Abstract
Dietary intake of live microbes may benefit human health, but less is known about the role in insulin resistance. This study was developed with the goal of evaluating potential relationships between IR and dietary live microbes. The National Health and Nutrition Examination Survey (NHANES) dataset was leveraged to collect data from 6,333 subjects 18 + years of age. The Sanders system for the classification of dietary live microbe intake (containing Low (< 104 CFU/g), Medium (104–107 CFU/g), or High (> 107 CFU/g) levels of live microbes) was then used to separate these patients into three groups (low, medium, or high). Fasting blood glucose and insulin levels were used to approximate IR based on the homeostasis model of insulin resistance (HOMA-IR). Weighted linear regressions were used to assess the relationship between IR and live microbe intake. After fully adjusting for confounding factors, subjects in the groups exhibiting medium and high levels of live microbe intake exhibited HOMA-IR scores that were below those of subjects in the low group. The relationship between live microbe intake and HOMA-IR scores was also potentially impacted by ethnicity. In summary, a negative correlation was detected between dietary live microbe intake and HOMA-IR values.
Similar content being viewed by others
Introduction
While microbe-free sterile food products are generally sought after for their potential benefits to public health, the lack of exposure to microbes can also have unforeseen negative health effects1. Encountering symbiotic or harmless microbes generally has no adverse impact on human health, and can instead serve a beneficial immunostimulatory role2,3. When live microbes are consumed as a part of the diet, they can reach the gut and colonize this compartment, interacting with other resident microbes and potentially exerting positive effects4,5. Benefits associated with such microbial colonization can include enhanced intestinal function, reduced inflammation and oxidative stress, superior immunoregulatory activity, and greater insulin sensitivity6,7. The World Health Organization and the UN Food and Agriculture Organization have defined probiotics as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”, highlighting the increasingly important role that these microbes play in shaping human health8,9.
Type 2 diabetes mellitus (T2DM) and other metabolic diseases, such as obesity and hyperlipidemia, are often closely associated with pathogenic insulin resistance10,11,12, which is a partially modifiable risk factor. The gold-standard approach to defining IR is based on a hyperinsulinemic-euglycemic clamp strategy, but the homeostasis model assessment of insulin resistance (HOMA-IR)13 was designed as an alternative strategy that can be more readily implemented. As a result, HOMA-IR values are widely used in both epidemiological and clinical settings, providing insight into IR status based on measurements of fasting values of insulin and glucose14,15. A previous meta-analysis indicated that probiotics and synbiotics consumption has favorable effects on improving fasting plasma glucose, fasting insulin serum level, hemoglobin A1c (HbA1c) and HOMA-IR16. A significant reduction in HOMA-IR, triglycerides, and high-density lipoprotein levels was also observed in fecal microbiota transplantation group17,18. The consumption of Kefir, a fermented beverage rich in live microbe, can reduce fasting blood sugar and insulin level19. However, live microbes are not only present in fermented foods, but also in a diverse range of other food items, such as raw, unpeeled vegetables and fruits20,21,22. Although extensive research has been conducted on the relationship between probiotics and diabetes mellitus and IR23,24,25, less attention has been given to the live microbe in food26. The association between IR and live microbe intake has not been explored explicitly. As such, the present study was developed with the goal of examining the relationship between the dietary intake of live microbes and IR among healthy nondiabetic American adults. These analyses were conducted using data from the large-scale nationally-representative NHANES 2003–2020 study in the US, providing cross-sectional survey data enabling the examination of the effects of a wide variety of potential confounding variables on this link between IR status and microbe intake.
Materials and methods
Data sources and study subjects
Conducted by the US National Center for Health Statistics (NCHS), the National Health and Nutrition Examination Survey (NHANES) enrolled a representative participant population through a stratified, multistage cluster probability sampling approach to recruitment. The goal of this study was to enable the monitoring and evaluation of the health and nutrition of members of the US population. The study received approval from the Ethics Review Committee of the NCHS, and all participants provided written informed consent.
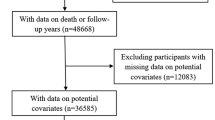
The 2003–2020 NHANES cycles enrolled 57,456 total adults 18 + years of age. For the present study, participants were excluded if they (1) had been diagnosed with T1DM or T2DM as HOMA-IR values may not be indicative of true IR status in these patients (n = 14,865), (2) participants using medications, some of which may impact IR (including metabolic analogs, antiretroviral agents, antineoplastic agents, antipsychotics, and antidiabetic drugs) (n = 20,684), (3) participants with missing fasting insulin or fasting blood glucose (FBG) data, thus preventing the calculation of HOMA-IR values (n = 13,639), (4) participants with missing data regarding the dietary intake of live microbes (n = 293), and (5) participants missing important data including data related to current smoking status, education, body mass index (BMI), recreational activity, or income-to-poverty ratio (PIR) values (n = 1396).
Dietary live microbe intake analyses
In the NHANES database, 24-h dietary data were used to estimate nutrient and energy intake levels using the US Department of Agriculture Food Surveys Nutrient Database. Four experts in this field (CH, RH, MES, and MLM) estimated the live microbe levels present per gram for each of 9,388 food codes across 48 subgroups included in this dataset27. Given the inherent variability in these microbe levels, foods were broadly classified as containing Low(< 104 CFU/g, referring to pasteurized foods), Medium (104–107 CFU/g, referring to fresh fruits and vegetables eaten unpeeled), or High (> 107 CFU/g, referring to unpasteurized fermented foods and probiotic supplements) levels of live microbes. These determinations were made with reference to published studies, definitive reviews, and inferences made based on knowledge of pasteurization and other techniques used in the context of food processing (for example, pasteurization). Any discrepancies were resolved through discussion within the team and consultation with Fred Breidt, an expert microbiologist from the USDA27. Subsequently, we categorize participants' food based on their corresponding live microbial content, as outlined in Supplementary Table S1. Participants were ultimately stratified into three groups, including low, participants exclusively consumed foods categorized as Low levels of live microbes content; medium, participants consumed any foods categorized as Medium levels of live microbes content, but not High; high, participants consumed any foods categorized as High levels of live microbes content.
Analyses of insulin resistance
HOMA-IR values were used to establish the IR status of study participants. For the present analyses, IR was defined by HOMA-IR values above the 75th percentile, which was equal to 3.0128. The formula for HOMA-IR score calculation was as follows: HOMA-IR = [fasting insulin (μU/L) × fasting glucose (mmol/L)]/22.5. Given that insulin secretion is impaired in diabetic patients such that HOMA-IR values may not adequately capture the IR status of these individuals, diabetic subjects were excluded from this study. Diabetes was diagnosed by self-reported diagnosis, self-reported use of insulin or other diabetes medications, fasting blood glucose ≥ 126 mg/dL, HbA1c ≥ 6.5%, or serum glucose levels ≥ 200 mg/dL at 2 h following a 75 g oral glucose load29.
Covariates
Covariates with the potential to impact the association between HOMA-IR values and the dietary intake of live microbes were selected with reference to published studies and clinical experiences. These potential confounding factors included sex (male or female); age; ethnicity (Mexican American, Non-Hispanic Black, Non-Hispanic White, Other Hispanic, Other); familial PIR values (< 1.2, or ≥ 1.2); education (below high school, high school, or greater than high school); participation in recreational activities (no or yes); current smoking status (no or yes), energy intake (kcal/day); BMI (kg/m2); fasting insulin levels (µU/L); FBG levels (mmol/L); HbA1c levels, fasting levels of total cholesterol (mg/dL); and fasting triglyceride levels (mg/dL). Fasting blood samples were collected via venipuncture for appropriate analyses of serum and plasma parameters. PIR values were computed by dividing individual or family income by poverty standards for a particular year. BMI was computed as body weight (kg) divided by height (m) squared. Recreational activity included individuals participating in moderate/vigorous activity and muscle strengthening activity.
Statistical analyses
To account for the complex multi-stage cluster design of the underlying data, appropriate NHANES sample weights were applied. Categorical data were reported as numbers (weighted percentages) and compared with chi-squared tests, whereas continuous variables were reported as means ± standard error (SE) and compared with Student’s t-tests. Associations between these groupings and HOMA-IR scores were assessed using multiple linear regression analyses with appropriate survey weighting under two different models. Model 1 was adjusted for age, gender, ethnicity, education, current smoking status, and BMI (kg/m2). Model 2 was adjusted for these same variables as well as levels of recreational activity, total fasting cholesterol, fasting triglycerides, HbA1c, and energy intake.
The potential impact of obesity (BMI ≥ 30 kg/m2) on study results was evaluated through subgroup analyses. Stratified regression analyses were employed to account for differences as a function of gender, ethnicity, PIR, education, current smoking status, recreational activity levels, and obesity status. All analyses were performed with R (v 4.2.2), and P < 0.05 served as the cut-off to define statistical significance.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by The National Center for Health Statistics Research Ethics Review Board. All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki).
Results
Participant characteristics
In total, this study enrolled 6333 subjects (weighted n = 29,505,658). These subjects were stratified into three groups based on levels of dietary live microbe intake, and baseline characteristics for participants in each group are presented in Table 1. Subjects in the high group were more likely to be female and were older than those in the low group, in addition to being more likely to be non-Hispanic white individuals, current non-smokers, to engage in more recreation activity, and to exhibit higher levels of energy intake, higher PIR values, and a lower BMI (P < 0.05).
Participant classification based on IR status
Participant HOMA-IR values were used for stratification into two groups based on whether these values were below or above the cut-off threshold of 3.01. The characteristics of subjects in these groups are presented in Table 2. Individuals with a HOMA-IR ≥ 3.01 were more likely to be males, non-hispanic white individuals, to engage in less recreational activity, to exhibit a higher education level, to have a higher PIR, and to have a higher BMI (P < 0.05), while also being likely to exhibit a lower level of dietary live microbe intake (P < 0.0001).
Relationships between dietary live microbe intake and HOMA-IR classification
Next, a series of univariate and multivariable weighted linear regression analyses were conducted to analyze relationships among variables of interest (Table 3). Univariate linear regression revealed that participants in the groups with high and intermediate levels of dietary live microbe intake exhibited lower HOMA-IR values than individuals in the group with low dietary live microbe intake. Using a model adjusted for age, sex, ethnicity, education, BMI, and current smoking status (Model 1), HOMA-IR scores in the medium and high groups remained significantly below those in the group with low levels of dietary live microbe intake (P < 0.05) After further adjustment for energy intake, recreational activity, fasting total cholesterol, fasting triglycerides, and HbA1c levels (Model 2), this relationship remained significant. The results of univariate weighted linear regression analyses for the association between all covariates and HOMA-IR values are provided in Supplementary Table S2. Smooth curve fitting was further used to examine the association between high levels of dietary microbe intake, in grams (adjusted as in Model 2), and HOMA-IR values (Fig. 1).
The Smooth curve fitting results for the relationship between high levels of dietary live microbe intake (in grams) and HOMA-IR values. At dietary live microbe intake levels below 184.67 g, HOMA-IR values trended downward with increases in microbe intake. When these levels exceeded 184.67 g, however, HOMA-IR values gradually trended upwards.
Stratified regression analyses
Lastly, stratified regression analyses were conducted using study data (Table 4). Relative to participants in the group with low levels of dietary microbe intake, individuals that were males, Mexican–American, had a lower income, exhibited higher levels of educational attainment, were current non-smokers, were obese, and engaged in recreational activity exhibited lower HOMA-IR scores in the group with high levels of dietary microbe intake. The interaction between ethnicity and the relationship between HOMA-IR values and dietary live microbe grouping was significant (P for interaction = 0.04). No other significant interactions were detected for other study variables.
Discussion
The present analyses revealed a negative association between the dietary intake of live microbes and the FBG, fasting insulin, and HOMA-IR values of healthy adults in the USA. These results remained consistent even when conducting stratified analyses based on smoking status, BMI, recreational activity levels, gender, and PIR. In analyses stratified by ethnicity, this negative relationship was only significant for Mexian-American individuals.
Foods can contain a range of live microorganisms such as bacteria, yeasts, and other fungi, with the levels thereof being impacted by food preparation and processing techniques as well as the methods and duration of food storage30. Fermented foods without subsequent processing usually exhibit very high levels of live microbes (> 107 CFU/g), whereas freshly prepared fruits and vegetables that have been peeled exhibit very low microbe levels (< 104 CFU/g)21, as do heat-treated fools. The three largest dietary sources of microorganisms include fermented dairy products, fruits, and vegetables27. Probiotic geniuses include lactic acid-producing bacteria (Streptococcus, Bifidobacterium, and Lactobacillus spp.) as well as other geniuses of bacteria including Bacillus and Propionibacterium31,32. In addition, certain species of probiotic yeasts have been identified (Saccharomyces) as well as non-spore-forming and non-flagellated rods or coccobacilli. The most prominent groups of probiotics are Bifidobacterium and Lactobacillus, both of which are lactic acid-producing groups. Mechanistically, probiotics can influence the metabolism of glucose by generating short-chain fatty acids including acetate, butyrate, and propionate. The resultant secretion of incretin hormones can impact levels of glucose. In a prior report, Yadav et al. found that in mice, probiotic-derived butyrate can induce the secretion of the incretin hormone glucagon-like peptide 1 (GLP-1) in mouse models33. L-cell-derived GLP-1 can suppress glucagon production while simultaneously favoring the release of insulin34. Probiotics may exert their antidiabetic effects through other mechanisms including the enhancement of immunity and the production of anti-inflammatory cytokines, the suppression of oxidative stress35, and reductions in levels of intestinal permeability36. Live Lactobacillus reuteri have been shown to suppress nuclear NF-κB translocation and to prevent pro-inflammatory cytokine production, in addition to promoting the upregulation of nerve growth factor, thereby regulating pancreatic β-cell proliferation and inflammation37. Ejtahed et al. found that the intake of probiotics (Lactobacillus acidophilus and Bifidobacterium lactis) resulted in improved erythrocyte superoxide dismutase and glutathione peroxidase activity together with greater overall antioxidant activity38. However, considering the higher availability of food compared to probiotic supplements, research on live microbes in food has gradually attracted the attention of scientists in recent years39,40,41.
The present results suggest that the intake of foods rich in live microbes may be beneficial in adults owing to a negative correlation with IR. Smooth-curve fitting (Fig. 1) further indicated a negative association between the intake of high level live microbe food and IR when intake < 184.67 g. This negative trend gradually decreased as the intake increased. The relationship tended to stabilize when the intake was greater than 184.67 g. A significant association between the live microbe intake group and ethnicity was also detected, suggesting that ethnicity may also have an impact on the relationship between the intake of these microbes and HOMA-IR values. Mexican Americans were more likely to exhibit IR, and increases in dietary live microbe intake in this ethnic group were associated with HOMA-IR reductions (P < 0.01), whereas the same was not evident for other ethnic groups included in this study cohort. Longitudinal population-based research indicated that the blood glucose levels and HOMA-IR values of Mexican Americans were higher than those of other ethnic groups42. A genetic study focused on Mexican Americans also identified an important region on chromosome 12q24 containing several diabetes-related candidate genes. We found more pronounced associations between live microbe intake and IR in patients with obesity (BMI > = 30) compared to BMI < 30. These specific differences could reflect gut microbiota dysbiosis in obesity patients43. A steeper negative relationship was observed in no current smokers compared to current smokers. Smoking dysregulates the gut microbial profile, which could be the main reason for this phenomenon44. In addition, those with recreational activity demonstrated a more obvious negative association between live microbes intake and HOMA-IR, possibly due to activity optimizing human gut microbiota compositional and function45,46. However, this negative association was not observed in those less than high school education. This study is the first to our knowledge evaluating the link between the dietary intake of live microbes and IR in a large nationally representative group of adults from the USA. As these data were derived from the NHANES study, they were subject to extensive quality control for validation purposes. However, there are also certain limitations to these analyses. For one, the NHANES study was cross-sectional in design such that it is not possible to establish temporal or causal relationships among variables. In addition, the classification of dietary live microbe intake was based on expert opinions and consultation with microbiologists. Efforts to directly detect or culture these microbes may yield more reliable and precise results, although the high costs and substantial time investments that this would entail make this approach unrealistic. Third, the simplistic classification of patients into three groups based on levels of dietary live microbe intake may contribute to some degree of bias in these results, emphasizing a need for the investigation of other potential approaches to calculating such microbial intake. In addition, HOMA-IR values were used as an alternative to more reliable gold-standard measures of IR such as hyperinsulinemic-euglycemic clamping results. Moreover, this study only enrolled healthy subjects such that these results may not be generalizable to other populations. Lastly, these models were adjusted for covariates, but other undetected confounding variables still have the potential to affect these results.
Conclusions
In summary, these data suggest that the degree of dietary live microbe consumption is negatively related to HOMA-IR values. However, ethnicity, lifestyle, and physical condition differences appear to impact the magnitude of this relationship. Further studies with a larger sample size and more detailed dietary assessment and other confounding factors are needed to verify and extend our findings.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes.
References
Hill, C. et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. https://doi.org/10.1038/nrgastro.2014.66 (2014).
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137-4153.e4114. https://doi.org/10.1016/j.cell.2021.06.019 (2021).
Dimidi, E., Cox, S. R., Rossi, M. & Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients https://doi.org/10.3390/nu11081806 (2019).
Marco, M. L. et al. Should there be a recommended daily intake of microbes?. J. Nutr. 150, 3061–3067. https://doi.org/10.1093/jn/nxaa323 (2020).
Savaiano, D. A. & Hutkins, R. W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 79, 599–614. https://doi.org/10.1093/nutrit/nuaa013 (2021).
Rad, A. H. et al. The future of diabetes management by healthy probiotic microorganisms. Curr. Diabetes Rev. 13, 582–589. https://doi.org/10.2174/1573399812666161014112515 (2017).
Marco, M. L. & Sanders, M. E. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 18, 196–208. https://doi.org/10.1038/s41575-020-00390-5 (2021).
Roselli, M. et al. Colonization ability and impact on human gut microbiota of foodborne microbes from traditional or probiotic-added fermented foods: A systematic review. Front. Nutr. 8, 689084. https://doi.org/10.3389/fnut.2021.689084 (2021).
Taylor, B. C., Lejzerowicz, F., Poirel, M. & Shaffer, J. P. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems https://doi.org/10.1128/mSystems.00901-19 (2020).
Schwartz, M. W. & Kahn, S. E. Insulin resistance and obesity. Nature 402, 860–861. https://doi.org/10.1038/47209 (1999).
Brož, J. et al. Prevalence of diabetes and prediabetes and its risk factors in adults aged 25–64 in the Czech Republic: A cross-sectional study. Diabetes Res. Clin. Pract. 170, 108470. https://doi.org/10.1016/j.diabres.2020.108470 (2020).
Torres-Fuentes, C., Schellekens, H., Dinan, T. G. & Cryan, J. F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2, 747–756. https://doi.org/10.1016/s2468-1253(17)30147-4 (2017).
Wallace, T. M. & Matthews, D. R. The assessment of insulin resistance in man. Diabet. Med. 19, 527–534. https://doi.org/10.1046/j.1464-5491.2002.00745.x (2002).
Rudvik, A. & Månsson, M. Evaluation of surrogate measures of insulin sensitivity: Correlation with gold standard is not enough. BMC Med. Res. Methodol. 18, 64. https://doi.org/10.1186/s12874-018-0521-y (2018).
Kawada, T. Preliminary report: Homeostasis model assessment of insulin resistance, an indicator of insulin resistance, is strongly related to serum insulin: Practical data presentation and the mathematical basis. Metabolism 59, 1044–1046. https://doi.org/10.1016/j.metabol.2009.10.028 (2010).
Naseri, K. et al. Probiotics and synbiotics supplementation improve glycemic control parameters in subjects with prediabetes and type 2 diabetes mellitus: A GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Pharmacol. Res. 184, 106399. https://doi.org/10.1016/j.phrs.2022.106399 (2022).
Yang, Y. et al. Efficacy of fecal microbiota transplantation in type 2 diabetes mellitus: A systematic review and meta-analysis. Endocrine 84, 48–62. https://doi.org/10.1007/s12020-023-03606-1 (2024).
Proença, I. M. et al. Fecal microbiota transplantation improves metabolic syndrome parameters: Systematic review with meta-analysis based on randomized clinical trials. Nutr. Res. 83, 1–14. https://doi.org/10.1016/j.nutres.2020.06.018 (2020).
Salari, A. et al. Effect of kefir beverage consumption on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. Complement. Ther. Clin. Pract. 44, 101443. https://doi.org/10.1016/j.ctcp.2021.101443 (2021).
Jeddi, M. Z. et al. Microbial evaluation of fresh, minimally-processed vegetables and bagged sprouts from chain supermarkets. J. Health Popul. Nutr. 32, 391–399 (2014).
Valentin-Bon, I., Jacobson, A., Monday, S. R. & Feng, P. C. Microbiological quality of bagged cut spinach and lettuce mixes. Appl. Environ. Microbiol. 74, 1240–1242. https://doi.org/10.1128/aem.02258-07 (2008).
Kase, J. A., Borenstein, S., Blodgett, R. J. & Feng, P. C. Microbial quality of bagged baby spinach and romaine lettuce: Effects of top versus bottom sampling. J. Food Prot. 75, 132–136. https://doi.org/10.4315/0362-028x.jfp-11-097 (2012).
Bock, P. M., Martins, A. F. & Schaan, B. D. Understanding how pre- and probiotics affect the gut microbiome and metabolic health. Am. J. Physiol. Endocrinol. Metab. https://doi.org/10.1152/ajpendo.00054.2024 (2024).
Salgaço, M. K., Oliveira, L. G. S., Costa, G. N., Bianchi, F. & Sivieri, K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl. Microbiol. Technol. 103, 9229–9238. https://doi.org/10.1007/s00253-019-10156-y (2019).
Patterson, E. et al. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 92, 286–300. https://doi.org/10.1136/postgradmedj-2015-133285 (2016).
Homayoni Rad, A., Mehrabany, E. V., Alipoor, B., Mehrabany, L. V. & Javadi, M. Do probiotics act more efficiently in foods than in supplements?. Nutrition 28, 733–736. https://doi.org/10.1016/j.nut.2012.01.012 (2012).
Marco, M. L. & Hutkins, R. A classification system for defining and estimating dietary intake of live microbes in US adults and children. J. Nutr. 152, 1729–1736. https://doi.org/10.1093/jn/nxac074 (2022).
Kernan, W. N. et al. Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374, 1321–1331. https://doi.org/10.1056/NEJMoa1506930 (2016).
Classification and Diagnosis of Diabetes. Standards of Medical Care in Diabetes-2019. Diabetes Care 42, S13-s28. https://doi.org/10.2337/dc19-S002 (2019).
Alp, D. & Bulantekin, Ö. The microbiological quality of various foods dried by applying different drying methods: A review. Eur. Food Res. Technol. A 247, 1333–1343. https://doi.org/10.1007/s00217-021-03731-z (2021).
Kim, Y. A., Keogh, J. B. & Clifton, P. M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 31, 35–51. https://doi.org/10.1017/s095442241700018x (2018).
Michels, N. et al. Human microbiome and metabolic health: An overview of systematic reviews. Obes. Rev. 23, e13409. https://doi.org/10.1111/obr.13409 (2022).
Yadav, H., Lee, J. H., Lloyd, J., Walter, P. & Rane, S. G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 288, 25088–25097. https://doi.org/10.1074/jbc.M113.452516 (2013).
Drucker, D. J. & Nauck, M. A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705. https://doi.org/10.1016/s0140-6736(06)69705-5 (2006).
Ejtahed, H. S. et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28, 539–543. https://doi.org/10.1016/j.nut.2011.08.013 (2012).
Zhai, L., Wu, J. & Lam, Y. Y. Gut-microbial metabolites, probiotics and their roles in type 2 diabetes. J. Mol. Sci. 22, 12846. https://doi.org/10.3390/ijms222312846 (2021).
Paszti-Gere, E. et al. Metabolites of Lactobacillus plantarum 2142 prevent oxidative stress-induced overexpression of proinflammatory cytokines in IPEC-J2 cell line. Inflammation 35, 1487–1499. https://doi.org/10.1007/s10753-012-9462-5 (2012).
Ejtahed, H. S. et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 94, 3288–3294. https://doi.org/10.3168/jds.2010-4128 (2011).
Chen, J., Fang, S., Huo, J. & Yang, N. The chain-mediating effect of Crp, BMI on the relationship between dietary intake of live microbes and hyperlipidaemia. Lipids Health Dis. 23, 130. https://doi.org/10.1186/s12944-024-02107-y (2024).
Lin, S., Zhu, N., Zhu, Y., Mao, H. & Zhang, S. Exploratory analysis on the association of dietary live microbe and non-dietary prebiotic/probiotic intake with serum cotinine levels in the general adult population. Front. Nutr. 11, 1405539. https://doi.org/10.3389/fnut.2024.1405539 (2024).
Yan, K. et al. Higher dietary live microbe intake is associated with a lower risk of sarcopenia. Clin. Nutr. 43, 1675–1682. https://doi.org/10.1016/j.clnu.2024.05.030 (2024).
Lorenzo, C., Hazuda, H. P. & Haffner, S. M. Insulin resistance and excess risk of diabetes in Mexican-Americans: The San Antonio Heart Study. J. Clin. Endocrinol. Metab. 97, 793–799. https://doi.org/10.1210/jc.2011-2272 (2012).
Sáez-Lara, M. J., Robles-Sanchez, C., Ruiz-Ojeda, F. J., Plaza-Diaz, J. & Gil, A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int. J. Mol. Sci. 17, 928. https://doi.org/10.3390/ijms17060928 (2016).
Redondo-Useros, N. & Nova, E. Microbiota and lifestyle: A special focus on diet. Nutrients 12, 1776. https://doi.org/10.3390/nu12061776 (2020).
Allen, J. M. et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50, 747–757. https://doi.org/10.1249/mss.0000000000001495 (2018).
Bayat, Z., Damirchi, A., Hasannejad-Bibalan, M. & Babaei, P. Metabotropic effect of probiotic supplementation and high-intensity interval training in menopause-induced metabolic syndrome in rats. J. Menopausal. Med. 29, 29–39. https://doi.org/10.6118/jmm.22037 (2023).
Acknowledgements
We would like to thank the NHANES team for providing the data. We would also like to thank Zhang Jing (Second Department of Infectious Disease, Shanghai Fifth People's Hospital, Fudan University) for his work (nhanesR package and webpage) on the NHANES database.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YJS, SCG, CYJ, ZJY, WYY and CQW—data collection and analysis; SCG, YJS and CYJ—wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, S., Jiang, C., Yu, Z. et al. The relationship between dietary intake of live microbes and insulin resistance among healthy adults in the US: a cross-sectional study from NHANES 2003–2020. Sci Rep 14, 17666 (2024). https://doi.org/10.1038/s41598-024-68243-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68243-8
- Springer Nature Limited