Abstract
Increasing seawater temperatures coupled with more intense and frequent heatwaves pose an increasing threat to marine species. In this study, the New Zealand green-lipped mussel, Perna canaliculus, was used to investigate the effect of genetics and ontogeny on thermal resilience. The culturally and economically significant mussel P. canaliculus (Gmelin, 1971) has been selectively-bred in New Zealand for two decades, making it a unique biological resource to investigate genetic interactions in a temperate bivalve species. Six selectively-bred full sibling families and four different ages, from early juveniles (6, 8, 10 weeks post-fertilisation) to sub-adults (52 weeks post-fertilisation), were used for experimentation. At each age, each family was exposed to a three-hour heat challenge, followed by recovery, and survival assessments. The shell lengths of live and dead juvenile mussels were also measured. Gill tissue samples from sub-adults were collected after the thermal challenge to quantify the 70 kDa heat shock protein gene (hsp70). Results showed that genetics, ontogeny and size influence thermal resilience in P. canaliculus, with LT50 values ranging between 31.3 and 34.4 °C for all studied families and ages. Juveniles showed greater thermotolerance compared to sub-adults, while the largest individuals within each family/age class tended to be more heat sensitive than their siblings. Sub-adults differentially upregulated hsp70 in a pattern that correlated with net family survival following heat challenge, reinforcing the perceived role of inducible HSP70 protein in molluscs. This study provides insights into the complex interactions of age and genotype in determining heat tolerance of a key mussel species. As marine temperatures increase, equally complex selection pressure responses may therefore occur. Future research should focus on transcriptomic and genomic approaches for key species such as P. canaliculus to further understand and predict the effect of genetic variation and ontogeny on their survival in the context of climate change.
Similar content being viewed by others
Introduction
Climate change is an increasing threat to the persistence of aquatic and terrestrial species on Earth. The most recent report published by the Intergovernmental Panel on Climate Change (IPCC) illustrates that climate change is substantially accountable for observed impacts on natural ecosystem structure, such as shifts in species distribution and phenology, as well as on human ecosystems, including food production 1. Globally, air temperature has increased by 0.95–1.2 °C in the last decade relative to the pre-industrial period, and is projected to continue increasing even further (1–5 °C) based on a range of greenhouse gas emission scenarios1. The increase in air temperature and CO2 emissions has increased ocean temperatures and decreased pH, as well as increasing the frequency and intensity of extreme climate and weather events such as heatwaves and cyclic phenomena such as the El Niño-Southern Oscillation (ENSO)1,2,3. Several studies have shown a slow increase in seawater temperature in New Zealand in the last few decades4,5,6,7. Additionally, New Zealand is experiencing more regular marine heatwave events8,9, and projections indicate that these events will become more intense and longer by the end of the century10.
Marine invertebrates are ectothermic organisms that rely on environmental temperature to perform in optimal conditions11,12. All biological functions, including physiological and biochemical processes, operate according to the physiological performance curve, and work within a defined temperature range for optimal metabolism13,14,15,16,17. The upper limits to optimum thermal windows are defined by the upper pejus temperature, at which biological functions start to deteriorate, and all energy is diverted to maintain essential functions15,18. However, when organisms experience even higher temperatures, they enter the pessimum range, in which all energy is destined for basal maintenance, with the organism potentially switching from aerobic to anaerobic metabolism; in this range, survival of the organism becomes time-dependent15. The temperature ranges at which an organism experiences its optimal, pejus and pessimum ranges are species-specific14,19. Moreover, within a species, different developmental stages also respond differently to changes in environmental temperature, with reproductive adults, eggs and larvae having narrower thermal windows, and lower thermotolerance than juveniles of the same species14. The intraspecific variation in thermal tolerance may be driven by distinct energetic requirements at each ontogenetic stage and individual size, and inherent genetic population differences14,20,21. Consequently, increasing seawater temperatures due to climate change can greatly impact the performance and survival of marine species, particularly sessile marine invertebrates.
An established and straightforward proxy to evaluating the thermal tolerance of a particular organism is the median lethal temperature (LT50); the upper or lower threshold temperature at which 50% of the studied population is predicted to die12. Physiologically, exact thermal tolerance limits to extreme temperatures cannot be determined as tolerance depends on the duration of exposure to a particular temperature12. Therefore, the determination of the upper LT50 can be used as a tool to determine the ability of an organism or a certain age and genetic background to cope with increasing temperatures. While the calculation of LT50 quantifies a population response, more specific approaches (e.g., genetic mutation analysis, quantification of molecular chaperone response, etc.) can be also used to complement and understand how an individual is able to cope with extreme temperatures. For example, heat shock proteins (HSPs) are a family of chaperone proteins that are synthesised to help protect cells against damaging stressors, avoiding aggregation of damaged proteins and degrading denatured proteins22,23,24. High HSP levels have been associated with natural adaptation of species to warmer environments as they provide protection against oxidative stress and enhancing cytoprotection25, and as a contingency for unpredictable stress26. In particular, the 70 kDa family of heat shock proteins (HSP70) has been related to thermal stress responses, specifically to increased thermal tolerance and potential for adaptation to environmental stress26,27,28,29. One of the mechanisms by which HSP70 and other HSPs can help organisms and cells to cope with stress-related damage is via their intrinsic proteolytic activity role in proteolysis, which effectively eliminates degraded proteins28. However, some coastal invertebrate species (e.g., Carcinus maenas) appear to have evolved novel cellular survival strategies to allow increased thermal tolerance without increased synthesis of HSP7030, so species specific investigations are required to confirm the role of such proteins.
Sessile coastal invertebrates include taxa of particular interest in the elucidation of environmental resilience. These organisms occupy a volatile and disproportionately climate change-influenced environment and are typically reliant upon individual resilience (phenotypic plasticity) and genetic diversity (polymorphism) to support individual- or population-level success, respectively. Juvenile life stages of mytilid mussels represent a particularly intriguing research focus, as these ostensibly sessile individuals retain the capacity to undertake bysso-pelagic drifting, allowing substantial distances to be covered 31.
The present study aimed to investigate the influence of genetics and juvenile development stage on the thermal tolerance of a key mytilid, the green-lipped mussel, Perna canaliculus (Gmelin, 1971). P. canaliculus is endemic to New Zealand, is found from the middle intertidal zone to depths of over 50 m, and is a foundation species that controls biodiversity on rocky reefs and in soft-sediment habitats32,33,34. The successful and wide-spread distribution of P. canaliculus around a mid-latitude New Zealand coastline exposes it to a wide range of thermal maxima, currently covering a range of about 8 °C (Fig. 1). However, with current temperature increases measured at up to 0.3 °C per decad 7, northern (sub-tropical) populations of P. canaliculus are likely to experience greater thermal stress in the future.
Maximum sea surface temperatures (SST) around New Zealand over 1 Jan 2003 to 1 Jan 2024. Data modelled from single daily measurements from the MODIS-Aqua sensor (processed by NASA's Ocean Biology Processing Group). The underlying Level 3 data is a standard daily SST product with 4 km resolution, as sourced from NASA's Ocean Colour web portal (https://oceancolor.gsfc.nasa.gov/). Map projection: NZTM2000.
Perna canaliculus is also one of the most important seafood species in New Zealand, with northern populations particularly important for the provision of wild-sourced juveniles for aquaculture35. The risks posed from wild-sourced stock to a growing aquaculture industry has led to the development of an established selective breeding programme. This programme includes selection of traits such as shell length, meat weight, shell weight and a semi-quantitative, visually-assessed meat condition score20,36. In addition to the selection of commercial traits, selective breeding programmes have been suggested as a viable method to produce more heat-tolerant species that are more likely to adapt to heatwaves and climate change37. Genetic variability, as well as divergence in phenotypic plasticity, are key in adaptive responses to changing environmental condition and climate change through time38,39, and thus a crucial element for the success of selective breeding programmes. The current selective breeding programme for P. canaliculus represents a unique opportunity for scientists to test hypotheses about genetic interactions and expression near maximum thermal limits.
In this study, upper thermal tolerance at different developmental stages, from early juveniles (6, 8, 10 weeks post-fertilisation) to sub-adults (52 weeks post-fertilisation), was determined for populations of P. canaliculus from six different selectively-bred families. Acute thermal tolerance of each mussel family was based on the calculation of LT50 values and survival curve analysis after mussels were exposed to a three-hour heat challenge at a range of temperatures40,41. Additionally, expression of the 70 kDa heat shock protein gene (hsp70) was evaluated in sub-adult mussels to determine whether a relationship exists between the molecular regulation of the hsp70 gene and thermal resilience on this important mussel species.
Material and methods
Mussel source and experimental overview
Full-sibling green-lipped mussel, Perna canaliculus, from genetically distinct groups (families) and at different ages (juveniles and sub-adults) were obtained from the two-decade long selective breeding programme36. This programme is maintained by Breedco Ltd, the commercial hatchery SPATnz and Sanford Ltd, New Zealand. Mussels in this family-based programme have been selected for commercial traits relating to production and were not specifically selected for thermal tolerance. Families for this study were created with single-parent mating within the 2021 breeding cohort using the system and procedures described by Ragg et al. 42. Family represented the range of genetic variation present in the cohort; they were not selected on the basis of performance. Juveniles and sub-adults were exposed to a heat challenge and then allowed to recover. Net survival was assessed at the end of the recovery period to create population-level survival probability models and estimate LT50 values. Additionally, daily survival was recorded for sub-adults to estimate time-to-death and the probability of death for each family. This study did not require ethical approval in accordance with the local legislation and institutional requirements.
Thermal challenge I: juveniles
Assessments focused on early juveniles, representing the post-metamorphosis life stages where growth and development are fastest, and mussels are potentially more vulnerable to changes in temperature. A cohort of green-lipped mussel juveniles was surveyed at different ages (6, 8 and 10 weeks post-fertilisation) from six genetically diverse families provided by a commercial hatchery (SPATnz). Mussels of each age group were collected from the hatchery (~ 18–20 °C at the time of collection for the different ages) and transported to an adjacent laboratory at the Cawthron Aquaculture Park. For each age group, mussels from each family were separated into 10 subgroups, which were used for the thermal challenge immediately after collection. The size and the number of mussels for each family and age used in this study are shown in Table 1.
The thermal challenge was performed using a purpose-built aluminium block connected to a cooling and a heating unit, creating a customised thermal gradient43,44. The aluminium block consisted of 60 chambers that held 20-ml glass scintillation vials, in a 10 × 6 design (10 temperatures, six replicates per temperature, one for each family). Temperatures for the challenge ranged between 23 and 39 °C (Table 2), and were based on previous research to purposely include upper pejus and pessimum temperatures40.
For each thermal challenge, 60 glass scintillation vials were filled with 18 ml of 5-µm filtered seawater (FSW) and left to reach the desired temperature. Once the temperatures were stable, each subgroup of mussels was placed into a vial and challenged for 3 h40,41. The temperature of each glass vial was measured three times during the thermal challenge using a thermocouple.
After the thermal challenge, mussels from each vial were placed in circular sieves (mesh size = 200 µm for 6-week-old mussels, and 500 µm for 8- and 10-week-old mussels). The sieves were placed in a shallow tank with flowing 5-µm filtered seawater at 18 ± 0.2 °C to allow the mussels to recover for 44 h after the thermal challenge. The mussels were supplied with a continuous water flow containing a 50:50 cell mix of Tisochrysis lutea and Chaetoceros muelleri (23,040 ± 4,958 cells ml−1; n = 24) as a source of food during recovery.
After 44 h, mussels from each sieve were collected into a 35-ml plastic container for survival assessment. Survival was evaluated using the Fast Green staining method45. For this, mussels in the containers were immersed in 2 ml of freshwater for two minutes to induce valve closure, then 1 ml of 1% Fast Green solution was added to each container and left for two minutes. Non-viable mussels that were unable to close their valves after immersion in freshwater were stained green. After staining, mussels were rinsed thoroughly, observed under the dissecting microscope, and scored as dead (stained) or alive (unstained). For most chambers, all mussels were either alive or dead. Where a chamber contained both live and dead mussels (i.e., ~ 34 °C chamber in Table 2), these were separated into live and dead categories, and their shell lengths were measured using a dissecting microscope and the Olympus cellSens imaging software.
The thermal challenge and survival assessments were repeated three times (Runs 1–3) for each age group, with a total of nine times for the full experimental design using juvenile mussels. In total, 55,802 mussels were assessed for all age groups and families (Table 1) to build family-level survival probability models and estimate LT50 values for each family and age combination.
Thermal challenge II: sub-adults
Three hundred sub-adult mussels (52 weeks post-fertilisation; Table 1) belonging to the same families used in the juvenile experiments (A-F) were collected from a mussel farm located in the Marlborough Sounds, New Zealand. Seawater temperature at the time of collection was ~ 18 °C, but annually P. canaliculus can be exposed to a seawater temperature range between 10 and 18 °C in the Marlborough Sounds region, with a maximum of around 20 °C in summer46. After collection, mussels were transported in chilled containers approximately 70 km to the laboratory of the Cawthron Aquaculture Park. In the laboratory, mussels from each family were separated into mesh bags (~ 15 mussels per bag, 19 bags in total per family) and placed in 170-l tanks of flowing seawater at 18.9 ± 0.1 °C for one week to allow the mussels to recover from collection and handling prior to the thermal challenge. During this recovery period, the mussels were fed continuously with a 50:50 mix of Tisochrysis lutea and Chaetoceros muelleri (1.75e+04 ± 1.00e+04 cells ml−1; n = 15). Prior to the thermal challenge, five mussels from each family were randomly sampled to evaluate their reproductive condition using histology47,48. For this, mussels were dissected, the tissue was placed in cassettes, fixed in 4% formalin and transferred to 70% ethanol after 48 h. Dehydration, clearing and embedding of the tissue were performed following standard methods for wax histology49, using haematoxylin and eosin as stains and sectioned at 3–5 μm. Histological sections were observed using a compound microscope (Olympus BX53) at 40 × magnification.
Due to the larger size of sub-adult mussels, the thermal challenge system consisted of six static 100-l tanks, each containing seawater at a different temperature (Table 2), controlled by either a submersible titanium heater or submersible stainless-steel coil connected to a water bath. Temperatures for the challenge ranged between 25 and 39 °C, based on previous research to purposely include upper pejus and pessimum temperatures41. An additional tank was set up at 25 °C to pre-warm mussels (1-min immersion) before they were placed into the respective thermal challenge to mitigate any potential tank temperature reduction due to the introduction of the mussels coming from 19 °C water. The experimental tanks were supplied with strong aeration to maintain a homogeneous temperature within the tank. For the thermal challenge, the mussel bags were immersed for 3 h in each temperature41 and then moved to shallow tanks for recovery with flowing seawater at 18.6 ± 0.4 °C, and continuous food supply as described above. Mussels were kept in recovery for two weeks, a period previously determined as sufficient to identify acute heat shock-induced mortalities41,50. Survival was monitored daily during the recovery time using the British Standard Squeeze (i.e., mussels unable to close their valves after ten rapid squeezes to stimulate closure were considered dead)41,51,52. Times of death for all individuals were recorded and shell length was measured, either at the time of death or at the end of the recovery period for the surviving mussels. The thermal challenge and survival assessments were repeated three times (Run 1–3) over a period of three days, with a total of 1,620 sub-adult mussels individually assessed for all family groups.
Hsp70 gene expression in sub-adults
During the heat challenge, an extra bag per family containing five mussels each was placed in the tank set at 31 °C at the same time as the survival assessment bags. These extra replicates allowed sampling of gill tissue from mussels exposed to the three-hour heat challenge at 31 °C, followed by a one-hour recovery at 19 °C. Additionally, gill tissue samples were collected from five control mussels per family; these mussels were not exposed to the heat challenge, but instead were maintained in a 100-l tank set at 19 °C. Gill tissue was excised and placed in a 1.7-ml cryo-tube, flash frozen in liquid nitrogen and stored at − 80 °C. Gill tissue from a total of five mussels per treatment (control and heat shocked) per family were used for RNA extraction and gene expression analysis.
Frozen gill tissue was cryogenically ground and homogenised using a Mixer Mill (MM400; Retsch). Aliquots (30–50 mg) of the homogenised frozen tissue powder were collected for RNA extraction, cDNA synthesis and subsequent reverse transcription quantitative polymerase chain reaction (RT-qPCR) for the gene corresponding to the inducible isoform of the heat shock protein 70 kDa (hsp70).
TRIzol Reagent (Invitrogen) (1 ml) was added to the frozen tissue powder, and RNA extracted following the manufacturer's protocol with the addition of a second ethanol wash step to ensure unwanted organic compounds had been removed completely from the RNA sample. Any residual genomic DNA was removed using TURBO™ DNase (Invitrogen) following the manufacturer’s protocol. RNA concentration and purity were determined using a NanoPhotometer® N60/N50 (Implen GmbH). First-strand cDNA synthesis was conducted using 3 μg of total RNA extract with oligoDT, according to the SuperScript™ IV First-Strand Synthesis System (Invitrogen) protocol. Samples were stored at − 20 °C until RT-qPCR analyses.
RT-qPCR reactions were performed in 10-μl volumes containing 5 μl PowerUp™ SYBR™ Green Master Mix, 3 μl DNase free water, 1 μl cDNA diluted four-fold, and 0.5 μl of each hsp70 primer diluted in the final reaction concentration to 0.5 μM. The hsp70 primers used in this study have been specifically developed and validated for Perna canaliculus (Accession number OK318441). The 5′-3′ sequence for the hsp70 primers were: forward: CAG CCT GGT GTG TTG ATC CA; reverse: TGC TGG TGG GAT TCC TGT CA, resulting in an amplicon size of 99 bp. Thermal cycling conditions consisted of an initial denaturation at 95 °C for 2 min, followed by 45 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 15 s. The samples were analysed in triplicate and included no template control reactions. Data were reported as normalized relative expression (fold change) compared to control samples and elongation factor-1 (EF1) as reference gene using the ∆∆Ct method. EFI was chosen as it was found to be a stable reference gene in a previous study53.
Statistical analysis
Survival analysis and median lethal temperature (LT50)
To understand the role of genetics and ontogeny in thermal resilience, the survival probability of mussels in the thermal challenges was modelled using a Bayesian multilevel framework assuming the likelihood of observing the experimental data is given by a binomial distribution. We modelled the probability of survival as a function of the thermal stress that the mussels were exposed to (Temperature) and the number of weeks post-fertilization (Age), while allowing parameters to vary by genetic lineage (Family). We called this model the ‘survival model’. The multilevel structure of the survival model allowed us to account for both fixed effects (the average effect of Temperature and Age across all Families) and random effects (Family-specific deviations from the average effect). This and all subsequent Bayesian models were performed using the programming language R 55 and the “brms” package56. A comprehensive mathematical specification of the model, along with details on convergence and model evaluation, is provided in the Supplementary Information (Supplementary Information: Survival Model).
The posterior distribution of parameter values from the survival model was used to calculate the distribution of the median lethal temperature (LT50) across all Age groups and Families. This method allowed us to make specific calculations for different Family groups as well as propagating the uncertainty of parameter values into our predictions. See supplementary information for a more detailed description of the calculations and mathematical specifications for the calculation of LT50 (Supplementary Information: Median Lethal Temperature).
For sub-adults, survival functions (time-to-death analysis) were also performed for daily survival data and were computed according to Kaplan and Meier57, using the “Survival” R package58. The Chi-square test was used to identify the effect of Family and Temperature on the time-to-death data. Survival time curves were compared using the Cox regression model 59 after adjusting for Temperature and Family, excluding survival data from the 39 °C treatment, because mussels from all families died at the second day of recovery, impeding the multiple comparisons analysis. The proportionality of hazards (PH) was checked based on Schoenfeld residuals60.
Juvenile mussel size
Mussel size for each age group (6, 8 and 10-week-old mussels) was analysed to determine size differences among families. Size data for 6-week-old mussels were analysed using a one-way ANOVA, while size data for 8 and 10-week-old mussels were analysed using Kruskal–Wallis ANOVA on Ranks test as these data violated normality and homoscedasticity (Shapiro–Wilk and Brown-Forsythe tests, respectively). Significant differences among group means were identified using Tukey LSD pair-wise comparisons and Dunn’s tests (α = 0.05)61. These statistical analyses were performed using Sigma Plot 14.0 software (SYSTAT Software, Inc.).
Mussel size data obtained from live and dead juveniles observed at 34 °C (the vial temperature that most frequently contained both live and dead mussels) were used to determine the effect of shell size on mussel survival. To understand how size impacts survival once the effect of ontogeny is accounted for, a Bayesian multilevel model assuming the observed outcome followed a Bernoulli distribution was developed. The model considered the probability of survival as a function of shell size (Size) and ontogeny (Age), allowing each parameter to vary by the genetic lineage of each individual (Family). We denoted this model the ‘size model’. Additionally, we compared the size model to less complex models to understand if the covariates used were necessary to understand survival. Details on the mathematical specification of the size model, model comparison, evaluation and diagnostics can be found in the Supplementary Information (Supplementary Information: Size Model).
Hsp70 gene expression in sub-adults
Data for the gene expression of sub-adults were log10 transformed prior to analysis to meet analysis of variance assumptions. Data were then analysed with a two-way ANOVA using family and treatment (control/heat shock) as factors. Significant differences among group means were identified using Tukey LSD pair-wise comparisons (α = 0.05)61. These statistical analyses were performed using Sigma Plot 14.0 software (SYSTAT Software, Inc.). Additionally, a simple linear regression was performed to identify a correlation between mean hsp70 expression and mean LT50 values obtained for each family in sub-adult mussels.
Figure 1 was created in MATLAB (version 2021a) using the maximum sea surface temperatures (SST) over 1 Jan 2003 to 1 Jan 2024, as observed from single daily measurements from NASA’s MODIS-Aqua sensor and processed by NASA’s Ocean Biology Processing Group (OBPG). The underlying Level 3 data corresponds to the standard daily SST product at 4 km resolution, which was sourced from NASA’s Ocean Colour web portal. Remaining figures and plots were generated using the programming language R55 and Sigma Plot 14.0 software (SYSTAT Software, Inc.).
Results
Juvenile and sub-adult survival
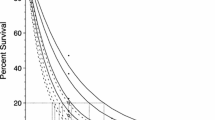
Survival of the juvenile mussels following a three-hour thermal challenge and a 44-h recovery period showed similar trends for all ages in all families (Fig. 2). The survival model explained a high proportion of the variance of the data (Bayesian R-squared = 0.90). Model predictions of survival of juvenile and sub-adult mussels following the three-hour thermal challenge showed that family group could strongly influence their response to temperature (survival model results shown in supplementary Table S1). In particular, a distinct separation among families was observed at 10 weeks and 52 weeks post-fertilization (Fig. 2).
Logistic curves fitted to the predicted survival for Perna canaliculus of different ages (6, 8, 10 and 52 weeks post-fertilisation) and six genetically diverse families (A-F). Mussels were exposed to a 3-h thermal challenge followed by recovery for 44 h at 18 °C (6, 8 and 10 weeks old mussels) or 14 days (52 weeks old mussels) at 19 °C. Solid coloured points represent observed survival rates during the experiment, while solid coloured lines and shaded areas represent the mean and 95% Percentile Interval (PI) of the posterior distribution of the survival model, respectively. Dashed black line demarks the 50% probability of survival.
The time-to-death analysis performed on 52-week-old mussels showed a significant survival effect due to family and temperature (Chi-square = 2171, df = 35, p < 0.001). A pairwise comparison matrix of survival curves is shown in supplementary Table S2. Generally, all families performed similarly at each temperature aside from Family C (Fig. 3), with survival at high temperatures (≥ 33 °C) being significantly different to survival at low temperatures (≤ 31 °C; Fig. 3, supplementary Table S2). A heat challenge at 31 °C and 33 °C had the strongest discriminating effects on survival when comparing different families (Fig. 3). At 31 °C, Family C showed significantly lower survival estimates compared to all the other families (p < 0.001). At 33 °C, most mussels died within the first seven days of recovery (Fig. 3), except for Families A, B and E, in which some mussels were alive at the end of the recovery period.
Survival time curves of six genetically diverse mussel families (A-F) at 52 weeks of age. Mussels experienced a three-hour heat challenge at different temperatures (25–39 °C). Curves were separated by family to aid with graph interpretation. Significant differences (p < 0.05) among temperature groups within each family are denoted by lower-case letters. Detailed pairwise comparison matrix is shown in supplementary Table S4.
Median lethal temperature (LT50)
Predictions of the LT50 using the survival model fitted to experimental data showed that both genetic group and ontogeny determined thermal tolerance in mussels (Fig. 4). Specifically, predictions showed that there were distinct differences in thermal tolerance among families as mussels aged, as can be seen in the lack of overlap in some predictive distributions in Fig. 4. Generally, 52-week-old mussels exhibited lower thermal tolerance compared to younger mussels, with the difference being most pronounced when compared to 6-week-old individuals. However, the effect of age in thermal tolerance was mediated by the family that the mussels belong to. For instance, Family F showed the highest LT50 when mussels were 10 weeks old, which was 3 °C higher than the lowest LT50, corresponding to Family C when mussels were 52 weeks old (Table 3).
Median lethal temperature (LT50) distributions from six genetically distinct families (A-F) at different ages (6, 8, 10 and 52 weeks post-fertilisation). Shaded areas show the probability density function of predicted LT50 values for each family, differentiated by age group. Solid points denote the mean predicted LT50 for each group. Thick horizontal lines denote the 66% percentile interval while thin horizontal lines extend up to the 95% percentile interval of the distribution of LT50 values.
Correlations of mean estimated LT50 values and mean shell length in mussels from different age groups showed varied relationships (Fig. 5). For 6- and 8-week-old juveniles, there was a strong positive relationship between mean LT50 and mean shell length (Fig. 5A, B), with larger families having slightly higher thermal tolerance compared to smaller families. In contrast, there was no evidence of a relationship between mean LT50 and mean shell length for 10- and 52-week-old mussels (Fig. 5C, D).
Juvenile mussel size
The size of the juvenile mussels varied considerably among and within families. Within the age groups, mussel size was significantly different among families (6 weeks old: F(5,469) = 15.984, p < 0.001; 8 weeks old: H = 48.636, d.f. = 5, p < 0.001; 10 weeks old: H = 135.320, d.f. = 5, p < 0.001), with families A and C being significantly larger than the other families (p < 0.05) (supplementary Figs. S1–S4).
While the size model was a relatively good fit to the data compared to less complex models (supplementary Table S3), the model explained relatively low levels of variability in the data (Bayesian R-squared = 0.05;62), suggesting that both shell size and developmental stage were necessary but not sufficient to accurately predict survival outcomes. Nonetheless, the size model provides insight into how size might affect survival probabilities under thermal stress (size model results shown in supplementary Table S4). The size model showed that on average, survival probability decreases with mussel size (Fig. 6). However, the model suggested that the effect of shell size is mediated by age, as older mussels tended to have higher survival probabilities, even compared to mussels of the same size but of different age groups (Fig. 6). Importantly, model predictions indicated that survival probabilities consistently decreased with increasing shell size across all families (Fig. 6).
Survival probability as a function of shell size of Perna canaliculus of different ages (6, 8 and 10 weeks post-fertilisation) and from six genetically diverse families (A-F). Mussels were exposed to a three-hour thermal challenge at 34 °C followed by recovery for 44 h at 18 °C. Solid coloured points denote the mean observed survival rates at different size ranges in the experiments. Solid coloured lines and shaded areas denote the mean and 95% percentile interval of the posterior predictions of the size model, respectively. Each panel shows data and predictions for different developmental stages (A): 6 weeks post-fertilisation; (B): 8 weeks post-fertilisation; (C): 10 weeks post-fertilisation). Each plot within a panel corresponds to a different family as denoted on the labels.
Histological assessment of sub-adults
Histological assessment of sub-adult mussels (52 weeks post-fertilisation) showed that 40–100% of individuals from the different families were reproductively mature. Family A appeared the least developed, with 40% of mussels mature; in contrast, Family C and E showed all the assessed mussels to be in a mature stage. Sixty percent of mussels from Family F were mature, while Families B and D showed 80% maturity.
Hsp70 gene expression of sub-adults
The expression of the inducible hsp70 gene in gill tissue from the sub-adult mussels showed a significant interaction between family and treatment (F(5,45) = 3.740, p = 0.006; Fig. 7), with main effects of family and treatment also being statistically significant (Family: F(5,45) = 3.74, p = 0.006; Treatment: F(1,45) = 592.97, p < 0.001). Heat-challenged mussels showed a sixfold increase in hsp70 gene expression compared to control mussels (p < 0.001; Fig. 7). At the same time, family E and F had a significantly higher (approximately double) hsp70 gene expression after exposure to the heat challenge compared to families A (p = 0.016 and p = 0.022, respectively) and C (p < 0.001 for both comparisons) under the same conditions (Fig. 7).
Hsp70 gene expression variability in sub-adult Perna canaliculus from six genetically diverse families (A-F). Mussels were exposed to a three-hour thermal challenge at 31 °C followed by recovery for 1 h at 19 °C. Elongation factor-1 (EF1) was used as the reference gene for data normalisation. Solid and dotted lines within boxes show the median and mean value for each box and whiskers, respectively. Significant differences (p < 0.05) are denoted with lower-case letters above bars.
Tissue sampling for hsp70 expression was lethal, so direct correlation between gene expression and survival outcome could not be established. A positive linear correlation was, however, apparent between mean family LT50 and mean hsp70 expression levels following heat challenge (r2 = 0.530; Fig. 8).
Discussion
Effects of ontogeny and size on thermal tolerance
This study showed that ontogeny and size influence thermal tolerance in the green-lipped mussel, Perna canaliculus, with early juveniles showing greater heat tolerance compared to sub-adult mussels. Ontogeny has been demonstrated to be a factor influencing thermal tolerance in ectotherms including marine invertebrates. A recent meta-analysis found that early life stages of marine and terrestrial ectothermic species are more vulnerable to rising temperatures, and that aquatic ectotherms are more than three times more plastic than terrestrial ectotherms63. For example, early life stages of marine invertebrates such as the porcelain crab Petrolisthes cinctipes64, the dogwhelk Nucella ostrina65, the barnacle Balanus glandula65, and the mussel Mytilus trossulus65, can be more sensitive to temperature compared to later life stages. However, as in this study, there are exceptions where early stages have shown to be more thermotolerant than older individuals, as it has been shown in another porcelain crab, P. manimaculis64, the clam Laternula elliptica66, the sea cucumber Cucumaria georgiana66, the sea urchin Sterechinus neumayeri66, the seastar Odontaster validus66 and the barnacle Chthamalus dalli65; although the lower tolerance of adult C. dalli was attributed to active mating and the energetic costs associated with reproduction65.
The lower thermal tolerance observed in sub-adult mussels in this study could be attributed to the reproductive status of these mussels as the majority were shown to be reproductively active. In this respect, it has been shown that mature and reproductively active marine organisms have narrower thermal windows compared to other life stages of the same species14,65. This difference in thermal tolerance between juveniles and adults has also been suggested to result from the different aerobic scope between life stages, mainly attributed to organismal size66. Increasing temperatures reduce the aerobic scope faster in larger organisms compared to smaller animals66. Also, the oxygen supply in larger organisms is more likely to be diffusion limited, with a longer path from the site of uptake to the mitochondria, promoting a transition to anaerobic metabolism earlier than in smaller individuals66,67. These findings suggest that older (and therefore generally larger) animals will have a lower thermal tolerance compared to smaller, younger conspecifics.
Critically, individual size had an important role in determining the tolerance of mussels to thermal stress within each juvenile age group. In the present study, differences in sizes among families were evident, with some families being considerably larger than others of the same age, but there was also a large within-family size variation. At an early age (6 and 8 weeks post-fertilisation), families with larger juveniles seem to be more thermotolerant than families with smaller juveniles, however, this relationship changes when mussels reach 10 weeks post-fertilisation. For example, the skewed size-frequency distribution was most apparent in the 10-week-old juveniles (supplementary Fig. S3), where a small subset of individuals within each family displayed exceptional growth; all of these individuals were killed by a 34 °C challenge. In addition to the hypothesis described above regarding a shift to anaerobic metabolism, oxygen limitation also plays an important role in defining thermal tolerance. Oxygen availability becomes a limiting resource as oxygen solubility decreases in warmer water, and higher metabolism due to warmer temperatures increases oxygen demand in ectothermic species18,68. The increased oxygen demand relative to supply reduces the aerobic scope, especially in larger individuals, resulting in a greater metabolic stress affecting physiological performance69. A recent example of this was reported for the ragworm Hediste diversicolor, where larger individuals had lower thermal tolerance70.
It is also possible that larger mussels from a particular family may consume greater amounts of food71. A greater consumption of food can result in a more active metabolism, requiring larger energy expenditure to support basic functions, with ATP being the most common energy carrier in cells72,73. Higher ATP synthesis results in a higher production of reactive oxygen species (ROS) due to normal metabolism72,74,75. Subsequently, when larger individuals are exposed to stress, an overproduction of ROS could be expected, possibly overwhelming the cellular redox balance faster than in smaller individuals of the same age and exposed to the same stressful condition. This ROS overproduction hypothesis would require a targeted experimental design to demonstrate that larger mussels of the same age are more vulnerable to heat stress due to their higher metabolic rate and ROS production. While the best fit model (supplementary Table S3) showed that increments in size reduce survival probabilities for all families, our model suggests that survival probabilities can vary widely and are not entirely predicted by size and age (Fig. 6). This highlights the need to further understand the drivers of thermal tolerance in juvenile Perna canaliculus.
Genetic effects
Thermal tolerance (LT50) of Perna canaliculus juveniles and sub-adults varied among families, strongly suggesting a genetic effect76. However, there were few differences among families in thermal tolerance for younger (6 and 8 weeks post-fertilisation) juveniles, with family effects becoming apparent by 10 weeks post-fertilisation. At 54 weeks post-fertilisation, the interaction between family and age was more evident, with Family C being considerably less heat tolerant than the other families.
In this study, juvenile mussels showed higher tolerance to heat, with greater LT50s than sub-adult mussels. Sub-adult mussels were obtained from a sea-based farm after undergoing a full year of field conditions. It seems reasonable to suggest that natural selection of more resilient individuals would have occurred, in addition to acclimation or ‘hardening off’ in a variable thermal environment. The observed differences between juvenile and sub-adult resilience may therefore be modified if all individuals experience similar thermal histories prior to assessment, but this remains to be investigated.
Resilience of marine organisms is crucial to understand how the organisms may cope with environmental changes, especially under the current environmental pressures driven by climate change. In this context, investigating the genetics of marine organisms and the opportunities around breeding for resilience could help mitigate the potential effects of future climate change on culturally, economically and environmentally important marine species. Resilient genotypes are more likely to be able to cope with changes in the environment, increasing the likely persistence of the populations under future climate change conditions.
Genetic variability, including resilient and less tolerant genotypes, and phenotypic plasticity is fundamentally important to the adaptation of species38. Studies that include a genetic component such as the one investigated here, allow the adaptation potential of a species to be tested in laboratory conditions, allowing for the estimation of heritability and genetic variability in a particular population77. Estimation of heritability and genetic correlations with other traits (e.g., growth rate) were beyond the scope of this study, therefore, the confounding effects of early life history and other traits (e.g., shell length) could not be ruled out as factors influencing the observed differences in thermal resilience among families. Nonetheless, a plastic response of the different genotypes was observed in this study.
It is important that future studies focus on full quantitative genetic analyses comparing a larger number of families and considering the effects of both age and individual size in combination, which would allow better heritability estimates to determine if thermal tolerance is a heritable trait in P. canaliculus. Future studies should also investigate the degree of environmental sensitivity of a particular genotype by studying genotype-by-environment interactions78,79. The findings in this study are consistent with the hypothesis that there is genetic variation in the thermal resilience of mussels and therefore potential to selectively breed for this important trait. Additionally, it is important to consider the effects of non-genetic heritability such as epigenetics effects and transgenerational plasticity in P. canaliculus which might also be influenced by the genetic makeup of a particular family.
Molecular response: hsp70 expression
The hsp70 gene expression in sub-adult P. canaliculus showed that mussels from all families differentially upregulated this gene after being exposed to an acute heat stress. The lowest upregulation of hsp70 was observed in Family C, which aligns with reduced survival and LT50 results obtained for the same family, and a significant linear relationship is apparent between mean hsp70 upregulation and mean family LT50 for the 52-week-old mussels. This implicates a relationship between upregulation of the hsp70 gene and survival during thermal stress in P. canaliculus. Several other studies have related the increase in thermal tolerance in marine species with the upregulation of the hsp70 gene or an increase in the synthesis of the HSP70 protein25,80,81,82,83.
The hsp70 gene measured in this study corresponds to the inducible form of the HSP70 protein53. Chaperone proteins that are part of the HSP70 family have different regulatory patterns84. Strictly inducible proteins are absent or have low basal levels of expression under normal conditions, but are significantly upregulated under stressful conditions; constitutively expressed and moderately inducible proteins, which are expressed under normal conditions and moderately upregulated under stressful conditions; and solely constitutively expressed, which are proteins that are less stress-dependent84. Constitutive (hsc70) and inducible (hsp70) forms perform different roles, with the constitutive form being continuously expressed to maintain cellular homeostasis, and the inducible form being produced in response to external stressors82. Nonetheless, in other species such as Pseudodiamesa branickii (Diptera Chironomidae), larvae were able to upregulate both the constitutive (hsc70) and inducible (hsp70) forms with increasing temperature and time of exposure85. The role of the constitutive and inducible HSP70 forms in bivalves remains to be investigated, in particular to their relationship with resilient phenotypes.
Baseline levels of hsp70 expression were similar across all families but showed differential up-regulation when exposed to stress. This suggests that measuring the hsp70 gene expression levels in individuals from different families that are exposed to stress could potentially be used as a screening tool for selection of the most thermally resilient genotypes. However, many other genes are involved in response to thermal stress (and other stressors) in mussels86,87,88. In the present study, only the hsp70 gene was evaluated and therefore it is important to investigate other genes that may be contributing to thermal resilience in P. canaliculus.
Climate change implications
Increasing air and ocean temperatures together with more frequent, intense marine heatwaves are a dramatic result of anthropogenic climate change1,2,3. In New Zealand, seawater temperatures are increasing by 0.1–0.3 °C per decade6,7, with reports of more regular marine heatwave events8,9. Climate change is modifying community structure and affecting the distribution of species89,90,91,92. Survival and adaptation of species to increasing seawater temperatures and heatwaves may be determined by the integrated thermal history of the species and the frequency of exposure to warming events. For instance, recurrent exposure to heat stress decreases thermal tolerance in the mussel Mytilus edulis93; while chronic heat stress decreased growth, survival and affected immune response94. Additionally, stress-on-stress responses could also determine and influence performance, survival and potential for adaptation to a new environment95.
Investigating how marine species respond to rises in temperature is critical to understanding the mechanisms used by organisms to cope with higher temperatures, and the potential for adaptation to a new environment. In the current study, ontogeny and genetics were found to play an important role in determining thermally-resilient phenotypes, shaping thermal performance in P. canaliculus. The upregulation of hsp70 was associated with an increased capacity of P. canaliculus to cope with heat stress, while larger individuals within a family showed greater vulnerability to an acute heat stress than smaller individuals. The higher thermal tolerance (LT50) values obtained for some mussel families tested in this study indicate that those families are likely physiologically better prepared to cope with challenging conditions such as marine heatwave events. The greater potential of some mussel families to withstand warmer conditions has direct relevance for the aquaculture industry and could be captured using selective breeding programmes. Although mussels in aquaculture farms grow in subtidal conditions, selective breeding for heat-tolerant mussels could still be implemented to create climate-hardened stocks, with consideration of the life stage (i.e., age) at which selection is applied to maximise trait expression and effectiveness of selection.
Genetic variation also plays a fundamental role in the adaptation of organisms to a new environment in the wild. Some organisms will be able to cope with stressful conditions better than others, influencing natural selection. As seawater temperatures increase, natural selection of the more tolerant individuals within a population is likely to occur. In the wild, mussels have been dramatically affected by higher temperatures, evidenced by more frequent summer mortality events93,96,97,98. Intertidal mussel populations are expected to experience higher thermal stress compared to subtidal mussels, with intertidal mussels likely having higher thermal tolerances, as has been reported for mussels and other marine species64,99,100. Nonetheless, climate change poses a threat to both intertidal and subtidal populations which must cope with significant environmental stress in future climate change scenarios101.
In this study, the mussels used for experiments were subtidal mussels and their thermal tolerance (LT50) ranged between 31.3 and 34.4 °C for different families and age groups. These temperatures are unlikely to occur in subtidal environments because of a marine heatwave, or in the coming decades due to long-term increases in seawater temperature. However, air and seawater temperatures can increase rapidly in New Zealand’s intertidal zone, exposing intertidal mussels to extreme heat stress. For instance, in rock pools, seawater temperature can reach 37 °C102, while air temperature can increase up to 11 °C in 30 min100. This study showed that thermal tolerance varied across genetic groups, suggesting there is genetic variation available for adaption to increased temperatures.
Thermal tolerance is influenced by a complex interplay of physiological processes, and organisms in the wild will be exposed to rising temperatures in conjunction with other stressors such as food limitation, changes in pH and salinity. Physiological performance together with behavioural responses and interactions with other species may affect how marine species respond to increasing temperatures103,104. The organismal response to multiple stressors can be different depending on the level and combination of stressors having synergistic, antagonistic or additive effects105. Therefore, it is premature to anticipate effects on survival in the wild and changes in distribution of this species in the natural environment, even though this study provides valuable information about the thermal tolerance of P. canaliculus. In depth knowledge of genomic, transcriptomic and epigenetic responses of P. canaliculus exposed to several stressors, including warmer temperatures, would allow the prediction of the distribution and survival of this highly valuable mussel species in the future.
Conclusion
This study provides insights into the thermal response of different life stages and genotypes of a representative marine mussel species (Perna canaliculus) in the laboratory. Thermal tolerance in this mussel was influenced by both genetics and age, with older and larger individuals being more thermally sensitive. Although the heritability of this trait is unknown, this study showed that selective breeding of P. canaliculus for thermal resilience may be possible. In mussel aquaculture, selective breeding programmes frequently breed for fast growth and meat yield; however, this study showed that faster growers from within any given family are more sensitive to acute thermal stress. The size effect found in this study indicates that the aquaculture industry should consider thermal sensitivity in future selective breeding programmes if within-family selection is being undertaken. Important trade-offs may also occur in natural populations, with the competitive advantages of individual juveniles displaying faster growth potentially incurring a predisposition to thermal stress. In the context of climate change, natural selection of more thermal-tolerant phenotypes is likely to become increasingly apparent as the oceans warm.
Future research should focus on quantifying the heritability of resilience traits and genetic correlations with other (e.g., production) traits, as well as a more comprehensive molecular performance of P. canaliculus under stress. Transcriptomic and genomic research of P. canaliculus exposed to several stressors, and at environmentally relevant temperatures, may better inform the predictability of response of this species in the context of global climate change.
Data availability
The datasets generated during/or analysed during the current study are available from the corresponding author on reasonable request.
References
IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, 2022).
Oliver, E. C. J. et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9, 1324. https://doi.org/10.1038/s41467-018-03732-9 (2018).
Oliver, E. C. J. et al. Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Mar. Sci. 6, 1–12. https://doi.org/10.3389/fmars.2019.00734 (2019).
Schiel, D. R. The other 93%: trophic cascades, stressors and managing coastlines in non-marine protected areas. NZ J. Mar. Freshwat. Res. 47, 374–391 (2013).
Schiel, D. R., Lilley, S. A., South, P. M. & Coggins, J. H. J. Decadal changes in sea surface temperature, wave forces and intertidal structure in New Zealand. Mar. Ecol. Prog. Ser. 548, 77–95 (2016).
Shears, N. T. & Bowen, M. M. Half a century of coastal temperature records reveal complex warming trends in western boundary currents. Sci. Rep. 7, 14527. https://doi.org/10.1038/s41598-017-14944-2 (2017).
Sutton, P. J. H. & Bowen, M. Ocean temperature change around New Zealand over the last 36 years. NZ J. Mar. Freshwat. Res. 53, 305–326. https://doi.org/10.1080/00288330.2018.1562945 (2019).
Salinger, M. J. et al. The unprecedented coupled ocean-atmosphere summer heatwave in the New Zealand region 2017/18: drivers, mechanisms and impacts. Environ. Res. Lett. 14, 044023. https://doi.org/10.1088/1748-9326/ab012a (2019).
Salinger, M. J. et al. Unparalleled coupled ocean-atmosphere summer heatwaves in the New Zealand region: drivers, mechanisms and impacts. Clim. Change 162, 485–506 (2020).
Behrens, E. et al. Projections of future marine heatwaves for the oceans around New Zealand using New Zealand’s earth system model. Front. Clim. 4, 798287. https://doi.org/10.3389/fclim.2022.798287 (2022).
Hill, R. W. Animal Physiology 2nd edn. (Sinauer Associates, 2008).
Schmidt-Nielsen, K. Animal Physiology (Cambridge University Press, 1997).
Monaco, C. J. & Helmuth, B. Tipping points, thresholds and the keystone role of physiology in marine climate change research. Adv. Mar. Biol. 60, 123. https://doi.org/10.1016/b978-0-12-385529-9.00003-2 (2011).
Pörtner, H. O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008).
Sokolova, I. M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Compar. Biol. 53, 597–608. https://doi.org/10.1093/icb/ict028 (2013).
Sokolova, I. M., Frederich, M., Bagwe, R., Lannig, G. & Sukhotin, A. A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 79, 1–15. https://doi.org/10.1016/j.marenvres.2012.04.003 (2012).
Willmer, P. Environmental Physiology of Animals (Blackwell Publisher, 1999).
Pörtner, H. O. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Compar. Biochem. Physiol. Part A 132, 739–761. https://doi.org/10.1016/s1095-6433(02)00045-4 (2002).
Pörtner, H. O., Peck, L. & Somero, G. Thermal limits and adaptation in marine antarctic ectotherms: an integrative view. Philos. Trans. Biol. Sci. 362, 2233–2258. https://doi.org/10.2307/20210022 (2007).
Ibarrola, I., Hilton, Z. & Ragg, N. L. Physiological basis of inter-population, inter-familiar and intra-familiar differences in growth rate in the green-lipped mussel Perna canaliculus. Aquaculture 479, 544–555 (2017).
Ericson, J. A. et al. Differential responses of selectively bred mussels (Perna canaliculus) to heat stress -survival, immunology, gene expression and microbiome diversity. Front. Physiol. https://doi.org/10.3389/fphys.2023.1265879 (2023).
Evgen’ev, M. B., Garbuz, D. G. & Zatsepina, O. G. Heat Shock Proteins and Whole Body Adaptation to Extreme Environments (Springer, 2014).
Sanders, B. M. Stress proteins in aquatic organisms: An environmental perspective. Critic. Rev. Toxicol. 23, 49–75. https://doi.org/10.3109/10408449309104074 (1993).
Sørensen, J. G., Kristensen, T. N. & Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. https://doi.org/10.1046/j.1461-0248.2003.00528.x (2003).
Oksala, N. K. J. et al. Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol. 3, 25–28. https://doi.org/10.1016/j.redox.2014.10.003 (2014).
Chen, B., Feder, M. E. & Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 27, 3040–3054. https://doi.org/10.1111/mec.14769 (2018).
Feder, M. E. & Hofmann, G. E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. https://doi.org/10.1146/annurev.physiol.61.1.243 (1999).
Parsell, D. A. & Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genetics 27, 437 (1993).
Tomanek, L. & Somero, G. N. Evolutionary and acclimation- induced variation in the heat- shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: Implications for limits of thermotolerance and biogeography. J. Exp. Biol. 202, 2925–2936 (1999).
Jost, J. A., Podolski, S. M. & Frederich, M. Enhancing thermal tolerance by eliminating the pejus range: A comparative study with three decapod crustaceans. Mar. Ecol. Progr. Ser. 444, 263–274 (2012).
Bayne, B. L. Primary and secondary settlement in Mytilus edulis L. (Mollusca). J. Anim. Ecol. 33, 513–523 (1964).
Jeffs, A. G., Holland, R. C., Hooker, S. H. & Hayden, B. J. Overview and bibliography of research on the greenshell mussel, Perna canaliculus, from New Zealand waters. J. Shellfish Res. 18, 347–360 (1999).
McLeod, I. M., Parsons, D. M., Morrison, M. A., Van Dijken, S. G. & Taylor, R. B. Mussel reefs on soft sediments: a severely reduced but important habitat for macroinvertebrates and fishes in New Zealand. New Zealand J. Mar. Freshwat. Res. 48, 48–59. https://doi.org/10.1080/00288330.2013.834831 (2014).
Menge, B. A., Daley, B. A., Sanford, E., Dahlhoff, E. P. & Lubchenco, J. Mussel zonation in New Zealand: An integrative eco-physiological approach. Mar. Ecol. Progr. Ser. 345, 129–140 (2007).
Chaput, R. et al. Identifying the source populations supplying a vital economic marine species for the New Zealand aquaculture industry. Sci. Rep. 13, 9344 (2023).
Camara, M. D. & Symonds, J. E. Genetic improvement of New Zealand aquaculture species: Programmes, progress and prospects. New Zealand J. Mar. Freshwat. Res. 48, 466–491. https://doi.org/10.1080/00288330.2014.932291 (2014).
Liu, J. et al. Genomic selection applications can improve the environmental performance of aquatics: A case study on the heat tolerance of abalone. Evolut. Appl. 15, 992–1001. https://doi.org/10.1111/eva.13388 (2022).
Bell, G. & Collins, S. Adaptation, extinction and global change. Evolut. Appl. 1, 3–16. https://doi.org/10.1111/j.1752-4571.2007.00011.x (2008).
Kelly, M. Adaptation to climate change through genetic accommodation and assimilation of plastic phenotypes. Philos. Trans R. Soc. B Biol. Sci. 374, 20180176. https://doi.org/10.1098/rstb.2018.0176 (2019).
Dunphy, B. J., Ragg, N. L. C. & Collings, M. G. Latitudinal comparison of thermotolerance and HSP70 production in F2 larvae of the greenshell mussel (Perna canaliculus). J. Exp. Biol. 216, 1202–1209. https://doi.org/10.1242/jeb.076729 (2013).
Dunphy, B. J., Watts, E. & Ragg, N. L. C. Identifying thermally-stressed adult green-lipped mussels (Perna canaliculus Gmelin, 1791) via metabolomic profiling. Am. Malacol. Bull. 33, 127–135. https://doi.org/10.4003/006.033.0110 (2015).
Ragg, N. L. C., King, N., Watts, E. & Morrish, J. Optimising the delivery of the key dietary diatom Chaetoceros calcitrans to intensively cultured Greenshell™ mussel larvae Perna canaliculus. Aquaculture 306, 270–280. https://doi.org/10.1016/j.aquaculture.2010.05.010 (2010).
Delorme, N. J. & Sewell, M. A. Temperature limits to early development of the New Zealand sea urchin Evechinus chloroticus (Valenciennes, 1846). J. Therm. Biol. 38, 218–224. https://doi.org/10.1016/j.jtherbio.2013.02.007 (2013).
Sewell, M. A. & Young, C. M. Temperature limits to fertilization and early development in the tropical sea urchin Echinometra lucunter. J. Exp. Mar. Biol. Ecol. 236, 291–305. https://doi.org/10.1016/S0022-0981(98)00210-X (1999).
Webb, S. C. & Heasman, K. G. Evaluation of fast green uptake as a simple fitness test for spat of Perna canaliculus (Gmelin, 1791). Aquaculture 252, 305–316. https://doi.org/10.1016/j.aquaculture.2005.07.006 (2006).
Broekhuizen, N., Plew, D. R., Pinkerton, M. H. & Gall, M. G. Sea temperature rise over the period 2002–2020 in Pelorus Sound, New Zealand – with possible implications for the aquaculture industry. New Zealand J. Mar. Freshwat. Res. 55, 46–64. https://doi.org/10.1080/00288330.2020.1868539 (2021).
Copedo, J. S. et al. Histopathological changes in the greenshell mussel, Perna canaliculus, in response to chronic thermal stress. J. Therm. Biol. 117, 103699. https://doi.org/10.1016/j.jtherbio.2023.103699 (2023).
Alfaro, A. C., Jeffs, A. G. & Hooker, S. H. Reproductive behavior of the green-lipped mussel, Perna canaliculus, in northern New Zealand. Bull. Mar. Sci. 69, 1095–1108 (2001).
Howard, D. W. Histological techniques for marine bivalve mollusks and crustaceans. Vol. 5 (NOAA, National Ocean Service, National Centers for Coastal Ocean Service, Center for Coastal Environmental Helath and Biomolecular Research, 2004).
Dunphy, B. J., Ruggiero, K., Zamora, L. N. & Ragg, N. L. C. Metabolomic analysis of heat-hardening in adult green-lipped mussel (Perna canaliculus): A key role for succinic acid and the GABAergic synapse pathway. J. Therm. Biol. 74, 37–46. https://doi.org/10.1016/j.jtherbio.2018.03.006 (2018).
Webb, S. C. Aspects of stress with particular reference to mytilid mussels and their parasites PhD Thesis thesis, University of Cape Town, (1999).
Zamora, L. N. et al. Emersion survival manipulation in Greenshell™ mussels (Perna canaliculus): Implications for the extension of live mussels’ shelf-life. Aquaculture 500, 597–606. https://doi.org/10.1016/j.aquaculture.2018.10.057 (2019).
Baettig, C. G. et al. Development and validation of molecular biomarkers for the green-lipped mussel (Perna canaliculus). New Zealand J. Mar. Freshwat. Res., 1–20 (2023).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Statist. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
R Core Team, R. (R Foundation for Statistical Computing, Vienna, Austria, 2024).
Bürkner, P. An R package for Bayesian multilevel models using Stan. J. Statist. Softw. 80, 1–28 (2017).
Kaplan, E. L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Statist. Assoc. 53, 457–481. https://doi.org/10.2307/2281868 (1958).
Therneau, T. & Grambsch, P. M. Modeling Survival Data: Extending the Cox Model (Springer, 2000).
Cox, D. R. Regression nodels and life-tables. J. R. Statist. Soc. Ser. B 34, 187–202. https://doi.org/10.1111/j.2517-6161.1972.tb00899.x (1972).
Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 69, 239–241. https://doi.org/10.1093/biomet/69.1.239 (1982).
Underwood, A. J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance (Cambridge University Press, 1996).
Gelman, A., Goodrich, B., Gabry, J. & Vehtari, A. R-Squared for Bayesian Regression Models (The American Statistician, 2019).
Pottier, P. et al. Developmental plasticity in thermal tolerance: Ontogenetic variation, persistence, and future directions. Ecol. Lett. 25, 2245–2268. https://doi.org/10.1111/ele.14083 (2022).
Miller, N. A., Paganini, A. W. & Stillman, J. H. Differential thermal tolerance and energetic trajectories during ontogeny in porcelain crabs, genus Petrolisthes. J. Therm. Biol. 38, 79–85. https://doi.org/10.1016/j.jtherbio.2012.11.005 (2013).
Hamilton, H. J. & Gosselin, L. A. Ontogenetic shifts and interspecies variation in tolerance to desiccation and heat at the early benthic phase of six intertidal invertebrates. Mar. Ecol. Progr. Ser. 634, 15–28 (2020).
Peck, L. S., Souster, T. & Clark, M. S. Juveniles are more resistant to warming than adults in 4 species of Antarctic marine invertebrates. PLoS One 8, e66033. https://doi.org/10.1371/journal.pone.0066033 (2013).
Atkinson, D., Morley, S. A. & Hughes, R. N. From cells to colonies: At what levels of body organization does the ‘temperature-size rule’ apply?. Evol. Dev. 8, 202–214 (2006).
Pörtner, H. Climate change and temperature-dependent biogeography: Oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146 (2001).
Rubalcaba, J. G., Verberk, W., Hendriks, A. J., Saris, B. & Woods, H. A. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. USA 117, 31963–31968. https://doi.org/10.1073/pnas.2003292117 (2020).
Fernandes, J. F., Calado, R., Jerónimo, D. & Madeira, D. Thermal tolerance limits and physiological traits as indicators of Hediste diversicolor’s acclimation capacity to global and local change drivers. J. Therm. Biol. 114, 103577. https://doi.org/10.1016/j.jtherbio.2023.103577 (2023).
Fernández-Reiriz, M. J., Irisarri, J. & Labarta, U. Flexibility of physiological traits underlying inter-individual growth differences in intertidal and subtidal mussels Mytilus galloprovincialis. PLoS One 11, e0148245. https://doi.org/10.1371/journal.pone.0148245 (2016).
Berg, J. M., Tymoczko, J. L., Gatto, G. J. & Stryer, L. Biochemistry 9th edn, 1208 (W. H. Freeman and Company, 2019).
Randall, D. J., Burggren, W. & French, K. Eckert animal physiology 5th edn. (W. H. Freeman, 2002).
Loft, S., Astrup, A., Buemann, B. & Poulsen, H. E. Oxidative DNA damage correlates with oxygen consumption in humans. FASEB J. 8, 534–537. https://doi.org/10.1096/fasebj.8.8.8181672 (1994).
Yang, S. & Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 467, 1–12. https://doi.org/10.1007/s11010-019-03667-9 (2020).
Han, Z. et al. Heritability estimates for gonadal development traits and their genetic correlations with growth and heat tolerance traits in the Fujian oyster Crassostrea angulata. Front. Mar. Sci. 9, 986441. https://doi.org/10.3389/fmars.2022.986441 (2022).
Hoffmann, A. A. & Sgro, C. M. Climate change and evolutionary adaptation. Nature 470, 479 (2011).
Falconer, D. S. & Mackay, T. F. C. Introduction to Quantitative Genetics (Oliver & Boyd, 1996).
Evans, M. L., Neff, B. D. & Heath, D. D. Quantitative genetic and translocation experiments reveal genotype-by-environment effects on juvenile life-history traits in two populations of Chinook salmon (Oncorhynchus tshawytscha). J. Evol. Biol. 23, 687–698. https://doi.org/10.1111/j.1420-9101.2010.01934.x (2010).
Dong, Y. & Dong, S. Induced thermotolerance and expression of heat shock protein 70 in sea cucumber Apostichopus japonicus. Fish. Sci. 74, 573–578. https://doi.org/10.1111/j.1444-2906.2008.01560.x (2008).
Aleng, N. A., Sung, Y. Y., MacRae, T. H. & Abd Wahid, M. E. Non-lethal heat shock of the Asian green mussel, Perna viridis, promotes Hsp70 synthesis, induces thermotolerance and protects against Vibrio infection. PLoS One 10, e0135603. https://doi.org/10.1371/journal.pone.0135603 (2015).
Valenzuela-Castillo, A., Sánchez-Paz, A., Castro-Longoria, R., López-Torres, M. & Grijalva-Chon, J. Hsp70 function and polymorphism, its implications for mollusk aquaculture: A review. Latin Am. J. Aquat. Res. 47, 224–231. https://doi.org/10.3856/vol47-issue2-fulltext-2 (2019).
Sung, Y. Y., Liew, H. J., Ambok Bolong, A. M., Abdul Wahid, M. E. & MacRae, T. H. The induction of Hsp70 synthesis by non-lethal heat shock confers thermotolerance and resistance to lethal ammonia stress in the common carp, Cyprinus carpio (Linn.). Aquac. Res. 45, 1706–1712 (2014).
Yusof, N. A. et al. Can heat shock protein 70 (HSP70) serve as biomarkers in Antarctica for future ocean acidification, warming and salinity stress?. Polar Biol. 45, 371–394. https://doi.org/10.1007/s00300-022-03006-7 (2022).
Bernabò, P., Rebecchi, L., Jousson, O., Martínez-Guitarte, J. L. & Lencioni, V. Thermotolerance and hsp70 heat shock response in the cold-stenothermal chironomid Pseudodiamesa branickii (NE Italy). Cell Stress Chaperones 16, 403–410. https://doi.org/10.1007/s12192-010-0251-5 (2011).
Negri, A. et al. Transcriptional response of the mussel Mytilus galloprovincialis (Lam.) following exposure to heat stress and copper. PLoS One 8, e66802. https://doi.org/10.1371/journal.pone.0066802 (2013).
Nielsen, M. B., Vogensen, T. K., Thyrring, J., Sørensen, J. G. & Sejr, M. K. Freshening increases the susceptibility to heat stress in intertidal mussels (Mytilus edulis) from the Arctic. J. Anim. Ecol. 90, 1515–1524. https://doi.org/10.1111/1365-2656.13472 (2021).
Barrett, N. J. et al. Molecular responses to thermal and osmotic stress in arctic intertidal mussels (Mytilus edulis): The limits of resilience. Genes 13, 155. https://doi.org/10.3390/genes13010155 (2022).
Beaugrand, G., Reid, P. C., Ibañez, F., Lindley, J. A. & Edwards, M. Reorganization of north Atlantic marine copepod biodiversity and climate. Science 296, 1692–1694. https://doi.org/10.1126/science.1071329 (2002).
Drinkwater, K. F. et al. On the processes linking climate to ecosystem changes. J. Mar. Syst. 79, 374–388. https://doi.org/10.1016/j.jmarsys.2008.12.014 (2010).
Chevaldonne, P. & Lejeusne, C. Regional warming-induced species shift in north-west Mediterranean marine caves. Ecol. Lett. 6, 371–379 (2003).
Antao, L. H. et al. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 4, 927–933 (2020).
Seuront, L., Nicastro, K. R., Zardi, G. I. & Goberville, E. Decreased thermal tolerance under recurrent heat stress conditions explains summer mass mortality of the blue mussel Mytilus edulis. Sci. Rep. 9, 17498. https://doi.org/10.1038/s41598-019-53580-w (2019).
Ericson, J. A. et al. Chronic heat stress as a predisposing factor in summer mortality of mussels Perna canaliculus. Aquaculture 564, 738986. https://doi.org/10.1016/j.aquaculture.2022.738986 (2023).
Delorme, N. J. et al. Stress-on-stress responses of a marine mussel (Perna canaliculus): Food limitation reduces the ability to cope with heat stress in juveniles. Mar. Ecol. Progr. Ser. 644, 105–117 (2020).
Li, S., Alfaro, A. C., Nguyen, T. V., Young, T. & Lulijwa, R. An integrated omics approach to investigate summer mortality of New Zealand Greenshell™ mussels. Metabolomics 16, 1–16 (2020).
Tremblay, R., Myrand, B., Sevigny, J.-M., Blier, P. & Guderley, H. Bioenergetic and genetic parameters in relation to susceptibility of blue mussels, Mytilus edulis (L.) to summer mortality. J. Exp. Mar. Biol. Ecol. 221, 27–58 (1998).
Capelle, J. J., Garcia, A. B., Kamermans, P., Engelsma, M. Y. & Jansen, H. M. Observations on recent mass mortality events of marine mussels in the Oosterschelde, the Netherlands. Aquac. Int. 29, 1737–1751 (2021).
Somero, G. N. Thermal physiology and vertical zonation of intertidal animals: Optima, limits, and costs of living. Integr. Comp. Biol. 42, 780–789. https://doi.org/10.1093/icb/42.4.780 (2002).
Sorte, C. J. et al. Thermal tolerance limits as indicators of current and future intertidal zonation patterns in a diverse mussel guild. Mar. Biol. 166, 1–13 (2019).
Petes, L. E., Menge, B. A. & Murphy, G. D. Environmental stress decreases survival, growth, and reproduction in New Zealand mussels. J. Exp. Mar. Biol. Ecol. 351, 83–91 (2007).
Trowbridge, C. D. Life at the edge: Population dynamics and salinity tolerance of a high intertidal, pool- dwelling ascoglossan opisthobranch on New Zealand rocky shores. J. Exp. Mar. Biol. Ecol. 182, 65–84. https://doi.org/10.1016/0022-0981(94)90211-9 (1994).
Olabarria, C. et al. Response of two mytilids to a heatwave: The complex interplay of physiology, behaviour and ecological interactions. PloS One 11, e0164330 (2016).
Morón Lugo, S. C. et al. Warming and temperature variability determine the performance of two invertebrate predators. Sci. Rep. 10, 6780. https://doi.org/10.1038/s41598-020-63679-0 (2020).
Folt, C. L., Chen, C. Y., Moore, M. V. & Burnaford, J. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877 (1999).
Acknowledgements
This study was funded by the New Zealand Ministry of Business, Innovation and Employment under the Cawthron Shellfish Aquaculture Research Platform (ShARP; Contract CAWX1801). We thank Rodney Roberts, the SPATnz team (Luke Johnston and Andy Day), and BreedCo for supplying all mussels for experimentation. We also thank Joanna Copedo for histological assessments, and to Dayanitha Damodaran, Mena Welford, Martin Cheng and all Cawthron staff for their invaluable support during the experiments.
Funding
This study was funded by the New Zealand Ministry of Business, Innovation and Employment under the Cawthron Shellfish Aquaculture Research Platform (Contract CAWX1801).
Author information
Authors and Affiliations
Contributions
N.J.D. concenptualise the experiment and design, carried out experiments, analysed data and wrote the main manuscript text. N.K., A.C.L., J.A.E. and N.L.C.R. contributed with data analysis. P.M.S., L.N.Z. and J.A.E. contributed performing experiments. C.G.B. and K.F.S. performed molecular analysis. B.R.K. performed environmental data modelling for Figure 1. All co-authors contributed to the revision of drafts and final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Delorme, N.J., King, N., Cervantes-Loreto, A. et al. Genetics and ontogeny are key factors influencing thermal resilience in a culturally and economically important bivalve. Sci Rep 14, 19130 (2024). https://doi.org/10.1038/s41598-024-70034-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70034-0
- Springer Nature Limited