Abstract
A prospective observational study comparing mechanical power density (MP normalized to dynamic compliance) with traditional spontaneous breathing indexes (e.g., predicted body weight normalized tidal volume [VT/PBW], rapid shallow breathing index [RSBI], or the integrative weaning index [IWI]) for predicting prolonged weaning failure in 140 tracheotomized patients. We assessed the diagnostic accuracy of these indexes at the start and end of the weaning procedure using ROC curve analysis, expressed as the area under the receiver operating characteristic curve (AUROC). Weaning failure occurred in 41 out of 140 patients (29%), demonstrating significantly higher MP density (6156 cmH2O2/min [4402–7910] vs. 3004 cmH2O2/min [2153–3917], P < 0.01), lower spontaneous VT/PBW (5.8 mL*kg−1 [4.8–6.8] vs. 6.6 mL*kg−1 [5.7–7.9], P < 0.01) higher RSBI (68 min−1*L−1 [44–91] vs. 55 min−1*L−1 [41–76], P < 0.01) and lower IWI (41 L2/cmH2O*%*min*10−3 [25–72] vs. 71 L2/cmH2O*%*min*10-3 [50–106], P < 0.01) and at the end of weaning. MP density was more accurate at predicting weaning failures (AUROC 0.91 [95%CI 0.84–0.95]) than VT/PBW (0.67 [0.58–0.74]), RSBI (0.62 [0.53–0.70]), or IWI (0.73 [0.65–0.80]), and may help clinicians in identifying patients at high risk for long-term ventilator dependency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Determining a patient’s spontaneous breathing ability is one of the most challenging tasks during prolonged weaning. Patients unable to maintain autonomic breathing usually remain ventilator-dependent over the long term, resulting in reduced health-related quality of life1 and high mortality rates2. Previous studies on weaning predictors have focused on variables associated with spontaneous breathing and indexes derived from esophageal pressure measurements (e.g., diaphragmatic tension-time index)3,4, quantifying respiratory muscle workload, a key factor in prolonged weaning failure. However, most of these single parameters (e.g., respiratory rate, tidal volume) are not accurate at predicting weaning outcomes, most likely because they do not reflect weaning failure’s underlying pathophysiology, and the complexity of the procedure prevents esophageal catheters from being integrated into daily routines.
Mechanical power (MP) is the displacement work provided by the ventilator each minute5, with ventilatory variables determining MP adjusted by clinicians to maintain patient oxygenation and decarboxylation (e.g., positive end-expiratory pressure, minute ventilation). As such, MP could serve to estimate respiratory muscle workload during spontaneous, unassisted breathing in an indirect manner. There is preliminary evidence that MP normalized to dynamic lung-thorax compliance, essentially a pressure-rate index measuring the intensity of mechanical stress exerted on the respiratory system and consistent with MP density, may help separate patients experiencing weaning failure or success6,7. However, MP density has not yet been compared to spontaneous breathing indexes for predicting a patient’s autonomic breathing ability following prolonged mechanical ventilation.
The study objectives were (1) to evaluate MP density’s predictive performance compared to traditional breathing indexes assessed during a protocolized spontaneous breathing trial (SBT) regarding weaning failure in prolonged ventilated, tracheotomized patients, (2) to analyze the interactions between ventilatory and spontaneous breathing indexes reflecting respiratory muscle workload, and (3) to correlate these indexes with PaCO2 during spontaneous breathing at the end of weaning.
Methods
We conducted a prospective, single-center observational cohort study at a German national weaning center. The local institutional review board for human studies approved the project (Ethics Committee of the State Chamber of Physicians of Baden-Wuerttemberg, Germany, file number F-2021–118), which was performed following the Declaration of Helsinki. All patients or their legal representatives provided written informed consent.
Patient selection
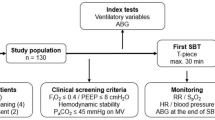
We included tracheotomized subjects referred from ICUs across Germany to our center between September 2021 and May 2023 for “prolonged weaning” according to the WIND criteria [Fig. 1]. By definition, these patients were still on mechanical ventilation 7 days after their first separation attempt, which in most cases was an SBT with or without subsequently attempted extubation in the referring ICU8. The referring facilities included both external and internal ICUs in a 50:50 ratio. Exclusion criteria were a diagnosis of neuromuscular disease, death during weaning, and declined consent for participation6.
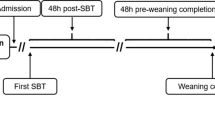
Patient flow diagram.*Refers to the WIND criteria for prolonged weaning8.
Data collection
Baseline characteristics, including demographics, clinical features, and comorbidities, were collected on patients upon weaning center admission. We created a protocol for the documentation of the first (start of weaning) and last (end of weaning) SBT [Supplementary file 1] and summarized the results of prolonged weaning. Finally, a clinical follow-up was conducted up to 7 days after weaning completion to determine if progressive hypercapnia developed during spontaneous breathing, requiring resumption of mechanical ventilation and indicating weaning failure [Fig. 2].
Ventilator weaning process
Upon weaning center admission, pressure-controlled, assist-control (A/C mode, without setting a target volume) mechanical ventilation (Elisa 600, Löwenstein Medical, Bad Ems, Germany) was used on all patients to unload the respiratory pump effectively during both an assisted and controlled breath. With this mode, an assisted breath does not necessarily indicate a high level of spontaneous activity. When the ventilator is triggered with minimal effort, it delivers a breath similar to controlled ventilation (passive insufflation occurs)9.
As described previously, a standardized method of ventilator liberation was employed as soon as clinical screening criteria for weaning readiness were met, including inspired oxygen fraction (FiO2) ≤ 0.4, positive end-expiratory pressure (PEEP) ≤ 8 cmH2O, stable hemodynamics without vasopressors or inotropic agents, and normocapnia with mechanical ventilation6. According to protocol, ventilator weaning always starts with a 30 min SBT. These weaning trials (using a T-piece) are usually conducted once daily, and their duration is typically extended by 2–3 h per day, aiming to achieve sustained autonomic breathing. Following ventilator weaning, patients were evaluated for decannulation, with those demonstrating persistent ventilatory failure (hypercapnia) transitioning to non-invasive ventilation whenever possible10.
Spontaneous breathing trials
Analyses were conducted on a protocolized SBT at the start (first SBT) and end of weaning (last SBT), which used continuous positive airway pressure (CPAP) to assess patients` spontaneous breathing variables.
As a first step, patients were placed in the semi-recumbent position while mechanically ventilated, “ventilatory variables” were recorded, and an arterial blood gas analysis (ABG) was performed. Next, we applied CPAP, which was set at the same level as PEEP during A/C ventilation. CPAP breathing was limited to 30/60 min at first/last SBTs [Fig. 2], with patients’ “spontaneous breathing variables” recorded from the ventilators display at the weaning trials beginning (after 5 min) and completion (after 30/60 min of the first/last SBT). Another ABG was performed at the end of each trial.
Ventilatory variables and indexes
According to protocol, ventilatory variables and ABG measurements were recorded immediately before the SBT. Variables collected included FiO2, respiratory rate (RR), tidal volume (VT), peak inspiratory airway pressure (Pmax), and PEEP, with the following parameters calculated: ∆Paw (dynamic driving pressure, defined as Pmax—PEEP), dynamic lung-thorax compliance (LTCdyn, defined as VT/∆Paw), ventilatory ratio (VR; a measure of ventilation efficiency, correlating with the pulmonary dead-space fraction)11, and MP utilizing a simplified formula for pressure-control ventilation12.
Mechanical power and MP density
With each mechanical breath, the pressure required to inflate the lungs and expand the thoracic cage is used to overcome the resistance of the airways (dissipating heat) and the elastance of the lungs and chest wall. As such, mechanical energy is the displacement work (pressure times volume) the ventilator provides per breath as determined by applied Pmax (including PEEP) and VT during pressure-controlled ventilation12. Concerning elastance, given that the lungs and thorax expand predominantly elastically, most of the energy is stored with each breath, which ultimately drives expiration (through respiratory systems’ elastic recoil pressure)13.
The same mechanical energy per breath can be achieved with various combinations of Pmax and VT, as determined by the amount of aerated lung tissue, chest wall elastance, and airway resistance. Dynamic respiratory system compliance provides a simple proxy for all these factors. Normalizing energy per breath to dynamic compliance estimates the relative contributions of Pmax and VT to a particular energy level. When compliance is low, higher pressure is required to achieve the same amount of energy as when compliance is high, in which, despite lower pressures, greater tidal volumes are generated. In other words, at a given tidal volume (strain), lower compliance results in more energy being transferred per breath, resulting in higher (strain) “energy density”. Given this, energy density mainly represents the stored energy per unit of (tidal) volume resulting from the body’s elastic deformation (resistive-elastic unicompartmental model)14.
Multiplying the energy per breath by the respiratory rate results in mechanical power, the amount of energy transferred to the respiratory system per minute. The “MP density” is calculated by multiplying the energy density—energy per breath normalized to dynamic compliance—with the respiratory rate, quantifying the energy transferred per minute with respect to the volume applied per minute.
This index measures the intensity of mechanical stress placed on the respiratory system, determined by peak airway pressure, dynamic driving pressure, and the frequency of their application (pressure application per time), quantifying the time rate (or speed) of energy transfer per unit of volume displaced. It has also been referred to as the “specific MP”, which is the power in relation to the ventilated lung volume15.
In a subsequent step, LTCdyn-MP was normalized to PaCO2 similarly to the corrected minute ventilation16 to approximate the MP density required for adequate decarboxylation. We used 45 mmHg as the target PaCO2 originally termed the respiratory system Power index6. For convenience, LTCdyn-MP and its PaCO2 normalized values will be referred to as “MP density indexes” throughout the manuscript. Detailed descriptions and equations for all computed variables are provided in the online supplement [Supplementary file 1].
Spontaneous breathing variables and indexes
We collected the following variables during CPAP breathing, as per protocol, at the weaning trials onset (after 5 min) and completion (after 30/60 min of first/last SBT): FiO2, peripheral oxygen saturation measured with pulse oximetry (SpO2), respiratory rate, tidal volume, minute ventilation, and the ratio of inspiratory to expiratory time. We calculated the following parameters using these variables: tidal volume normalized to the predicted body weight (VT/PBW), rapid shallow breathing index (RSBI; also referred to as frequency-to-tidal volume ratio)17, and a modified integrative weaning index (IWI modified; using LTCdyn instead of the static respiratory system compliance)18.
Classification of weaning outcomes
At the end of the ventilator weaning process, patients were categorized into two groups exclusively based on spontaneous breathing abilities: weaning failure and success6. Weaning failure is defined as “long-term ventilator dependence” due to persistent ventilatory failure with transitioning to domiciliary ventilation by face mask or tracheostomy tube. Ventilatory failure describes recurrent hypercapnia (PaCO2 > 45 mmHg) during daily weaning trials (observed on at least two consecutive days), preventing the extension of spontaneous unassisted breathing or persistent hypercapnia (on at least two successive occasions) occurring within seven days after weaning completion. These patients usually remain ventilator-attached at discharge. Conversely, the definition of successful weaning is sustained “autonomic breathing” (≥ 7 days) without concomitant signs of ventilatory failure (hypercapnia) after weaning completion (median PaCO2 ≤ 45.0 mmHg derived from the highest measured PaCO2 on each of the seven days), determined by the last day on which the patient was ventilated. These patients typically remain ventilator-detached at hospital discharge.
Primary and secondary study outcomes
The study`s primary objective was to compare MP density (before SBT) with spontaneous breathing indexes (during SBT) regarding prolonged weaning failure prediction. Secondary outcomes included an analysis of the interaction between ventilatory and spontaneous breathing indexes reflecting respiratory muscle workload and the parameters` correlation to spontaneous breathing PaCO2.
Statistical analysis and sample size determination
Descriptive and frequency statistics summarized patients` baseline demographics, clinical characteristics, and comorbidities. A Chi-square or Fisher’s exact test was used when comparing categorical variables. Depending on the continuous variables` homogeneity of variance, determined by the Kolmogorov-Smirnov normality test, differences were analyzed through Student’s t-test or Mann-Whitney U-test.
First, we performed bivariate comparisons of ventilatory and spontaneous breathing indexes between weaning failure and success patients at the onset and end of the weaning process. Next, we assessed the ability of these indexes to predict prolonged weaning failure by performing a receiver operating characteristic (ROC) curve analysis in the entire study population, with diagnostic accuracy expressed as the area under the ROC curve (AUROC). Moreover, we performed 2-time repeated, fivefold cross-validation to evaluate the internal validity of these indexes. The resulting diagnostic performance of each parameter was expressed as sensitivity, specificity, positive/negative predictive value, accuracy, positive/negative likelihood ratio, diagnostic odds ratio (DOR), F1 score, and Matthews` correlation coefficient (MCC). Rank correlations and linear regression analyses were conducted to determine interactions between ventilatory (e.g., dynamic compliance, power density) and spontaneous breathing indexes (e.g., RSBI) reflecting respiratory muscle workload. Finally, we correlated indexes assessed at last SBT with median spontaneous breathing PaCO2 at the end of weaning (during follow-up) using Spearmans` correlation coefficient (ρ).
We conducted sensitivity analyses by redefining the PaCO2 threshold for ventilatory failure (to > 50 mmHg) or restricting the analysis to patients without COVID-19 pneumonia as the leading cause of prolonged ventilation, as their respiratory mechanics may differ from those of other respiratory failure patients19. Furthermore, we reanalyzed rank correlations (ρ) between parameters and PaCO2 limited to weaning success patients.
Based on an α-level (type-I error) of 0.05 with power (1‒β) of 80%, an expected prevalence of prolonged weaning failure of 40%, and an expected MP density`s AUROC of 0.80–0.90 for prolonged weaning failure prediction (at the end of weaning)6, we calculated a sample size of about 140 patients for detecting AUROC differences of at least 10% between power density and spontaneous breathing indexes.
We performed two-tailed tests; statistical significance was indicated by P < 0.05. The analyses were conducted with MedCalc® statistical software v20.305 (MedCalc Software Ltd, Ostend, Belgium; http://www.medcalc.org; 2023).
Ethics approval and consent to participate
The study was approved by the local institutional review board for human studies (Ethics Committee of the State Chamber of Physicians of Baden-Wuerttemberg, Germany, approval number F–2021–118) and performed following the Declaration of Helsinki. Written informed consent was obtained from all patients or a legal representative.
Results
Of 150 consecutive patients screened between September 2021 and May 2023, 140 (93%) were included in the analyses. The reasons for exclusion were a confirmed diagnosis of neuromuscular disease in two patients, five patients died during weaning, and three patients declined consent [Fig. 1]. Clinical characteristics differed between patients with weaning failure and success, mainly regarding gender and associated PBW, smoking history, pre-existing domiciliary non-invasive ventilation, and chronic obstructive pulmonary disease (COPD) as comorbidity [Table 1].
Weaning failure occurred in 41 patients (29%) [Fig. 1, Supplementary file 1: Table S1], with significant differences between groups in ventilatory and spontaneous breathing variables at the first and last SBT [Supplementary file 1: Table S2-3].
Ventilatory variables and indexes
During mechanical ventilation before the onset of weaning, weaning failure patients showed lower dynamic compliance (median 32 mL/cmH2O with IQR [29–39] vs. 39 mL/cmH2O [33–47], P < 0.01) and higher PaCO2 (39 mmHg [35–43] vs. 33 mmHg [28–38], P < 0.01). Even though minute ventilation was lower in the failure group, these patients demonstrated significantly higher ventilatory ratios (1.65 [1.39–2.11] vs. 1.40 [1.26–1.73], P < 0.01). There was no difference in MP (21.6 Joule/min [17.8–26.3] vs. 22.3 Joule/min [19.1–27.4], P = 0.405), but MP density (measured by the Power indexrs2.0) was consistently higher in weaning failures (5116 cmH2O2/min [3601–7020] vs. 3004 cmH2O2/min [2034–4676], P < 0.01) [Supplementary file 1: Table S2]. Analyzing ventilatory variables before the last SBT yielded similar results [Fig. 3, Supplementary file 1: Table S3].
Between-group differences in selected ventilatory and breathing indexes at the end of weaning (last SBT). Comparison of selected ventilatory and spontaneous breathing indexes between weaning failure and success patients at the end of weaning (last SBT), as illustrated by Box-Whiskers plots. MP density refers to the Power indexrs2.0. VT/PBW tidal volume normalized to the predicted body weight, IWI modified modified integrative weaning index.
Spontaneous breathing variables and indexes
As weaning trials concluded, an increase in respiratory rate was apparent, accompanied by a drop in tidal volume, which was more prominent in the weaning failure group, resulting in significantly lower minute ventilation (9.7 L*min−1 [7.8–11.4] vs. 11.7 L*min−1 [9.5–13.9], P < 0.01), lower VT/PBW (5.8 mL*kg−1 [5.1–6.7] vs. 6.6 mL*kg−1 [6.0–7.9], P < 0.01), higher RSBI (69 min−1*L−1 [54–97] vs. 58 min−1*L−1 [43–73], P < 0.01), and lower IWI (43 L2/cmH2O*%*min*10−3 [31–58] vs. 62 L2/cmH2O*%*min*10−3 [46–91], P < 0.01) at the start of weaning [Supplementary file 1: Table S2]. The failure group also exhibited significantly higher PaCO2 (46 mmHg [42–51] vs. 36 mmHg [32–43], P < 0.01) [Supplementary file 1: Table S2]. Similar results were obtained from analyzing variables at the end of weaning [Fig. 3, Supplementary file 1: Table S3].
Diagnostic accuracy of ventilatory and breathing indexes
MP density (as measured by the Power indexrs2.0) demonstrated moderate diagnostic performance at the start of weaning (first SBT) (AUROC 0.76 [95%CI 0.68‒0.83]) and excellent accuracy at the end of weaning (last SBT) (AUROC 0.91 [0.84‒0.95], DOR 48, MCC 0.55) [Table 2, Supplementary file 1: Table S4]. According to AUROC measurements, MP density indexes at the start and end of weaning, as well as dynamic compliance, provided greater diagnostic accuracy in predicting weaning failure than spontaneous breathing indexes [Table 2, Fig. 4, Supplementary file 1: Table S4, Figure S1]. Similar results were obtained in the sensitivity analyses [Supplementary file 1: Table S5-6].
Comparison of ROC curves for selected ventilatory and breathing indexes used to predict weaning outcome at the end of weaning (last SBT). AUROC differed significantly between MP density (measured by the Power indexrs2.0) and mechanical power (P < 0.01), LTCdyn (P < 0.01), VT/PBW (P < 0.01), RSBI (P < 0.01), and IWI modified (P < 0.01). ROC receiver operating characteristic curve, SBT spontaneous breathing trial, LTCdyn dynamic lung-thorax compliance, IWI modified modified integrative weaning index, RSBI rapid shallow breathing index, VT/PBW tidal volume normalized to the predicted body weight, AUROC area under the receiver operating characteristic curve.
Correlations between ventilatory and breathing indexes
Rank correlations and linear regression analyses were used to analyze interactions between ventilatory (e.g., MP density, dynamic compliance) and spontaneous breathing indexes (e.g., RSBI) reflecting respiratory muscle workload. Specifically, MP density (ρ = 0.18 [95%CI 0.06–0.29] for the Power indexrs2.0; P < 0.01) and LTCdyn (ρ = − 0.33 [− 0.43 to − 0.22]; P < 0.01) revealed a significant correlation with the RSBI, as well as an independent relationship in linear regression analysis [Fig. 5].
The rapid shallow breathing index as a function of MP density and dynamic lung-thorax compliance: linear regression analysis. An analysis of 280 RSBI and MP density (measured by the Power indexrs2.0)/LTCdyn measurements at the onset (first SBT) and end of weaning (last SBT). RSBI rapid shallow breathing index, LTCdyn dynamic lung-thorax compliance.
Correlations with spontaneous PaCO2
Most ventilatory and breathing indexes correlated moderately with spontaneous breathing PaCO2 at weaning completion [Fig. 6, Supplementary file 1: Table S7], whereas MP density had a strong correlation (ρ = 0.73 [95%CI 0.64–0.80] for the Power indexrs2.0), maintained in a sensitivity analysis limited to 99 weaning success patients (ρ = 0.55 [95%CI 0.39–0.67]) [Supplementary file 1: Table S8].
Correlations of selected ventilatory and breathing indexes at the end of weaning (last SBT) with spontaneous PaCO2. The heat map of Spearman`s correlation coefficients (ρ) with the LOESS (Local Regression Smoothing) trendline. Some Y axes have logarithmic scales. MP density refers to the Power indexrs2.0. ρ Spearman`s correlation coefficient (with 95% confidence interval), VT/PBW tidal volume normalized to the predicted body weight, IWI modified modified integrative weaning index.
Exploratory analyses
COPD was significantly more prevalent among weaning failure patients. These patients mainly experienced significant differences in respiratory mechanics, MP density, and PaCO2 levels during mechanical ventilation and spontaneous breathing at the end of SBT [Supplementary file 1: Table S9].
Discussion
Study results can be summarized as follows: Following prolonged weaning, weaning failures differed significantly in ventilatory and spontaneous breathing indexes compared to patients who sustained autonomic breathing (weaning success). While MP did not differ between groups, MP density indexes were significantly higher in patients experiencing weaning failure. These parameters were more accurate at predicting weaning failures than traditional indexes assessed during spontaneous breathing (e.g., VT/PBW, RSBI, or IWI), demonstrating significant correlations and independent associations with parameters reflecting respiratory workload (e.g., RSBI). A strong correlation was observed between MP density indexes and spontaneous breathing PaCO2 at the end of the weaning process.
With pressure-controlled ventilation, dynamic lung-thorax compliance determines tidal volume, minute ventilation, and MP at a given respiratory rate, Pmax, and PEEP12. If the latter three variables remain constant, a decrease in compliance will result in a linear decline in MP. Adjusting Pmax to achieve the original (iso)-MP (at constant respiratory rate and PEEP) will result in reduced associated tidal volume and minute ventilation—compared to the values before compliance decreased—along with increased energy density and MP density. The reason is that every change in pressure is accompanied by an increase or decrease in tidal volume, which translates into an exponential variation in mechanical energy. In other words, as compliance decreases, ventilator-generated energy and airflow become uncoupled. Conversely, when applied to spontaneous breathing conditions, optimal coupling allows most respiratory pump energy to convert into airflow, rendering breathing more “efficient” (e.g., without ineffective efforts). As such, iso-MP may predict long-term ventilator dependence or autonomic breathing based on whether it is generated by high pressures along with low tidal volumes (consistent with high energy density and low compliance) or low pressures and high tidal volumes (low energy density at high compliance), referring to the degree of (un)coupling between energy and airflows.
MP density was significantly more accurate than traditional indexes (e.g., minute ventilation, VT/PBW, RSBI, or IWI) in predicting weaning outcomes, most likely because it better reflects prolonged weaning failure`s underlying pathophysiology, apparently an excessive respiratory muscle effort for carbon dioxide elimination. This aligns with previous studies on MP and weaning, in which MP density (e.g., LTCdyn-MP) has consistently demonstrated the most robust predictive ability regarding failure to wean6,7,20. This index incorporates respiratory rate and airway pressures, factors that also have been independently associated with weaning failure in the recent multicenter WEAN SAFE study21. MP density has also been shown to be an accurate predictor of prolonged ventilation after double lung transplantation, with LTCdyn-MP demonstrating the strongest correlation with invasive ventilation time following a patient’s transfer from the operating room to the ICU22. However, further research is required as other studies have demonstrated conflicting findings23.
IWI showed reasonable diagnostic accuracy, averaging between RSBI and dynamic respiratory system compliance, which are factored into this index. Interestingly, high VT/PBW was associated with sustained autonomic breathing, unlike clinical settings involving acute respiratory failures, where higher values predict escalating respiratory treatment (e.g., to invasive ventilation)24. This index probably reflects different pathophysiological mechanisms in acute and chronic respiratory failure, indicating a high respiratory drive in the acute setting25 but increased lung capacity during prolonged weaning. Similarly, a recent study of patients with non-cardiac thoracic surgery revealed an increased risk of postoperative pulmonary complications (e.g., respiratory failure) that were independently associated with a lower VT/PBW and a higher MP in conjunction with reduced dynamic respiratory system compliance26.
Regarding RSBI, this index has been developed to test a mechanically ventilated patient’s capacity to tolerate unassisted breathing17. Individuals who cannot tolerate a weaning trial typically develop a breathing pattern characterized by a high respiratory rate and low tidal volume immediately after disconnecting from the ventilator27. A limited number of studies examined the RSBI in the context of prolonged weaning, revealing poor diagnostic accuracy in predicting weaning outcomes3,4,28. Based on the MP density concept, reducing tidal volume will cause muscle stress to decline exponentially, whereas increasing respiratory rate will increase stress intensity linearly. Given this, a likely explanation for developing a rapid and shallow breathing pattern in general (e.g., during acute respiratory failure)29 is that it imposes less respiratory muscle stress (reducing the oxygen cost of breathing) and protects the respiratory pump against (fatal) failure. Despite RSBI being higher in weaning failures in the present study, the threshold that discriminated best between groups (61 min−1 * L−1) was lower compared to prior studies of intubated patients17. This observation may indicate differences in fatigue thresholds for acute respiratory distress (during a short weaning trial) and ventilatory failure (chronic hypercapnia), depending on the degree of stress intensity placed on respiratory muscles30,31. Accordingly, we found a significant correlation and independent relationship between MP density, dynamic compliance (mainly determining MP density), and RSBI, and this may also help explain why factors such as female gender and smaller endotracheal tube sizes (dynamic compliance is reduced due to smaller lung size and increased artificial airway resistance) are associated with higher RSBI32.
Although studies have not evaluated the relationship between MP or MP density and spontaneous breathing PaCO2, previous research has demonstrated compromised respiratory mechanics associated with increased respiratory variables used to calculate MP in hypercapnic patients failing SBT27. Since MP density demonstrated the strongest correlation with PaCO2 in the present study, this index may be a key determinant of autonomic breathing.
Limitations
This study has limitations. Our computations for pressure-controlled ventilation were based on a simplified equation associated with a higher error rate than more comprehensive formulas33. Furthermore, ICU ventilators have significant inaccuracies in measuring pressures and flows compared to pressure transducers and flowmeters34, affecting breathing variables calculations. Another limitation is that no information is available regarding patients’ respiratory drive and inspiratory effort before and during SBT. Finally, we did not compare MP density with other measures of spontaneous work of breathing, such as esophageal manometry-derived tidal changes in esophageal pressure25, spontaneous mechanical power ratio and pressure-rate index (4DPRR)35, or changes in oxygen consumption during SBT36, which should be explored in future research.
Conclusions
Several ventilatory and spontaneous breathing indexes readily available at the bedside can provide a comprehensive picture of a patient’s respiratory condition and assist clinicians in evaluating a patient’s spontaneous breathing ability following prolonged weaning. Study findings suggest the critical factor underlying sustained autonomic breathing is the mechanical stress intensity required for decarboxylation. MP density, based on thermodynamic principles, may help define and quantify stress intensity via less invasive, indirect methods, and it was more accurate at predicting weaning failures than established indexes assessed during spontaneous breathing.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Huttmann, S. E., Magnet, F. S., Karagiannidis, C., Storre, J. H. & Windisch, W. Quality of life and life satisfaction are severely impaired in patients with long-term invasive ventilation following ICU treatment and unsuccessful weaning. Ann. Intensive Care 8, 38 (2018).
Jubran, A. et al. Long-term outcome after prolonged mechanical ventilation. A long-term acute-care hospital study. Am. J. Respir. Crit. Care Med. 199, 1508–1516 (2019).
Purro, A. et al. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am. J. Respir. Crit. Care Med. 161, 1115–1123 (2000).
Carlucci, A. et al. Determinants of weaning success in patients with prolonged mechanical ventilation. Crit. Care 13, R97 (2009).
Gattinoni, L. et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 42, 1567–1575 (2016).
Ghiani, A., Paderewska, J., Walcher, S. & Neurohr, C. Mechanical power normalized to lung-thorax compliance predicts prolonged ventilation weaning failure: A prospective study. BMC Pulm. Med. 21, 202 (2021).
Yan, Y. et al. Mechanical power is associated with weaning outcome in critically ill mechanically ventilated patients. Sci. Rep. 12, 19634 (2022).
Beduneau, G. et al. Epidemiology of weaning outcome according to a new definition. The WIND study. Am. J. Respir. Crit. Care Med. 195, 772–783 (2017).
Telias, I. et al. The physiological underpinnings of life-saving respiratory support. Intensive Care Med. 12, 1–13 (2022).
Ghiani, A. et al. Incidence, causes, and predictors of unsuccessful decannulation following prolonged weaning. Ther. Adv. Chron. Dis. 13, 20406223221109656 (2022).
Sinha, P., Fauvel, N. J., Singh, P. & Soni, N. Analysis of ventilatory ratio as a novel method to monitor ventilatory adequacy at the bedside. Crit. Care 17, R34 (2013).
Chiumello, D. et al. Bedside calculation of mechanical power during volume- and pressure-controlled mechanical ventilation. Crit. Care 24, 417 (2020).
Bates, J. H. T., Kaczka, D. W., Kollisch-Singule, M., Nieman, G. F. & Gaver, D. P. Mechanical power and ventilator-induced lung injury: What does physics have to say?. Am. J. Respir. Crit. Care Med. 209, 787–788 (2024).
Faffe, D. S. & Zin, W. A. Lung parenchymal mechanics in health and disease. Physiol. Rev. 89, 759–775 (2009).
Marini, J. J., Rocco, P. R. M. & Gattinoni, L. Static and dynamic contributors to ventilator-induced lung injury in clinical practice. Pressure, energy, and power. Am. J. Respir. Crit. Care Med. 201, 767–774 (2020).
Wexler, H. R. & Lok, P. A simple formula for adjusting arterial carbon dioxide tension. Can. Anaesth. Soc. J. 28, 370–372 (1981).
Yang, K. L. & Tobin, M. J. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N. Engl. J. Med. 324, 1445–1450 (1991).
Nemer, S. N. et al. A new integrative weaning index of discontinuation from mechanical ventilation. Crit. Care 13, R152 (2009).
Ghiani, A. et al. Ventilatory ratio and mechanical power in prolonged mechanically ventilated COVID-19 patients versus respiratory failures of other etiologies. Ther. Adv. Respir. Dis. 17, 17534666231155744 (2023).
Yan, Y. et al. Development and validation of a mechanical power-oriented prediction model of weaning failure in mechanically ventilated patients: A retrospective cohort study. BMJ Open 12, e066894 (2022).
Pham, T. et al. Weaning from mechanical ventilation in intensive care units across 50 countries (WEAN SAFE): A multicentre, prospective, observational cohort study. Lancet Respir. Med. 11, 465–476 (2023).
Ghiani, A. et al. Mechanical power density predicts prolonged ventilation following double lung transplantation. Transpl. Int. 36, 11506 (2023).
Tartler, T. M. et al. High mechanical power and driving pressure are associated with postoperative respiratory failure independent from patients’ respiratory system mechanics. Crit. Care Med. 52, 68–79 (2024).
Carteaux, G. et al. Failure of non-invasive ventilation for de novo acute hypoxemic respiratory failure: Role of tidal volume. Intensive Care Med. 44, 282–290 (2016).
Tonelli, R. et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure. A pilot study. Am. J. Respir. Crit. Care Med. 202, 558–567 (2020).
Elefterion, B. et al. Intraoperative mechanical power and postoperative pulmonary complications in noncardiothoracic elective surgery patients: A 10 year retrospective study. Anesthesiology 140, 399–408 (2024).
Jubran, A. & Tobin, M. J. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am. J. Respir. Crit. Care Med. 155, 906–915 (1997).
Karakurt, Z. et al. Physiologic determinants of prolonged mechanical ventilation in patients after major surgery. J. Crit. Care 27, e9-16 (2012).
Acar, H. & Yamanoglu, A. Rapid shallow breathing index as a predictor for intubation and mortality in acute respiratory failure. Respir. Care 67, 562–571 (2022).
Bellemare, F. & Grassino, A. Effect of pressure and timing of contraction on human diaphragm fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 53, 1190–1195 (1982).
Laghi, F. et al. Is weaning failure caused by low-frequency fatigue of diaphragm?. Am. J. Respir. Crit. Care Med. 167, 120–127 (2003).
Epstein, S. K. & Ciubotaru, R. L. Influence of gender and endotracheal tube size on preextubation breathing pattern. Am. J. Respir. Crit. Care Med. 154, 1647–1652 (1996).
Trinkle, C. A., Broaddus, R. N., Sturgill, J. L., Waters, C. M. & Morris, P. E. Simple, accurate calculation of mechanical power in pressure-controlled ventilation (PCV). Intensive Care Med. Exp. 10, 22 (2022).
Garnier, M. et al. Multifaceted bench comparative evaluation of latest intensive care unit ventilators. Br. J. Anaesth. 115, 89–98 (2015).
Gattarello, S. et al. Mechanical power ratio and respiratory treatment escalation in COVID-19 pneumonia: A secondary analysis of a prospectively enrolled cohort. Anesthesiology 138, 289–298 (2023).
Lee, I. H. et al. Kinetics of oxygen uptake during unassisted breathing trials in prolonged mechanical ventilation: A prospective pilot study. Sci. Rep. 10, 14301 (2020).
Author information
Authors and Affiliations
Contributions
Study concept and design: AG. Acquisition of Data: AG, SW, AL, JP. Analysis and interpretation of data: AG, SW, AL, JP, SJ, NK, SSS, FCT, CN. First draft of the manuscript: AG. Critical manuscript revision for important intellectual content: AG, SW, AL, JP, SJ, NK, SSS, FCT, CN. Statistical analysis: AG. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghiani, A., Walcher, S., Lutfi, A. et al. Mechanical power density, spontaneous breathing indexes, and prolonged weaning failure: a prospective cohort study. Sci Rep 14, 16297 (2024). https://doi.org/10.1038/s41598-024-67237-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67237-w
- Springer Nature Limited