Abstract
Permanent residence at high-altitude and chronic mountain sickness (CMS) may alter the cerebrovascular homeostasis and orthostatic responses. Healthy male participants living at sea-level (LL; n = 15), 3800 m (HL3800m; n = 13) and 5100 m (HL5100m; n = 17), respectively, and CMS highlanders living at 5100 m (n = 31) were recruited. Middle cerebral artery mean blood flow velocity (MCAv), cerebral oxygen delivery (CDO2), mean blood pressure (MAP), heart rate variability and spontaneuous cardiac baroreflex sensitivity (cBRS) were assessed while sitting, initial 30 s and after 3 min of standing. Cerebral autoregulation index (ARI) was estimated (ΔMCAv%baseline)/ΔMAP%baseline) in response to the orthostatic challenge. Altitude and CMS were associated with hypoxemia and elevated hemoglobin concentration. While sitting, MCAv and LFpower negatively correlated with altitude but were not affected by CMS. CDO2 remained preserved. BRS was comparable across all altitudes, but lower with CMS. Within initial 30 s of standing, altitude and CMS correlated with a lesser ΔMAP while ARI remained unaffected. After 3 min standing, MCAv, CDO2 and cBRS remained preserved across altitudes. The LF/HF ratio increased in HL5100m compared to LL and HL3800m from sitting to standing. In contrary, CMS showed blunted autonomic nervous activation in responses to standing. Despite altitude- and CMS-associated hypoxemia, erythrocytosis and impaired blood pressure regulation (CMS only), cerebral homeostasis remained overall preserved.
Similar content being viewed by others
Introduction
The Peruvian high-altitude city of La Rinconada, with an estimated population of 40,000–60,000 permanent inhabitants, is the highest city in the world and is situated at 5100–5300 m. Most of the residents migrated from the Altiplano region (~ 4000 m); however, despite their high-altitude origins, these highlanders experience the full impact of chronic severe hypoxia and low arterial oxygenation (hypoxemia) associated with the approximately 47% lower barometric pressure at 5100 m compared to sea level1. Additionally, Andean high-altitude residents are prone to developing chronic mountain sickness (CMS), a syndrome involving excessive erythrocytosis (EE, defined by hemoglobin concentration values ≥ 21 g/dl for men or ≥ 19 g/dl for women), severe hypoxemia, neurological disorders2 and other signs and symptoms leading to reduced quality of life and premature death3. In La Rinconada, the CMS prevalence has been reported to be 14%4.
Since CMS is associated with neurological symptoms2,5, understanding the cerebral homeostasis during the manifestation process of CMS (before CMS manifestations, as well as during the progression of CMS) is of high clinical relevance to better recognize, prevent and treat CMS in highlanders who are unable to relocate to lower altitudes. Since cerebral functioning requires continuous supply of oxygen, this vulnerable organ is particularly challenged under chronic hypoxic conditions and with CMS6. Surprisingly, despite severe hypoxemia and impaired cardiac baroreflex sensitivity reported in Andean highlanders with CMS7,8, quantitative data are not available to support the dogma that cerebrovascular homeostasis and reactivity is impaired. One prospective study conducted at 3600 m (La Paz, Bolivia) has reported impaired cerebrovascular reactivity in response to CO2 and O2 alterations but preserved cerebral oxygen delivery in highlanders with and without CMS when compared to a low altitude control group from the United Kingdom5. Another study conducted at 4338 m (Cerro de Pasco, Peru) found no differences in the response of the cerebral circulation to hypoxia and hypocapnia between highlanders with and without CMS, either at high altitude or within 24 h of arriving at sea level9. However, neither study compared cerebrovascular homeostasis to ethnically matched populations living at lower altitudes or tried to distinguish between different CMS severities, while albeit in a different context, generic differences can affect susceptibility to cerebrovascular diseases10. Therefore, for the interpretation and contextuation of revealed differences in the cerebrovascular homestasis and reactivity, comparisons of ethnically matched control groups living at different altitudes is paramount, especially when investigating the adaptation of Andeans, a genetically adapted high-altitude population.
Therefore, the purpose of the current study was to investigate the cerebrovascular homeostasis and reactivity in relation to the living altitude of Andean male highlanders and to elucidate the role of CMS on its functionality. We hypothesized that at 5100 m, (1) highlanders free from CMS, present impaired cerebrovascular homeostasis and reactivity in comparison to highlanders at 3800 m and lowlanders; and that (2) in highlanders at 5100 m, CMS compared to non-CMS further deteriorates cerebrovascular homeostasis and reactivity.
Methods
Design and setting
This cross-sectional study took place in Lima (sea-level, barometric pressure [PB] = 760 mmHg), Puno (3800 m, PB = 480 mmHg) and La Rinconada (5100–5300 m, PB = 405 mmHg), Peru, and was part of the Expedition 5300 research program, which investigates the (patho)physiological consequences of long-term exposure to very high altitude4,11,12,13. The study was approved by the ethics committee of the Universidad National Mayor de San Marcos (CIEI-2019-002) and was performed in accordance with the Declaration of Helsinki. Participants in all three locations were recruited through street and radio advertisements and word of mouth.
Participants
Due to the higher risk of CMS manifestation in men compared to women and due to the challenging field conditions of recruitment, this study was conducted in men only4.
Non-smoking Peruvian males, permanently living (i) in Lima at < 1000 m (SL), (ii) in Puno at 3800 m (HL3800m) or (iii) La Rinconada at 5100–5300 m (HL5100m), aged between 18 and 60 years, without any regular intake of medication and no documented medical history of cardiorespiratory, metabolic, or neurological diseases were included in this study.
At 5100 m highlanders were categorized into no (HL5100m), mild (CMSmild) and moderate-to-severe (CMSmod.-sev.) CMS groups based on the Qinghai CMS questionnaire score (< 6; 6 to 10; and > 10, respectively)3. All participants gave their written informed consent prior to participation in this study.
Protocol overview
Medical history was assessed, and all participants had clinical examinations assessing heart rate, blood pressure and arterial oxygen saturation (SpO2). Hemoglobin concentration ([Hb]; HemoCue Hb201 + , HemoCue AB, Ängelholm, Sweden) was determined from a cubital venous blood sample while the patients were resting in the supine position and prior to the experimental procedur. To diagnose CMS, highlanders completed the Qinghai CMS questionnaire as described above.
Thereafter, participants were instructed to quietly sit in a comfortable chair for 10 min prior to instrumentation and the start of the protocol, which consisted in an active orthostatic challenge. After 5 min of baseline, participants were given a count down and instructed to stand up in one steady move within 1 s and without any assistance from their arms. They subsequently kept this upright position for a further 3 min. The experimental procedure is illustrated in Fig. 1. Participants were instructed not to talk, move or fall asleep during the sitting period as well as the active tilt test.
Experimental procedure. Participants remained seated in a comfortable chair for at least 10 min. After 5 min of baseline, participants were given a count down and instructed to stand up in one steady move within 1 s without any assistance from their arms. They subsequently kept this upright position for 3 more minutes. Red shaded areas indicate the 2.5-min intervals for heart rate variability and cardiac baroreflex sensitivity assessments. Gray shaded areas indicate the 30-s intervals for other physiological measurements.
Continuous beat-by-beat blood pressure was measured by finger cuff photoplethysmography (ADInstruments, Oxford UK) and oxygen saturation by finger pulse oximetry throughout the test. The changes in the vertical distance between the hand and the heart were accounted for by the height correction unit non-invasive blood pressure monitor.
Middle cerebral artery (MCA) blood flow velocity (MCAv) was measured through insonation of the temporal window with a 2 MHz pulsed Doppler ultrasound transducer (Multi-Dop-T, DWL, Elektronische Systeme, Singen, Germany). Throughout the procedures, the placement and angle of the ultrasound transducer were held constant using a headset (DiaMon, DWL, Elektronische Systeme, Singen, Germany). In this position the systolic (sMCAv), mean (mMCAv) and diastolic (dMCAv) MCAv were averaged over the time periods illustrated in Fig. 1. Cerebrovascular conductance (CVC, sMCAv/mean arterial pressure [MAP]) and resistance (CVR, MAP/sMCAv) indices were calculated. An index of cerebral O2 delivery (CDO2) was calculated as the product of oxygen content (CaO2; 1.39 × hemoglobin concentration x SpO2/100) x sMCAv5.
Data collection and analysis
Throughout the protocol, analogue signals were sampled and continuously recorded at 1 kHz, using an analogue to digital converter (Powerlab/16SP ML795, ADInstruments, Oxford UK), and stored for offline analysis (Chart version 7.2.2, ADInstruments, Oxford, UK). Sitting values were analyzed in the last 30 s of the 5-min baseline period (except HRV and cBRS – see below). The effect of the orthostatic challenge was assessed by identifying and averaging a 1-s interval around the nadir of the sMCAvand MAP, respectively. Standing values were analyzed in the last 30 s of the 3 min standing period (except HRV and cBRS – see below).
The cerebral autoregulation index (ARI)
The ARI was defined by the ability of the CA to maintain a constant sMCAv despite a change in MAP and was calculated for the orthostatic challenge when the direction of ΔsMCAv / ΔMAP were positively correlated14 as follows:
Therefore, higher ARI values represent larger MAP-induced MCAv fluctuations corresponding to impaired CA, whereas smaller values in ARI correspond to efficient CA.
Heart rate variability (HRV)
HRV parameters were calculated with the HRV LabChart Module (ADInstruments Ltd, Oxford, UK) during the last 2.5 min of the sitting and standing phases by applying the Lomb-Scargle periodogram for spectral analysis15 (Fig. 1). Spontaneous breathing was allowed, and ectopic beats and artefacts were visually and automatically detected and deleted but not replaced, since the Lomb-Scargle periodogram is capable of dealing with gaps and unevenly spaced samples16. These 2.5-min intervals allowed to analyse absolute and normalized low frequency (LF) (0.04–0.15 Hz) and high frequency (HF, 0.15–0.60 Hz) power as well as the LF/HF ratio17. The HF band was post-hoc extended from an upper limit of 0.40 Hz to 0.60 Hz and to address for chronic hyperventilation observed in highlanders at 5100 m. This extension allowed to compare HF activity between all altitudes.
Spontaneous cardiac baroreflex sensitivity
The cardiac baroreflex sensitivity (cBRS) was calculated using the sequence technique described by Kardos et al.18 In brief, during the last 2.5 min while sitting and standing (Fig. 1), the time series of systolic blood pressure (SBP) and pulse interval (PI) were automatically scanned. Only simultaneous increments in PI and SBP (+ PI/ + SBP) or decrements in PI and SBP (-PI/-SBP) over a minimum of 3 consecutive beats were included in the analysis, and if the minimum change in the rise or fall of SBP exceeded 1 mmHg and the correlation coefficient was > 0.85 for each individual sequence. In case of at least 3 valid sequences of PI/SBP within the 2.5 min, they were averaged and the linear correlation between PI/SBP was computed. The obtained value represented the cBRS. In accordance to the approach described by Kardos et al.18, cBRS was calculated for lag 0 and 1 of the PI interval and the lag with the largest number of valid sequences was included in the analysis.
Outcomes
Main outcomes of interest were the cerebrovascular indices while sitting, in response to the orthostatic challenge and while standing for 3 min (Fig. 1), in relation to the altitude of residence and CMS severity. Additional outcomes were obtained from cBRS and HRV analyses, giving insights into the mechanisms and allowing a comprehensive interpretation of the main outcomes.
Statistical analysis
This study being part of the larger research program falling under the umbrella of Expedition 5300, therefore no formal sample size estimation was performed for this substudy. Demographic characteristics are presented as mean ± standard deviation (SD), whereas values and their precision obtained from regression analyses are presented in mean ± standard error (SE). To analyse absolute and percentage changes from baseline values of continuous outcomes, linear mixed regression analyses were applied with the dependent variable in absolute number or in percent from baseline. Dependent variables were tested for normality (Shapiro Wilk test), and visually verified with Kernel density plots. In case of non-normal distribution, log transformation (CVC, CVR, ARI, Total power, LFpower, normalized LF, HFpower, LF/HF, cBRS) or 1/sqrt (sMCAv) was applied. Independent variables were altitude (sea level, 3800 m and 5100 m), CMS severity (no, mild and moderate to severe) and intervention (sitting, orthostatic challenge and standing), and the interaction between altitude*intervention*CMS severity as fixed effects and participants as random effects. Single missing values were not replaced. Statistical significance was assumed with P values < 0.05 and when the 95% confidence intervals of the mean difference did not include zero (STATA 16, StataCorp LLC, Texas, USA).
Results
Participants
Among a total of 76 male participants included in this study, 15 were SL, 13 HL3800m and 48 were highlanders living at 5100 m, respectively. Highlanders at 5100 m had been living in La Rinconada for 15 ± 8 years, and 17 (35%) were categorized as HL5100m, 11 (23%) CMSmild and 20 (42%) CMSmod.-sev., respectively (Table 1). All participants had similar anthropometric characteristics (height and weight) but HL5100m were significantly older and had elevated hemoglobin than HL3800m and SL groups. At 5100 m, CMSmod.-sev. had the highest hemoglobin concentrations.
Cardiovascular and cerebrovascular homeostasis while sitting
In HL5100m, living at 5100 m was associated with hypoxemia, lower PetCO2 and higher mean and diastolic blood pressure compared to SL (Table 2). Systolic MCAv and CVC was lower, and CVR higher with the altitude of residence while CDO2 was slightly reduced in HL5100m compared to HL3800m (P = 0.028) but comparable to SL (P = 0.253, Table 2). Frequency domain analysis of the HRV revealed altitude-dependent decrements in LF (P = 0.005 between HL5100m vs. SL), a trend in lower HF power (P = 0.123 between HL5100m vs. SL) and unchanged LF/HF ratio (P = 0.383 between HL5100m vs. SL). Cardiac BRS was similar across all altitudes.
At 5100 m, the CMSmod.-sev. was associated with more pronounced hypoxemia but similar degree of hypocapnia (lower end-tidal partial pressure of CO2) compared to HL5100m and CMSmild (Table 2). Blood pressure and cerebrovascular indices such as sMCAv, CVC, CVR and CDO2 were unaffected by CMS (Table 2) and no differences were found in HRV. In contrast cBRS was markedly reduced in CMSmod.-sev. compared to HL5100m.
Cardiovascular and cerebrovascular responses to an orthostatic challenge
In SL, HL3800m and HL5100m, the orthostatic challenge induced a transient drop in blood pressure. In contrast, the orthostatic challenge elevated the HR, but, these elevations were lower in HL5100m compare to SL and HL3800m (Table 3). However, when expressed in ∆percent from sitting, HL3800m showed a similar or milder reduction in MAP (% sitting) compared to HL5100m and SL, respectively (Table 3). The orthostatic challenge induced a similar fall in sMCAv across SL, HL3800m and HL5100m when expressed in percent change from sitting (Table 3) and resulted in lower CVC and increased CVR compared to sitting position. The calculated ARI expressed as Δ%sMCAv/Δ%MAP and Δ%sMCAv/ΔmmHg MAP were similar in those groups.
At 5100 m, the orthostatic challenge induced a decrease in MAP in HL5100m (P < 0.05), while MAP did not change significantly in CMSmild (P = 0.285) or CMSmod.-sev. (P = 0.396, Table 4). Although MAP changes differed across CMS groups with the orthostatic challenge, heart rates moderately increased and a similar decrease in absolute and percent change from baseline in the sMCAv was observed (Table 4). The calculated ARI was not altered by CMS severity (Table 4).
Cardiovascular and cerebrovascular homeostasis while standing
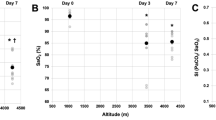
Despite persistently reduced sMCAv in HL5100m compared to SL and HL3800m (Table 5), no differences in CDO2 were observed. When expressed as a ∆percent from sitting, the changes in sMCAv while standing was similar between SL, HL3800m, HL5100m (Fig. 2B). Unlike what was observed while sitting, HRV-derived total power and LF were similar between groups while standing, mainly due to a larger increase in LF in HL5100m compared to HL3800m and SL (Fig. 2D and F). The cBRS did not differ between altitudes.
Physiological changes while standing expressed in percentage from sitting values in healthy lowlanders and highlanders. Panel (A) heart rate; Panel (B) middle cerebral artery peak blood flow velocity; Panel (C) cardiac baroreflex sensitivity; Panel (D) normalized low frequency power; Panel (E) normalized high frequency power; Panel (F) ratio between low frequency (LF) and high frequency (HF). Dots represent the mean change, whiskers the 95% confidence interval. Significant changes from resting are present when the 95% confidence interval does not include the value zero. *P < 0.05 indicate significant different changes compared to lowlanders; ¶P < 0.05 indicate significant different changes compared to highlanders at 3800 m.
At 5100 m, MCAv metrics, CDO2 and HRV-derived values were not different between HL5100m, CMSmild and CMSmod.-sev. while standing (Table 5). When expressed in ∆percent from sitting, however, CMSmild and CMSmod.-sev. showed no increase in LF (Fig. 3D), no decrease in HF (Fig. 3E) and, therefore, no increase in LF/HF compared to HL5100m (Fig. 3F). The cBRS remained unchanged in HL5100m and CMSmild during standing but increased in CMSmod.-sev. compared to HL5100m (P = 0.011) and CMSmild (P < 0.001) (Fig. 3C).
Physiological changes while standing expressed in percentage from sitting in highlanders living at 5100 m. CMS chronic mountain sickness. Panel (A) heart rate; Panel (B) middle cerebral artery peak blood flow velocity; Panel (C) cardiac baroreflex sensitivity; Panel (D) normalized low frequency power; Panel (E) normalized high frequency power; Panel (F) ratio between low frequency (LF) and high frequency (HF). Dots represent the mean change, whiskers the 95% confidence interval. Significant changes from resting are present when the 95% confidence interval does not include the value zero. #P < 0.05 indicate significant different changes compared to highlanders without CMS; †P < 0.05 indicate significant different changes compared to highlanders with mild CMS.
Discussion
Main findings
This comprehensive study assessed for the first time the cerebrovascular homeostasis at three different altitudes and in highlanders with different CMS scores severity.
Preserved cerebral homeostasis and CDO2 while sitting was achieved by elevated CaO2 to compensate for reduced cerebral perfusion in HL3800m and HL5100m compared to SL (Table 2). Interestingly, although HL5100m already showed pathological hemoglobin concentrations (> 21 g/dl, Table 1), these highlanders did not develop any CMS-related neurological symptoms (i.e. headache, tinnitus). Moreover, HL5100m had maintained cerebrovascular and cardiovascular responsiveness during the orthostatic challenge (Table 3) and during standing (Fig. 2) compared to HL3800m and SL residents. While standing, this was achieved by altered orthostatic hypotension, elevated sympathetic nervous system activation and maintained cardiac baroreceptor sensitivity (Table 5).
While sitting, CMSmild and CMSmod.-sev. presented comparable cerebrovascular homeostasis and CDO2 compared to HL5100m, albeit using different strategies (Table 2). Indeed, CMSmod.-sev. maintained their CDO2 due to further excessive erythrocytosis, which was associated with altered blood pressure regulation, and ventilatory (unchanged level of PetCO2 despite severe hypoxemia) and autonomic cardiovascular function (Tables 1 and 2). Interestingly, in CMSmild and CMSmod.-sev., the cerebrovascular reactivity in response to the orthostatic challenge was comparable to HL5100m; however, their baroreflex sensitivity and sympathethic/parasympathetic activity in response to standing for 3 min were impaired (Table 5, Fig. 3). Despite unaltered cerebrovascular homeostasis and reactivity in highlanders with vs. without CMS, this pathology has been traditionally associated with neurological symptoms; therefore, other underlying mechanisms might be involved in its manifestation and expression, e.g. cerebral edema19.
Effect of altitude
As mentioned above, our findings in HL5100m, while sitting showed maintained cerebral homeostasis and CDO2 compared to SL residents (Table 2). Only one study by Bailey et al.5 looked at cerebral homeostasis while resting in Andean highlanders with and without CMS living at 3600 m, La Paz, Bolivia, and compared their results to UK lowlanders. By using the same CDO2 equation as in our study, they showed reduced CDO2 in highlanders compared to lowlanders (850 vs. 983 ml/cm/s, P < 0.05). However, the CDO2 in highlanders reported by Bailey et al. was lower than those in the present study (1314 and 1087 ml/cm/s in HL3800m and HL5100m, respectively). This discrepancy is caused by the higher sMCAv and hemoglobin concentrations found in our populations (Tables 1 and 2) (54.1 cm/s and 18.9 g/dl at 3800 m; 42.9 cm/s and 22.1 g/dl at 5100 m compared to 39 cm/s and 17.2 g/dl at 3600 m reported by Bailey et al.). Interestingly, in the cited study, they employed transfer function analysis to investigate static CA while resting quietly. They reported no difference in coherence, phase and gain in the very low and low frequency bands between highlanders and lowlanders, suggesting maintained CA functionality. However, based on the exaggerated oxidative-inflammatory nitrosative stress and corresponding decrease in vascular NO bioavailability combined with reduced CDO2, they concluded that the cerebrovascular function is impaired in highlanders compared to lowlanders while resting. The differences between the cited study and our findings could be due to the chosen control group and, therefore, the comparator for the definition of “normal” CA functionality. In our study, an ethnically matched control group was chosen, therefore, comparisons of CA functionality in Peruvian highlanders compared to Peruvian lowlanders was not influenced by any factors related to ethnicity.
We found altered orthostatic hypotension but similar physiological changes in HL3800m and HL5100m compared to SL (Table 3). Interestingly, highlanders were less prone to hypotension during the orthostatic challenge, which has been reported previously20. Gulli et al. examined orthostatic tolerance and hypotension by using head-up tilting in highlanders living in Cerro de Pasco (4338 m), Peru, and compared their results to Ethiopian highlanders and UK sea-level controls. Similar to our results, Andean highlanders had no drop in systemic blood pressure with tilting (114 mmHg supine to 116 mmHg while tilted for 20 min), whereas Ethiopian highlanders as well as the lowlanders had slight decrements in blood pressure. On another hand, Gulli et al. also showed impaired autonomic cardiovascular activation in response to 20 min of 60 degree head-up tilting in Peruvian highlanders. This was mainly shown by an absence of LF-increase and HF-decrease in response to tilting by using autoregressive monovariate model with automated time series analysis. These results are in contrast with the present findings showing a normal rise in LF and drop in HF frequency after standing for 3 min compared to sitting. The passive vs active nature of the tilt test, the analysis method of the autonomic cardiovascular activity and more generally different study design between Gulli et al. and the present work most likely contribute to the discrepancy in the results.
In accordance with previous reports in lowlanders, the orthostatic challenge by standing-up is normally associated with reduced cBRS21 and elevated sympathetic activity22. We observed the same pattern of responses across altitudes, except for HL5100m who had pronounced LF activation when standing up (Fig. 2). This latter might be necessary to achieve comparable peripheral, cardiac and cerebrovascular responses under severe hypoxic conditions compared to normoxia23. One potential underlying factor might be the hypoxia-related dilatation of blood vessels24 requiring higher sympathetic activity levels to vasoconstrict, but further studies are required to clarify these cardiovascular responses.
Effect of CMS
Although CMSmild and CMSmod.-sev. were associated with further hypoxemia and excessive erythrocytosis (Table 1), CDO2 remained comparable to HL5100m (Table 2). The underlying cause for the comparable CDO2 most likely was the excessive hemoglobin concentration, while sMCAv remained stable and SpO2 was only mildly reduced (Table 2). Indeed, Bailey et al.5 also found similar CDO2 values between highlanders with and without CMS at 3600 m. Similarly, comparisons of CA functionality showed no difference between highlanders with and without CMS at 3600 m5, or, indeed at 5100 m, in the present study. These results suggest that other, yet to be established, CMS-related alterations explain neurological symptoms traditionnaly associated with CMS. In contrary, previous findings by Appenzeller et al.25 showed no response in cerebral blood velocity to an exogenous NO donor in Peruvian highlanders compared to reduced cerebral blood volumes in Ethiopian low- and highlanders, indicating impaired CA functionality in Peruvian highlanders. Unfortunately, owing to the small sample size, and methodological limitations (change in MCA diameter due to exogenous NO donor), this latter study did not separate analyses by CMS (4 out of 9 participants had CMS); therefore, the authors could not conclude about the possible role of CMS in the impaired CA functionality.
Aside from the maintained CDO2 in highlanders with CMS, CMS has clear deleterious effects on the cardiovascular and sympathetic/parasympathetic activity. The observed lower cBRS and therefore, lower blood pressure regulation while sitting (Table 2) is in accordance with a previous report comparing highlanders with hematocrit values > 65% and high CMS scores (cBRS 3.9 ± 2.1 ms/mmHg) to highlanders with hematocrit < 60% and lower CMS scores (cBRS 11.2 ± 8.5 ms/mmHg)8. Mechanisms behind the compromised blood pressure regulation might be impaired vascular compliance, lower cBRS sensitivity or, as previously shown negative correlation between blood volume and cBRS activity26,27.
When rapidly standing-up (Table 4), CMS was associated with altered orthostatic hypotension as suggested by the absence of decrease or even the increase in blood pressure but comparable ARI and sMCAv in comparison to HL5100m. However, it remains debatable whether the observed increase in blood pressure in response to the orthostatic challenge is an adequate response28. Blood pressure falls are thought to be the consequence of gravitationally driven redistribution of blood from the central circulation to the periphery and predominantly venous vasculature29. Highlanders living at 5100 m have large blood volumes11, and therefore a postural change might not cause the classically observed blood volume shift, blood vessel dilatation and blood pressure fall30. Although no study has examined the effect of hypervolemia on blood pressure regulation and orthostatic hypotension at high-altitude, our findings are in accordance with the widely accepted theory that hypovolemia causes orthostatic intolerance by exaggerated hypotension31. Despite hypervolemia and potentially limited capability to dilate the peripheral venous vasculature causing a minimal blood volume shift, the hydrostatic pressure still was likely to increase due to the orthostatic challenge. As seen in the elevation of heart rate in CMSmild and CMSmod.-sev. during the orthostatic challenge (Table 4), the potential hydrostatic shift caused sympathetic excitation and parasympathetic inhibition through reduced pressure on the arterial baroreflex receptors, as described previously32. Assuming that these symphathetic/parasympathetic alterations affect the cerebral vasculature32, then cerebrovascular vasoconstriction, decrease in MCAv and increase in CVR would be the consequences – which we observed in CMS compared to HL5100m (Table 4, reduction in mean and diastolic MCAv), despite no change in MAP with the orthostatic challenge.
While standing (Fig. 3), CMSmild and CMSmod.-sev. were associated with blunted autonomic cardiovascular activity (no change in heart rate, LF, HF or LF/ HF ratio), indicating that the impaired system was not able to adapt to the postural changes. This pattern was uniquely observed in CMSmild and CMSmod.-sev., however, challenging to comprehend. Indeed, excessive erythrocytosis cannot be the driving factor behind this observation, since HL5100m have comparable hemoglobin concentrations (at least compared to CMSmild), however, they are solely asymptomatic. Moreover, in a previously published paper in the same participants, we found no difference in hematocrit, blood viscosity or blood volume between HL5100m and CMSmild11,12. Therefore, the reduced sympathetic/parasympathetic activation has another origin. For instance, we observed no change or even increased cBRS sensitivity in response to standing in CMS, which suggests impaired blood pressure regulation, possibly through reduced cBRS sensitivity. Indeed, it could be hypothesized that the failure to activate cBRS in highlanders with CMS might have resulted in the failure of the baroreflex to activate the classical autonomic nervous system.
The selfish brain theory in CMS?
It seems to be appropriate to highlight that the brain is the most important and valuable organ in the human body and, due to its inability to store resources such as oxygen and glucose, constantly demands supply from the body33,34. The simple rationale is that when the brain dies, the organism dies, therefore, the better alternative is to extract as much resources as possible from the body – whatever the cost. In terms of highlanders living in the highest city in the world, being severely hypoxemic and suffering from CMS causing further hypoxemia, the brain is chronically under stress of not receiving enough oxygen to keep functioning. In our comprehensive study, we have insights, that the oxygen delivery to the brain is maintained by pathologically high hemoglobin concentrations especially in CMS11, as well as pathological alterations in several body functions. These consequences might be the direct cause of the brain-pull, maybe being the ultimate cause of CMS symptoms, impaired body functions, organ damage and premature death. However, it is certain that without this “selfish” behaviour, the brain would suffer substantial damage. Therefore, whether the observed changes in ventilatory, systemic and autonomic cardiovascular activation are “beneficial” or “detrimental” differ by what should be more prioritized – the brain or the body?
Limitations
This study was conducted only in men, although, CMS can also manifest in women just to a much lower extend4. The rationale behind studying only men was the unpredicatable and challenging recruitment procedure of highlanders living in La Rinconada as well as the complexity and multidimensionality of the study, not allowing to sufficiently characterize women related to their hormonal profile, menstrual cycle phase and pre-/postmenopause. Although this cross-sectional study conducted at different altitudes and in highlanders with and without CMS provides original data regarding the physiological effects of permanent high-altitude residence and CMS, longitudinal studies are warranted to further elucidate the (lack of) adaptations of the cerebral, cardiovascular and autonomic systems, as well as blood pressure regulation in highlanders. Furthermore, whether the observed CMS-related specificities are continuous or related to the CMS severity classifications (no, mild, moderate-to-severe), and whether hypervolemia and baroreflex function play an important role, requires further investigation. The chosen examination techniques were adapted and carefully chosen according to the challenging field conditions in La Rinconada. In absence of any hospital nearby, non-invasive over invasive measurements for assessing cerebral regulation and autonomic cardiovascular activation were preferred. Furthermore, assessed TCD indexes of the MCA and calculated CDO2 provide only partial insights into cerebral homeostasis, while potentially confounding factors such as differences in the MCA diameter among populations due to hypervolemia or PetCO2 differences remain to be elucidated. Standing-up as an orthostatic stress was chosen to simulate ecological condition with results directly applicable to daily life, however, the relatively mild change in blood pressure might have masked physiological responses potentially revealed with higher stress levels, i.e. exercise. As per Table 1, one can notice that dwellers from La Rinconada were overall older than those residing at lower altitudes, which could in and of itself influence cerebrovascular function. However, the present results were not modified by the inclusion of age as covariate.
Conclusion
In conclusion, we showed in male residents without symptoms of CMS living at sea-level, 3800 m and 5100 m, that the cerebral oxygen delivery and cerebrovascular reactivity are comparable during sitting and an orthostatic stress. This was achieved by ventilatory compensation, altered orthostatic hypotension, elevated sympathetic activation while standing and maintained blood pressure regulation through cardiac baroreflex function. At 5100 m, male highlanders with CMS showed comparable CDO2 and cerebral reactivity compared to highlanders living at 5100 m free of CMS. However, CMS, especially moderate-to-severe CMS, was associated with highly excessive erythrocytosis, absent of ventilatory compensation for severe hypoxemia, impaired blood pressure regulation and impaired sympathetic/parasympathetic activation. Whether these various pathological consequences developed due to CMS and maladaptation to chronic hypoxia or whether in some individuals the “selfish” brain under chronic oxygen deprivation caused the observed CMS symptoms and body dysfunction remains to be elucidated.
Data availability
Data available: Yes, Data types: De-identified participant data, How to access data: michael.furian@usz.ch, When available: With publication, Who can access the data: Anonymized data underlying this study can be requested by qualified researchers providing an approved proposal.
Abbreviations
- ARI:
-
Cerebral autoregulation index
- CA:
-
Cerebral autoregulation
- cBRS:
-
Spontaneous cardiac baroreflex sensitivity
- CDO2 :
-
Cerebral oxygen delivery
- CMS:
-
Chronic mountain sickness
- CMSmild :
-
Highlanders with mild chronic mountain sickness
- CMSmod.-sev. :
-
Highlanders with moderate-to-severe chronic mountain sickness
- CVC:
-
Cerebrovascular conductance
- CVR:
-
Cerebrovascular resistance
- EE:
-
Excessive erythrocytosis
- HF:
-
High frequency
- HL3800m :
-
Highlanders living at 3800 m
- HL5100m :
-
Highlanders living at 5100 m
- HRV:
-
Heart rate variability
- LF:
-
Low frequency
- MAP:
-
Mean artery pressure
- MCAv:
-
Middle cerebral artery blood flow velocity
- PB:
-
Barometric pressure
- PI:
-
Pulse interval
- SBP:
-
Systolic blood pressure
- SL:
-
Sea-level residents
- SpO2 :
-
Arterial oxygen saturation assessed by finger oximetry
References
West, J. B. The physiologic basis of high-altitude diseases. Ann. Intern. Med. 141, 789–800 (2004).
Monge-C, C., Arregui, A. & Leon-Velarde, F. Pathophysiology and epidemiology of chronic mountain sickness. Int. J. Sports Med. 13, S79–S81 (1992).
León-Velarde, F. et al. Consensus statement on chronic and subacute high altitude diseases. High Alt. Med. Biol. 6, 147–157. https://doi.org/10.1089/ham.2005.6.147 (2005).
Hancco, I. et al. Excessive erythrocytosis and chronic mountain sickness in dwellers of the highest city in the world. Front. Physiol. 11, 773. https://doi.org/10.3389/fphys.2020.00773 (2020).
Bailey, D. M. et al. Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J. Physiol. 597, 611–629. https://doi.org/10.1113/jp276898 (2019).
Hoiland, R. L., Bain, A. R., Rieger, M. G., Bailey, D. M. & Ainslie, P. N. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R398-413. https://doi.org/10.1152/ajpregu.00270.2015 (2016).
Champigneulle, B. et al. Excessive erythrocytosis and chronic mountain sickness in the highest city in the world: A longitudinal study. Chest 161, 1338–1342. https://doi.org/10.1016/j.chest.2021.11.030 (2022).
Keyl, C. et al. Autonomic cardiovascular function in high-altitude Andean natives with chronic mountain sickness. J. Appl. Physiol. (1985) 94, 213–219. https://doi.org/10.1152/japplphysiol.01258.2001 (2003).
Norcliffe, L. J. et al. Cerebrovascular responses to hypoxia and hypocapnia in high-altitude dwellers. J. Physiol. 566, 287–294. https://doi.org/10.1113/jphysiol.2005.086629 (2005).
Griessenauer, C. J. et al. Genetic susceptibility to cerebrovascular disease: A systematic review. J. Cereb. Blood Flow Metab. 38, 1853–1871. https://doi.org/10.1177/0271678x18797958 (2018).
Oberholzer, L. et al. Reevaluation of excessive erythrocytosis in diagnosing chronic mountain sickness in men from the world’s highest city. Blood 136, 1884–1888. https://doi.org/10.1182/blood.2019004508 (2020).
Stauffer, E. et al. Blood viscosity and its determinants in the highest city in the world. J. Physiol. https://doi.org/10.1113/jp279694 (2020).
Doutreleau, S. et al. Cardiac remodelling in the highest city in the world: Effects of altitude and chronic mountain sickness. Eur. J. Prev. Cardiol. https://doi.org/10.1093/eurjpc/zwac166 (2022).
Numan, T. et al. Static autoregulation in humans: A review and reanalysis. Med. Eng. Phys. 36, 1487–1495. https://doi.org/10.1016/j.medengphy.2014.08.001 (2014).
Lomb, N. R. Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 39, 447–462. https://doi.org/10.1007/BF00648343 (1976).
Moody, G. B. In Proc. of Computers in Cardiology Conference, 715–718.
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Health 5, 258–258. https://doi.org/10.3389/fpubh.2017.00258 (2017).
Kardos, A. et al. Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension 37, 911–916. https://doi.org/10.1161/01.hyp.37.3.911 (2001).
Bao, H. et al. Cerebral edema in chronic mountain sickness: A new finding. Sci. Rep. 7, 43224. https://doi.org/10.1038/srep43224 (2017).
Gulli, G. et al. Autonomic regulation during orthostatic stress in highlanders: Comparison with sea-level residents. Exp. Physiol. 92, 427–435. https://doi.org/10.1113/expphysiol.2006.035519 (2007).
Steptoe, A. & Vögele, C. Cardiac baroreflex function during postural change assessed using non-invasive spontaneous sequence analysis in young men. Cardiovasc. Res. 24, 627–632. https://doi.org/10.1093/cvr/24.8.627 (1990).
Pomeranz, B. et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 248, H151-153. https://doi.org/10.1152/ajpheart.1985.248.1.H151 (1985).
Simpson, L. L., Steinback, C. D., Stembridge, M. & Moore, J. P. A sympathetic view of blood pressure control at high altitude: New insights from microneurographic studies. Exp. Physiol. 106, 377–384. https://doi.org/10.1113/EP089194 (2021).
Tremblay, J. C. et al. Global REACH 2018: High blood viscosity and hemoglobin concentration contribute to reduced flow-mediated dilation in high-altitude excessive erythrocytosis. Hypertension 73, 1327–1335. https://doi.org/10.1161/hypertensionaha.119.12780 (2019).
Appenzeller, O. et al. Cerebral vasodilatation to exogenous NO is a measure of fitness for life at altitude. Stroke 37, 1754–1758. https://doi.org/10.1161/01.STR.0000226973.97858.0b (2006).
Mtinangi, B. L. & Hainsworth, R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp. Physiol. 84, 121–130. https://doi.org/10.1111/j.1469-445X.1999.tb00077.x (1999).
El-Sayed, H. & Hainsworth, R. Relationship between plasma volume, carotid baroreceptor sensitivity and orthostatic tolerance. Clin. Sci. (Lond.) 88, 463–470. https://doi.org/10.1042/cs0880463 (1995).
Jordan, J., Ricci, F., Hoffmann, F., Hamrefors, V. & Fedorowski, A. Orthostatic hypertension: Critical appraisal of an overlooked condition. Hypertension 75, 1151–1158. https://doi.org/10.1161/hypertensionaha.120.14340 (2020).
Sheriff, D. D., Nådland, I. H. & Toska, K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J. Appl. Physiol. 1985(108), 523–532. https://doi.org/10.1152/japplphysiol.01185.2009 (2010).
Claydon, V. E. et al. Orthostatic tolerance and blood volumes in Andean high altitude dwellers. Exp. Physiol. 89, 565–571. https://doi.org/10.1113/expphysiol.2004.027698 (2004).
Stewart, J. M. Mechanisms of sympathetic regulation in orthostatic intolerance. J. Appl. Physiol. (Bethesda, Md.1985) 113, 1659–1668. https://doi.org/10.1152/japplphysiol.00266.2012 (2012).
Ogoh, S. & Tarumi, T. Cerebral blood flow regulation and cognitive function: A role of arterial baroreflex function. J. Physiol. Sci. 69, 813–823. https://doi.org/10.1007/s12576-019-00704-6 (2019).
McBryde, F. D., Malpas, S. C. & Paton, J. F. R. Intracranial mechanisms for preserving brain blood flow in health and disease. Acta Physiol. (Oxf.) 219, 274–287. https://doi.org/10.1111/apha.12706 (2017).
Peters, A. et al. The selfish brain: Competition for energy resources. Neurosci. Biobehav. Rev. 28, 143–180. https://doi.org/10.1016/j.neubiorev.2004.03.002 (2004).
Funding
This study was supported by the Fonds de dotation AGIR pour les maladies chroniques, the Air Liquide Foundation, and by the French National Research Agency (ANR-12-TECS-0010) in the framework of the Investissements d’avenir programme (ANR-15-IDEX-02). Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, P5R5PM_210361.
Author information
Authors and Affiliations
Contributions
Concept and design: Verges, Brugniaux. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Furian and Brugniaux. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Furian. Obtained funding: Verges, Brugniaux.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Furian, M., Ulliel-Roche, M., Howe, C.A. et al. Cerebral homeostasis and orthostatic responses in residents of the highest city in the world. Sci Rep 14, 17732 (2024). https://doi.org/10.1038/s41598-024-68389-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68389-5
- Springer Nature Limited