I. W. & Von Stockar, U. The importance of ammonia in mammalian cell culture. J. Biotechnol. 46, 161–185 (1996).
Willkomm, L. et al. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res. 12, 742–753 (2014).
Salbitani, G. & Carfagna, S. Ammonium utilization in microalgae: A sustainable method for wastewater treatment. Sustainability 13, 956 (2021).
Cohen, J. E. et al. An innovative biologic system for photon-powered myocardium in the ischemic heart. Sci. Adv. 3, e1603078 (2017).
Yin, H. et al. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics 9, 2678–2693 (2019).
Schenck, T. L. et al. Photosynthetic biomaterials: A pathway towards autotrophic tissue engineering. Acta Biomater. 15, 39–47 (2015).
Bartek, T., Makus, P., Klein, B., Lang, S. & Oldiges, M. Influence of l-isoleucine and pantothenate auxotrophy for l-valine formation in Corynebacterium glutamicum revisited by metabolome analyses. Bioprocess Biosyst. Eng. 31, 217–225 (2008).
Haraguchi, Y., Okamoto, Y. & Shimizu, T. A circular cell culture system using microalgae and mammalian myoblasts for the production of sustainable cultured meat. Arch. Microbiol. 204, 615 (2022).
Ghosh, J., Haraguchi, Y., Asahi, T., Nakao, Y. & Shimizu, T. Muscle cell proliferation using water-soluble extract from nitrogen-fixing cyanobacteria Anabaena sp. PCC 7120 for sustainable cultured meat production. Biochem. Biophys. Res. Commun. 682, 316–324 (2023).
McDermott, C. M., Nho, C. W., Howard, W. & Holton, B. The cyanobacterial toxin, microcystin-LR, can induce apoptosis in a variety of cell types. Toxicon 36, 1981–1996 (1998).
Lakshmana Rao, P. et al. Involvement of caspase and reactive oxygen species in cyanobacterial toxin anatoxin-a-induced cytotoxicity and apoptosis in rat thymocytes and Vero cells. Arch. Toxicol. 76, 227–235 (2002).
Acknowledgements
This study was supported by the Cabinet Office, Government of Japan, Moonshot Research and Development Program for Agriculture, Forestry and Fisheries (funding agency: Bio-oriented Technology Research Advancement Institution).
Author information
Authors and Affiliations
Contributions
SC, YH, TA, YK, AK, TH, and TS discussed and defined the project. SC initiated and led the project in experimental design, and procurement and performed most of the measurements. YH, TA, YK, AK, TH, and TS discussed results and interpretation and prepared and finalized the manuscript. SC wrote the manuscript in consultation with YH, YK, AK, TH, and TS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Tokyo Women’s Medical University has received research funds from IntegriCulture Inc. The part of this manuscript describing the technique for culturing mammalian cells using culture supernatants is the subject of the following patent application: patent applicant (Tokyo Women’s Medical University and IntegriCulture, Inc.), name of inventors (T. Shimizu, Y. Haraguchi), application number (PCT/JP2023/019786), status of application (pending). The part of this manuscript describing the technique for L-lactate-assimilating cyanobacteria is the subject of the following patent application: patent applicant (Kobe University and Tokyo Women’s Medical University), name of inventors (T. Hasunuma, Y. Kato, A. Kondo, T. Shimizu, Y. Haraguchi), application number (PCT/JP2022/044962), status of application (Granted in Japan). S. Chu, and T. Asahi, declare no financial competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chu, S., Haraguchi, Y., Asahi, T. et al. A serum-free culture medium production system by co-culture combining growth factor-secreting cells and l-lactate-assimilating cyanobacteria for sustainable cultured meat production. Sci Rep 14, 19578 (2024). https://doi.org/10.1038/s41598-024-70377-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70377-8
- Springer Nature Limited