Abstract
The dingo is a wild dog endemic to Australia with enigmatic origins. Dingoes are one of two remaining unadmixed populations of an early East Asian dog lineage, the other being wild dogs from the New Guinea highlands, but morphological connections between these canid groups have long proved elusive. Here, we investigate this issue through a morphometric study of ancient dingo remains found at Lake Mungo and Lake Milkengay, in western New South Wales. Direct accelerated mass spectrometry (AMS) radiocarbon dates from an ancient Lake Mungo dingo demonstrate that dingoes with a considerably smaller build than the predominant modern morphotype were present in semi-arid southeastern Australia c.3000–3300 calBP. 3D geometric morphometric analysis of a near-complete Mungo cranium finds closest links to East Asian and New Guinean dogs, providing the first morphological evidence of links between early dingoes and their northern relatives. This ancient type is no longer extant within the range of modern dingo variability, but populations from nearby southeastern Australia show a closer resemblance than those to the north and west. Our results reaffirm prior characterisations of regional variability in dingo phenotype as not exclusively derived from recent domestic dog hybridisation but as having an earlier precedent, and suggest further that the dingo’s phenotype has changed over time.
Similar content being viewed by others
Introduction
The dingo is a canid endemic to Australia, with enigmatic origins and a heavily-debated specific taxonomic identity. Dingoes are closer genetic relatives of East Asian dogs than wolves or other wild canids, but simultaneously exhibit a number of behavioural and phenotypic traits comparable to wild canids that are absent or otherwise unusual in domestic dogs1,2. Dingoes are “dogs” which maintain stable wild populations entirely independent of reliance on human-derived resources and/or recruitment from domestic populations3, a uniquely naturalised status shared only by closely-related dogs found in the highlands of New Guinea4,5. As such, dingoes are considered by some to be amongst the earliest-established naturalised populations of any formerly domestic mammal, and certainly the oldest known example for Canis familiaris6.
Molecular dating estimates of the divergence of dingoes from New Guinean and Asian dogs suggests they arrived in Australia prior to 5000BP7, but the earliest directly-dated skeletal remains are less than 3500 years old, following closely the earliest remains of dogs in Indonesia and Near Oceania8. Nevertheless, genetic assessments of both ancient and modern canids widely agree that dingoes represent a surviving branch of the earliest southern foray of C. familiaris into the region of Australasia9. As such, knowledge of the dingo’s origins and relationship to non-Australian canids is of key importance to understanding the early history of dogs in the Asia–Pacific region, and by extension, the movement and relations of its’ resident human cultures10.
Before the era of wide-scale genetic and genomic applications to understanding the phylogenetic relations of dogs and their relatives, morphometric approaches struggled to find close matches to the dingo amongst the native dogs of the nearby Asia–Pacific region. Gollan11, in an examination of both modern and ancient samples from a wide area spanning from South Asia to Polynesia, suggested that dingoes were most closely and probably directly derived from Indian pariah dogs, rejecting any connection with Southeast Asian dogs. Later, Corbett12 suggested that Thai pariah dogs formed an intermediary stage between the early dingo and modern domestic dog. More recently, Gonzalez13, argued that dingoes derived from a particular type of pariah dog still extant in small numbers in India, which subsequently drifted towards an ancestral form (Canis lupus pallipes) during isolation and natural selection in Australia. These morphometric studies all rejected any morphological relation of dingo to the wild dogs found in the New Guinea highlands, on the basis of major differences in size and overall form between the two.
Conversely, genetic and genomic research unanimously rejects all of the above suppositions, clearly indicating that the dingo’s closest living relatives are New Guinean canids: the Singing Dogs (NGSD) and recently-described Highland Wild Dog (HWD) of Irian Jaya14,15,16,17,18,19,20. More broadly, the dingo, NGSD and HWD (grouped henceforth as NGC = New Guinea Canid) belong to a lineage of East Asian dogs reaching back at least as far as the early Neolithic of eastern China20, but are unique in having avoided admixture from other dog lineages until the introduction of domestic dogs by European colonists21. Members of this lineage previously existing in Mainland and Island Asia appear to have been replaced or assimilated by more recent East Asian and then West Eurasian lineages16, which constitute the present-day dog populations of these regions.
Recent phylogenies emphasise a split between dingoes found in northwestern and southeastern regions of Australia, which may be linked to separate introduction events15,16,20. Interestingly, they also place NGSDs within the dingo clade altogether, as a sister branch to southeastern dingo. Preliminary application of 3D geometric morphometric approaches to dingo cranial morphology have supported this phylogenetic division, finding that the cranial form of southeastern dingoes also bears a closer resemblance to NGCs than their northwestern conspecifics22. However, as some of the similarities between dingoes and NGCs are also shared with domestic dogs, the strength of these conclusions is somewhat limited by the possibility of recent domestic dog hybridisation resulting in equifinality of cranial forms between southeastern dingoes and NGCs.
Accordingly, there is currently a major disjunction between the impressions of dingo ancestry and population history given by genetic/genomic and morphometric approaches respectively, suggesting that the early history of canids in the southern Asia–Pacific region involved shifts in phenotype both in Australia and the broader Asia–Pacific. This issue could be better informed by study of ancient dingoes, but this has thus far been very limited as available specimens are very rare, generally highly incomplete and/or fragmentary, and often represent subadults. The only comparisons of reasonably intact crania in particular were performed several decades ago by Gollan11, using traditional caliper-based measurements, and mainly concern individuals from recent pre-contact times (< 1000BP).
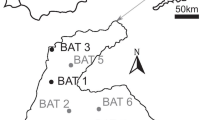
Here, we address this gap by presenting results of dating and morphological analysis of the remains of several ancient dingoes from lunette contexts in southwestern New South Wales (Table 1) at Lake Mungo and Lake Milkengay. These individuals were discovered and excavated from 1979 to 1981 as their positions within older (Pleistocene) strata were revealed by ongoing erosion and deflation of the dried lakes’ lunettes23. Some of these—MD1, MD2 and MLK1—were briefly studied by Gollan11, but the others were discovered after his work. One such individual (MD4) comprises a near-complete skeleton with an intact cranium (Fig. 1), which offered the first opportunity for 3D geometric morphometric comparison of an ancient dingo with present-day dingoes and their international relatives.
Results
AMS dating and dietary stable isotopes
AMS dates of 3035 ± 18BP (3329–3071 calBP) and 2878 ± 18BP (3060–2866 calBP) were obtained from MD1 and MLK1 respectively (Table 2). The calibrated age ranges for MD1 and MLK1, at approximately 3000BP, are both considerably lower than their sedimentary contexts might have initially suggested. It is likely that a similar situation applies to the undated MD4. MD1 is near-contemporaneous with other early, directly-dated specimens from Australia, also in semi-arid environments but over 1500 km away: Madura Cave (3069 ± 27BP8; and Koonalda Cave (3031 ± 34BP)24. Like the Mungo and Milkengay specimens, the Madura and Koonalda fossil specimens appear to have accumulated in the caves naturally rather than having been domestically associated with humans. Together, these dates suggest that wild dingoes were numerous enough by 3000BP to be frequently integrated into the archaeological record.
Dietary δ13C and δ15N stable isotopes were also obtained for MD1. In absence of directly comparable data from this area of Australia, these were compared with 99 published isotopes from dingoes and ancient dogs from elsewhere (Fig. 2). Of these, MD1 is very similar to the younger Madura Cave dingo8, but the older Madura individual is differentiated by very high δ15N comparable to Cook Islands and Pacific Northwest dogs heavily reliant on marine resources25,26,27. There is no indication of similarity to Neolithic Chinese dogs or the Matja Kuru 2 (Timor) village dog that were provisioned on agricultural produce13,28,29,30. When compared to recent dingoes from the tropics31, MD1’s δ13C score appears consistent with this sample, but it scores amongst the highest δ15N. This is consistent with most of Morrant et al.’s sample31 reflecting dingoes foraging in mixed/forest environs (C3 plants common) with a smaller proportion from open areas, as opposed to the mixed grassland/heath/Mallee scrub at Lake Mungo. The latter biome is rich in C4 plants, which results in comparably enriched δ15N for local foragers32.
Previous research has demonstrated that Late Holocene human remains from riverine sites to the southwest of Lake Mungo exhibit higher mean δ15N scores further inland (13.4 ± 1.2) than closer to the coast (10.1 ± 1.1). This is because the former region afforded greater access to terrestrial fauna that subsist on semi-arid C4-rich grasses32. Both ancient riverine populations however exhibit near identical mean δ13C (− 20 ± 0.8 and − 20.1 ± 1.2 respectively), reflecting C3 derived from additional heavy aquatic fish and shellfish consumption32. These observations contextualise MD1’s dietary isotopic results and indicate they are likely to reflect the normal foods of a wild dingo in the semi-arid zone—large marsupials, reptiles and birds33.
3D geometric morphometrics
The relationship between the cranial morphology of MD4, modern dingoes and other Asia–Pacific dogs is visualised in a neighbour-joining tree denoting Procrustes distances (Fig. 3a). Surprisingly, MD4 falls within a cluster of East Asian dogs; the nearest branches observed are two Japanese dogs and the next two are Chinese dogs, with the portion of New Guinean dogs representing Southern Highlands NGSD forming a sister branch to this group. Timorese village dogs cluster with a number of unverified dogs from the Eastern Highlands of New Guinea11, and, interestingly, with a number of dingoes from the southeast coast of NSW. A broad split in the tree between most northwestern and most southeastern dingoes is apparent, with the latter more closely associated with the branches containing New Guinean and Asian dogs, and MD4. Accordingly, modern dingoes near in cranial form to MD4 are exclusively from southeastern Australia, but MD4 is closer to several East Asian and New Guinean dogs than to any sampled dingo.
The population-level relationships between dingoes, New Guinean and East Asian dogs, and MD4 are further elucidated in a grouped-mean neighbour-joining tree (Fig. 3b). Modern dingoes cluster and separate in a northwest-southeast fashion, with southeastern dingoes intermediate to New Guinean and Asian dogs. MD4’s branch is closest to East Asian and New Guinean dogs, but maintains a substantial distance from either. This is because these two populations are groupings of convenience, being internally heterogeneous with higher in-group variability (dispersion) than any sampled dingo population (with southeastern dingoes intermediate to these dogs and northwestern dingoes; Table S2).
The traits evident in MD4 responsible for these associations are, compared to modern dingoes: a smaller size; overall shorter and broader shape; a dorsally-rotated (airorhynchous) palate with slightly curved toothrow; a prominent and anterior-rotated frontal-orbital (facial) area; and an elevated cranial height. These traits are evident in the population-mean cranial morphs for dingo and non-dingo groups (Fig. 3c). However, MD4 has an exceptionally well-developed and prominent sagittal crest and large auditory bullae, which are generally rare in East Asian and New Guinean dogs, but strongly characteristic of dingo11. Hence, MD4 displays mixed traits of dingoes and their northern relatives.
The East Asian population includes breeds and landraces from several different regions, whilst variability within New Guinean dogs has been previously noted22 and may reflect different introductions of dog to New Guinea. MD4 is close in conformation to only some of the members of these groups, as illustrated by the lowest Procrustes distances to this individual (Table 3). Of the ten nearest crania, four are Japanese dogs, and five are New Guinean dogs, from the original Southern Highlands breeding group4 and a 19th century Central Province specimen originally classified as “Canis papuensis”34. MD4 is conversely far-removed from the gracile Timorese village dogs and similarly gracile wild dogs from the Mt Wilhelm-Eastern Highlands collection areas in PNG11.
When the major axes of variation are examined using PCA MD4 also falls outside of the range of modern dingoes from either northwestern or southeastern Australia22, and within the range of Asian and New Guinean dogs (Fig. 4a). MD4’s centroid size tends towards the lowest end of modern dingoes, and is greater than all New Guinean canids (Fig. 4b), but very similar to some Asian dogs (in particular to a dog from Guangzhou, southern China). Linear regression of size on shape determined that the morphological trajectories described by PC1 (20.84%) and PC2 (8.74%) have an allometric component, with size contributing 10.90% of overall form, and 42.38% of PC1 in particular compared to just 3.72% of PC2 (all results significant, p < 0.0001). A secondary (Fig. 4c) PCA using the residuals of this regression to correct for influence of size reveals the association of MD4 with NGCs and Asian dogs, whilst a tertiary PCA (Fig. 4d) with the allometric components of form removed reveals that MD4’s association with Asian dogs specifically is stronger when only shape is considered. These results indicate some of the similarity between MD4 and New Guinean dogs is due to convergence in their overall sizes, which occur within a range outside that observed within most modern dingoes. These observations of similarity between the cranial form of MD4 and Asian dogs were tested through predictive classification using a linear discriminant analysis optimised for unequal sample sizes (sensu lato Evin et al.35; code available Hulme-Beaman36). This returned a predominantly Asian Dog identification followed by NGC, and lastly with a few consecutive combinations of principal components occasionally returning southeastern Australia dingo (see SI for full breakdown of all returned classifications from all consecutive combinations of principal components, and distributions of classification probability based on all resampling iterations). The outstanding position of MD4 relative to modern dingoes cannot be attributed to demographic factors. The specimen is categorically adult as indicated by closed and fused sutures throughout the posterior cranium and its fully erupted (and worn) adult teeth. The very well-developed sagittal crest in particular indicates that it is not a young adult. Although there is some sexual dimorphism in the cranial form of dingoes, this is very minor compared to variation on the basis of geography22. Our modern sample includes numerous individuals of both sex from several geographic populations. There are no signs of pathology or developmental issues in MD4 which might have affected its cranial form. Similarly, there are no indications that the specimen has been deformed by taphonomic processes; although the zygomatic arch was broken and has been digitally reattached, the areas of the cranium which are important in distinguishing MD4 from modern dingoes are located elsewhere in the intact regions of the cranium.
Size of body and carnassial teeth
The estimated shoulder height of ancient Lake Mungo dingoes ranged between approximately 40–47.5 cm (Fig. 5a). MD4 and MD1 are shorter than any of the 117 modern dingoes from around Australia for which height was reconstructed using the same method (46.37–61.50 cm, x̄ = 54.22 ± 29.87 cm), whilst the tallest individual, MD6, is shorter than 97% of these. MD4 and MD6 are taller than all 12 New Guinean canids for which limb bones were available (33.24–41.76 cm, \({\overline{\text{x}}}\) = 37.5 ± 29.87 cm), though MD1 is very close in height to the largest three: two NGSD from the Southern Highlands and one wild dog of uncertain affiliations from the Central Province mountains4. According to prior research, the intermediate space between dingoes and New Guinean dogs is occupied by various Southeast Asian dogs11,12,13. Particularly interesting is the closeness of MD1 to the Matja Kuru 2 (Timor) village dog dated to 2967 ± 58BP, at 39.3 cm tall13; and that of MD4 to the Hoekgrot (Java) pariah-type dog from a deposit aged 2655 ± 60 to 3265 ± 55 BP, which was 45 cm tall37.
Estimated body masses of MD4 and MLK1 were 14.53 kg and 14.27 kg respectively, falling within the range and close to the mean estimates for 95 sampled dingo crania (11.39–22.57 kg, \({\overline{\text{x}}}\) = 14.25 ± 1.69), also above almost all of the 20 New Guinean dogs sampled (2.49–14.28 kg, \({\overline{\text{x}}}\) = 8.58 ± 2.90 kg) but comparable to many Southeast Asian dogs11,13,38. Considering MD4’s mass together with its substantially shorter height suggests a stockier build compared to the usual lithe, lean frame of most modern dingoes. As MD4’s palate is unusually broad for its length, a proportion which strongly deviates from modern dingo, there is a possibility it has resulted in an inflated mass estimate.
Ancient Mungo and Milkengay dingoes exhibit maxillary (Fig. 5b) and mandibular (Fig. 5c) carnassial lengths near the minimum of the range of modern dingoes and within the range of many Asian and most New Guinean dogs, but generally maintain a breadth consistent with modern dingo. The exception is MD1 which possesses an exceptionally slim upper carnassial tooth, with equivalents in the study sample only amongst small New Guinean wild dogs of uncertain classification22, and Timorese village dogs. The differences between the dated specimens MD1 and MLK1 suggest substantial variability in the body size of dingoes from the same area within a period of 100–200 years.
Discussion
The dating and morphometric characterisation of ancient dingoes from western NSW informs several key aspects of the dingo’s early history long obscured by prior lack of materials suitable for study. Firstly, our results demonstrate that early dingoes were often smaller than modern dingoes, some intermediate in terms of both body size and dentition to these and New Guinean and Asian dogs. Reduced size for early dingoes was at one time suggested by the smaller teeth of archaeological specimens, but later retracted based on a revised assessment of individual age39. The validity of these prior suspicions are demonstrated through adult specimens of similar or greater antiquity in our study.
3D geometric morphometric characterisation of the MD4 Lake Mungo dingo cranium demonstrates the first clear links between ancient dingoes and dogs to the north of Australia; in particular, with East Asian and New Guinean dogs for which morphological connections were not previously evident. Prior efforts to elucidate the relationships of dingoes to non-Australian dogs by means of morphology have struggled largely due to an assumption that the ancestral dingoes must necessarily closely resemble their present-day descendants. Our results demonstrate that this is not the case, though there is no exact match for MD4 amongst modern-day East Asian or New Guinean dogs. MD4’s cranial morphology might reflect a form common to the ancestors of both dingoes and NGSDs, and by extension, that of an early lineage of East Asian dog which once existed across a large portion of the southern Asia–Pacific region and Australia20.
The divergence of modern dingoes from those described from Lake Mungo and Lake Milkengay suggests that dingoes have increased in size in Australia over the last 3000 years, a change accompanied by a drift in cranial form. An alternative possibility is that the ancient dingoes studied here represent an otherwise extinct lineage which did not successfully adapt, but was absorbed or replaced by an expansion of today’s dominant lineages. Modern dingoes sampled from the Mallee region, which is geographically and ecologically close to the Willandra and Anabranch lake systems, indeed appear as morphological intermediates to the ancient dingoes of this area and modern dingoes from the north and west, in terms of both cranial form and carnassial tooth size.
Finally, our results reveal the deep roots of regional variation in modern dingo morphology along a broadly northwestern-southeastern cline, in accordance with differential maternal and paternal haplotype distributions between these areas15,16. Features such as reduced height, mass and carnassial tooth size observed in modern southeastern dingoes have been explained as resulting from of recent admixture from European breed dogs40,41(Daniels and Corbett 2003; Radford et al. 2012), but have a precedent in dingoes which lived ~ 3000 years before this took place. Other “dog-like” aspects of cranial form in dingoes from southeastern Australia22 also have a precedent in the apparently non-Australian conformation of MD4. Though this of course does not entirely preclude a role of recent European dog admixture, some phenotypic differences observed in contemporary southeastern dingo may be of ancient origin and therefore unrelated.
Ongoing geometric morphometric investigations will further clarify the nature and direction of morphological drift between ancient and modern dingoes, how it relates to the wild and domestic relatives of the dingo, and how it may vary in different biogeographic areas of Australia. Of particular interest to continuing research is the degree to which modern dingoes resemble Asian wolves42, and which specific populations of ancient Asian dogs (a diverse group, like their present-day counterparts) bear the closest resemblance to ancient dingoes. Preliminary expeditionary studies of wild dogs in the Irian Jaya portion of the New Guinea highlands5, and the size of the lone lower carnassial tooth from this area sampled in this study suggests the local presence of an as-yet unstudied and more robust morphotype, which may prove to bear a closer resemblance to ancient dingoes such as MD4 than wild dogs from eastern and southeastern New Guinea.
Materials and methods
Discovery and excavation of ancient canid remains
Canid remains from Lake Mungo were originally identified from 1979 to 1985 in exposed positions eroding from the surface of the lake’s southern lunette, and were retrieved from these locations with minimal excavation. All were found eroding from the Zanci unit (24.5–16 ka), apart from MD4 which is from the older Arumpo unit (30–24.5 ka)43. Wombats became locally extinct before the twentieth century (potentially before European settlement began in the 1830s) but their burrows are common throughout the Mungo lunette. Many feature the bones of other terrestrial species which repurposed the burrows, including the dingoes reported here. The collapse of these burrows preserved their bones within the surrounding sediments, giving the impression of genuine in-situ provenance in Pleistocene-age strata23. At least one of the Mungo dingo skeletons (MD1) exhibits carnivore damage, suggesting it was already deceased for some time when the burrows collapsed.
The Milkengay dingo (MLK1) was discovered during archaeological survey in an eroded portion of the Lake Milkengay lunette containing lithic artefacts and shells, in an area with hearths dated to 3390 ± 500BP and 4590 ± 210BP. As such, it was considered one of, if not the oldest dingo remains in Australia by Gollan11. However, the actual association of the dingo with these archaeological dates was dubious considering that the ages of the dated charcoal samples were stratigraphically inverted, and that the dingo was laterally displaced from them by ~ 10 m11. Due to its fragmented and incomplete nature, study of MLK1 was limited to dental measurements and reconstruction of body mass.
Despite their provenance from lunette areas containing Aboriginal artefacts, more broadly identified as archaeological sites23, there is no indication of direct human association for the ancient dingo remains studied here11. The Mungo dingoes in particular appear to have been exhibiting denning behaviour at the time of their deaths within wombat burrows. The common frequency of skeletal remains of other species within collapsed/filled burrows or hollows in Willandra lunette archaeological sites, particularly bettongs and wombats, further suggests the deposition of these individuals was independent of human activity. However, the possibility that these individuals were tamed in young age before returning to the wild44, or lived in some commensal association with nearby people45, cannot be ruled out.
Community engagement and research design
Archaeological dingoes studied here come from the traditional lands of the Barkandji (Paakantyi), Mutthi Mutthi, and Ngiyampaa peoples. Representatives of these First Nations communities comprising the Willandra Lakes Region World Heritage Aboriginal Advisory Group were consulted during research design, data collection, analysis, results and interpretation, through in-person and digital meetings and progress updates from 2018 to 2021. With community feedback and comments considered at these stages, a final research proposal and submission manuscript was presented to and approved by the WLRWH AAG in 2023.
Dating and isotopes
Elements from each skeleton were subjected to FTIR (Fourier Transform Infrared Spectroscopy) analysis prior to sampling in order to identify those which most likely contain well-preserved collagen and thus yield radiocarbon dates. Based on the results of this analysis, seven samples were taken: from the astragalus of MD1; the mandible of MD3; the mandible and tibia of MD4; the femur of MD4b (a different individual from the same site); the calcaneus of MD5; and the canine tooth of MLK1. Approximately 1–2 g of bone from each specimen was collected for the sample using a Dremel drill.
AMS dating and dietary isotopic work of MD1 and MLK1 was performed at the University of Waikato Radiocarbon Dating Laboratory, Auckland, New Zealand (see Figures S5 and S6. for raw AMS plots). Despite promising FTIR results and good visual condition of sampled elements, the collagen preservation in all Mungo and Milkengay specimens was determined during AMS processing as very poor to non-existent. The only successful samples were from MD1 and MLK1; those submitted from MD3 and MD5 failed, as did two separate samples from the mandible and tibia of MD4. Calibrated ranges for successful samples were produced using the updated SHCal20 calibration curve for the southern hemisphere46.
Morphological analyses
MD4’s cranium was 3D scanned and landmarked at 45 points along the midline and left side of the cranium, following the methodology of Koungoulos22, which uses a Creaform 700 Handyscan to digitise crania and Stratovan Checkpoint47 to apply landmarks (Fig. S2). As the left zygomatic arch of MD4 was broken prior to excavation, this anatomical feature was digitally reassembled in Blender before landmarking following the protocols outlined by Lautenschlager48. MD4 was compared to a dataset of 475 dingo and dog crania scanned following Koungoulos22, excepting a portion of the East Asian sample which were obtained as open-source CT scans via KUPRI (Table S1). Modern dingo crania were assigned to 14 subpopulations based on the biogeographic area in which they were originally collected (Fig. S7). The dog populations were divided into dogs from New Guinea (including Irian Jaya and New Britain), and dogs from Asia.
Specimens were aligned using a Generalised Procrustes Analysis (GPA using Morpho49, and then between known modern group differences (i.e. removing the archaeological specimen) were tested using a permuted ANOVA using geomorph50. Dispersions (i.e. group morphological diversity) was tested using a permuted ANOVA on distances from the group centroids using geomorph50. The archaeological specimen was separated from the modern specimens; the raw data of the known specimens were resampled to equal sample size, and for each resampling iteration a new GPA was carried out and followed by a principal components analysis (PCA) and then a linear discriminant analysis on a reduced number of PCs. The archaeological specimen was projected into this GPA space based around equal sample size by aligning the archaeological specimen to the new GPA mean shape using Morpho functions49and then the specimen was projected using the predict function into PCA space, then LDA space for each iteration. Classification results vary depending on the number of PCs used, therefore the process was repeated for all consecutive combinations of PCs to the smallest known group sample size minus 1 (i.e. PCs 1–15, by testing each combination of PCs 1–2, then 1–3, 1–4 and so on). The posterior probability for the archaeological specimen was recorded in every iteration and PC combination and plotted as probability distributions for each PC combination. See SI R markdown for full work through of the data with all corresponding tests, and plots generated.
All 3D geometric morphometric analyses were performed in Rstudio (v.2022.07.2+576) using the packages ape51, geomorph50, GeoOrigins52, MASS53, Morpho49, and OptLDA36. A full record of all packages and versions used is located in the Supplementary Information. Unrooted neighbour-joining trees (BIONJ) based both on individual scores and population means were constructed to more clearly illustrate MD4’s relations in terms of cranial morphology54,55. Measurements of centroid size, and PCAs based on raw data and residuals from a regression of shape on size were performed to further explore these relationships and determine the role of size in characterisations of MD4’s nearest morphological relations.
Secondary metric analyses involved comparisons of three elements of body size for MD4 and other archaeological dingoes. Height at the shoulder was reconstructed from length of limb bones using Harcourt’s method56 for MD1, MD4 and MD6. The body mass of MD4 and MLK1 was estimated using measurements of the palate length and breadth, taken digitally from the 3D scan models, following the Lucas et al.57 dingo-specific method. These were compared with estimates for modern dingoes and New Guinean dogs obtained in the same manner from the crania sampled in this research. Measurements of carnassial teeth length and breadth were taken digitally from the 3D scans.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Jackson, S. M. et al. The Dogma of Dingoes—Taxonomic status of the dingo: A reply to Smith et al.. Zootaxa 4564(1), 198–212. https://doi.org/10.11646/zootaxa.4564.1.7 (2019).
Smith, B. et al. Taxonomic status of the Australian dingo: The case for Canis dingo Meyer, 1793. Zootaxa 4564(1), 173–197. https://doi.org/10.11646/zootaxa.4564.1.6 (2019).
Boitani, L. & Ciucci, P. Comparative social ecology of feral dogs and wolves. Ethol. Ecol. Evol. 7(1), 49–72. https://doi.org/10.1080/08927014.1995.9522969 (1995).
Koler-Matznick, J., Brisbin, I. L., Feinstein, M. & Bulmer, S. An updated description of the New Guinea singing dog (Canis hallstromi, Troughton 1957). J. Zool. 261(2), 109–118. https://doi.org/10.1017/S0952836903004060 (2003).
McIntyre, J. K., Wolf, L. L., Sacks, B. N., Koibur, J. & Brisbin, I. L. Jr. A population of free-living highland wild dogs in Indonesian Papua. Austral. Mammal. 42(2), 160–166. https://doi.org/10.1071/AM18039 (2019).
Gering, E. et al. Getting back to nature: Feralization in animals and plants. Trends Ecol. Evol. 34(12), 1–15. https://doi.org/10.1016/j.tree.2019.07.018 (2019).
Koungoulos, L. & Fillios, M. Hunting dogs down under? On the Aboriginal use of tame dingoes in dietary game acquisition and its relevance to Australian prehistory. J. Anthropol. Archaeol. 58, 101146. https://doi.org/10.1016/j.jaa.2020.101146 (2020).
Balme, J., O’Connor, S. & Fallon, S. New dates on dingo bones from Madura Cave provide oldest firm evidence for arrival of the species in Australia. Sci. Rep. 8, 9933. https://doi.org/10.1038/s41598-018-28324-x (2018).
Greig, K. et al. Complex history of dog (Canis familiaris) origins and translocations in the Pacific revealed by ancient mitogenomes. Sci. Rep. 8, 9130. https://doi.org/10.1038/s41598-018-27363-8 (2018).
Fillios, M. A. & Taçon, P. S. C. Who let the dogs in? A review of the recent genetic evidence for the introduction of the dingo to Australia and implications for the movement of people. J. Archaeol. Sci. Rep. 7, 782–792. https://doi.org/10.1016/j.jasrep.2016.03.001 (2016).
Gollan, K. Prehistoric Dingo (Doctor of Philosophy Thesis, Australian National University, 1982)https://doi.org/10.25911/5d6cfce36fe39.
Corbett, L. K. Morphological comparisons of Australian and Thai dingoes: A reappraisal of dingo status, distribution and ancestry. Proc. Ecol. Soc. Austral. 13, 277–291 (1985).
Gonzalez, A., Clark, G., O’Connor, S. & Matisoo-Smith, L. A 3000 year old dog burial in Timor-Leste. Austral. Archaeol. 76(1), 13–20. https://doi.org/10.1080/03122417.2013.11681961 (2013).
Ardalan, A. et al. Narrow genetic basis for the Australian dingo confirmed through analysis of paternal ancestry. Genetica 140(1–3), 65–73. https://doi.org/10.1007/s10709-012-9658-5 (2012).
Cairns, J. M., Brown, S. K., Sacks, B. N. & Ballard, J. W. O. Conservation implications for dingoes from the maternal and paternal genome: Multiple populations, dog introgression, and demography. Ecol. Evol. 7(22), 9787–9807. https://doi.org/10.1002/ece3.3487 (2017).
Cairns, K. M., Shannon, L. M., Koler-Matznick, J., Ballard, J. W. O. & Boyko, A. R. Elucidating biogeographical patterns in Australian native canids using genome wide SNPs. PLoS ONE 13, e0198754. https://doi.org/10.1371/journal.pone.0198754 (2018).
Oskarsson, M. C. R. et al. Mitochondrial DNA data indicate an introduction through Mainland Southeast Asia for Australian dingoes and Polynesian domestic dogs. Proc. R. Soc. B Biol. Sci. 279(1730), 967–974. https://doi.org/10.1098/rspb.2011.1395 (2011).
Savolainen, P., Leitner, T., Wilton, A. N., Matisoo-Smith, E. & Lundeberg, J. A detailed picture of the origin of the Australian dingo, obtained from the study of mitochondrial DNA. Proc. Natl. Acad. Sci. USA 101(33), 12387–12390. https://doi.org/10.1073/pnas.0401814101 (2004).
Surbakti, S. et al. New Guinea highland wild dogs are the original New Guinea singing dogs. Proc. Natl. Acad. Sci. https://doi.org/10.1073/pnas.2007242117 (2020).
Zhang, M. et al. Ancient DNA evidence from China reveals the expansion of Pacific dogs. Mol. Biol. Evol. 37(5), 1462–1469. https://doi.org/10.1093/molbev/msz311 (2020).
Bergström, A. et al. Origins and genetic legacy of prehistoric dogs. Science 370(6516), 557–564. https://doi.org/10.1126/science.aba9572 (2020).
Koungoulos, L. Old dogs, new tricks: 3D geometric analysis of cranial morphology supports ancient population substructure in the Australian dingo. Zoomorphology 139(2), 263–275. https://doi.org/10.1007/s00435-019-00475-z (2020).
Clark, P.M. Willandra Lakes World Heritage Area Archaeological Resource Study (NSW Department of Environment and Planning, Buronga, 1987).
Walshe, K. Koonalda Cave, Nullarbor Plain, South Australia—issues in optical and radiometric dating of deep karst caves. Geochronometria 44(1), 366–373. https://doi.org/10.1515/geochr-2015-0081 (2017).
Ames, K. M. et al. Stable isotope and ancient DNA analysis of dog remains from Cathlapotle (45CL1), a contact-era site on the Lower Columbia River. J. Archaeol. Sci. 57, 268–282. https://doi.org/10.1016/j.jas.2015.02.038 (2015).
Craig, J.A. Stable isotope analysis of prehistoric human and commensal diet on Aitutaki, southern Cook Islands (Doctor of Philosophy Thesis, University of Auckland, 2009).
Hillis, D., McKechnie, I., Guiry, E., St. Claire, D. E. & Darimont, C. T. Ancient dog diets on the Pacific Northwest Coast: Zooarchaeological and stable isotope modelling evidence from Tseshaht territory and beyond. Sci. Rep. 10, 15630. https://doi.org/10.1038/s41598-020-71574-x (2020).
Barton, L. et al. Agricultural origins and the isotopic identity of domestication in northern China. Proc. Natl. Acad. Sci. 106(14), 5523–5528. https://doi.org/10.1073/pnas.080996010 (2009).
Chen, X. L. et al. Raising practices of neolithic livestock evidenced by stable isotope analysis in the Wei River Valley, North China. Int. J. Osteoarchaeol. 26(1), 42–52. https://doi.org/10.1002/oa.2393 (2016).
Pechenkina, E. A., Ambrose, S. H., Xiaolin, M. & Benfer, R. A. Reconstructing northern Chinese Neolithic subsistence practices by isotopic analysis. J. Archaeol. Sci. 32(8), 1176–1189. https://doi.org/10.1016/j.jas.2005.02.015 (2005).
Morrant, D. S., Wurster, C. M., Johnson, C. N., Butler, J. R. A. & Congdon, B. C. Prey use by dingoes in a contested landscape: Ecosystem service provider or biodiversity threat?. Ecol. Evol. 7(21), 8927–8935. https://doi.org/10.1002/ece3.3345 (2017).
Pate, F. D. & Owen, T. D. Stable carbon and nitrogen isotopes as indicators of sedentism and territoriality in late Holocene South Australia. Archaeol. Oceania 49(2), 86–94. https://doi.org/10.1002/arco.5019 (2014).
Doherty, T. S. et al. Continental patterns in the diet of a top predator: Australia’s dingo. Mamm. Rev. 49(1), 31–44. https://doi.org/10.1111/mam.12139 (2018).
Parnaby, H. E., Ingleby, S. & Divljan, A. Type specimens of non-fossil mammals in the Australian Museum, Sydney. Rec. Austral. Museum 69(5), 277–420. https://doi.org/10.3853/j.2201-4349.69.2017.1653 (2017).
Evin, A. et al. Unravelling the complexity of domestication: A case study using morphometrics and ancient DNA analyses of archaeological pigs from Romania. Philos. Trans. R. Soc. B Biol. Sci. 370(166), 20130616. https://doi.org/10.1098/rstb.2013.0616 (2015).
Hulme-Beaman, A. OPTLDA: LDA optimisation with resampling to equal sample size. https://github.com/ArdernHB/OptLDA (2022).
Storm, P. Resten van een hond uit de vindplaats Hoekgrot (Java). Cranium 18(1), 31–44 (2001).
Corbett, L. K. The Dingo in Australia and Asia (Cornell University Press, 1995).
Macintosh, N. W. G. ’The origin of the dingo: An enigma. In The Wild Canids: Their Systematics, Behavioural Ecology and Evolution (ed. Fox, M. W.) 87–106 (Van Nostrand Reinhold Company, 1975).
Daniels, M. J. & Corbett, L. K. Redefining introgressed protected mammals: When is a wildcat a wild cat and a dingo a wild dog?. Wild. Res. 30(3), 213–218. https://doi.org/10.1071/WR02045 (2003).
Radford, C. G., Letnic, M., Fillios, M. & Crowther, M. S. An assessment of the taxonomic status of wild canids in south-eastern New South Wales: Phenotypic variation in dingoes. Austral. J. Zool. 60(2), 73–80. https://doi.org/10.1071/ZO12006 (2012).
Gonzalez, A. The Pariah Case: Some Comments on the Origin and Evolution of Primitive Dogs and on the Taxonomy of Related Species (Doctor of Philosophy Thesis, The Australian National University, 2012)https://doi.org/10.25911/5d5e766e646dc.
Jankowski, N. R., Stern, N., Lachlan, T. J. & Jacobs, Z. A high-resolution late Quaternary depositional history and chronology for the southern portion of the Lake Mungo lunette, semi-arid Australia. Quat. Sci. Rev. 233, 106224. https://doi.org/10.1016/j.quascirev.2020.106224 (2020).
Smith, B. P. & Litchfield, C. A. A review of the relationship between Indigenous Australians, dingoes (Canis dingo) and domestic dogs (Canis familiaris). Anthrozoös 22(2), 111–128. https://doi.org/10.2752/175303709X434149 (2013).
Brumm, A. Dingoes and domestication. Archaeol. Oceania 56(1), 17–31. https://doi.org/10.1002/arco.5226 (2021).
Hogg, A. G. et al. SHCal20 Southern Hemisphere Calibration, 0–55,000 Years cal BP. Radiocarbon 62(4), 759–778. https://doi.org/10.1017/RDC.2020.59 (2020).
Stratovan Corporation. Stratovan Checkpoint. 2018.08.07 edn.(2018).
Lautenschlager, S. Reconstructing the past: Methods and techniques for the digital restoration of fossils. R. Soc. Open Sci. 3(10), 160342. https://doi.org/10.1098/rsos.160342 (2016).
Schlager, S. Chapter 9 – Morpho and Rvcg – shape analysis in R: R-packages for geometric morphometrics, shape analysis and surface manipulations. In Statistical Shape and Deformation Analysis: Methods, Implementation and Applications (eds Zheng, G. et al.) 217–256 (Academic Press, 2017). https://doi.org/10.1016/B978-0-12-810493-4.00011-0.
Adams, D. C. & Otárola-Castillo,. geomorph: An r package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 4(4), 393–399. https://doi.org/10.1111/2041-210X.12035 (2013).
Paradis, E. & Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35(3), 536–528. https://doi.org/10.1093/bioinformatics/bty633 (2019).
Hulme-Beaman, A. et al. geoorigins: A new method and r package for trait mapping and geographic provenancing of specimens without categorical constraints. Methods Ecol. Evol. 11(10), 1247–1257. https://doi.org/10.1111/2041-210X.13444 (2020).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S 4th edn. (Springer, 2002).
Gascuel, O. BIONJ: An improved version of the NJ algorithm based on a simple model of sequence data. Mol. Biol. Evol. 14(7), 685–695. https://doi.org/10.1093/oxfordjournals.molbev.a025808 (1997).
Saitou, N. & Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4(4), 406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454 (1987).
Harcourt, R. A. The dog in prehistoric and early historic Britain. J. Archaeol. Sci. 1(2), 151–175. https://doi.org/10.1016/0305-4403(74)90040-5 (1974).
Lucas, T., Smith, B. P., Norris, R. M. & Henneberg, M. Reconstructing body mass of the Australian dingo (Canis dingo) from two simple measurements of the hard palate. J. Archaeol. Sci. Rep. 23, 534–539. https://doi.org/10.1016/j.jasrep.2018.11.018 (2019).
Acknowledgements
Acknowledgements are owed to the original excavators and collectors of the ancient dingo remains that form the focus of this paper: Peter Clark, Peter Brown, Klim Gollan, Jeanette Hope and Mike McIntyre, and to Allison Dejanovic of the Australian Museum who facilitated extended study access to the specimens. We thank the Barkandji (Paakantji), Mutthi Mutthi and Ngiyampaa communities of western NSW for their endorsement of this research, and are grateful to Dan Rosendahl, Tressna Martin, Aaron Fogel and Pat Faulkner for their invaluable assistance in facilitating community engagements. For facilitating data collection from the comparative specimens of modern dingoes and dogs, we thank Sandy Ingleby and Harry Parnaby (Australian Museum), Karen Roberts (Museum Victoria), Denise Donlon (Shellshear Museum), Leo Joseph and Chris Wilson (CSIRO National Wildlife Collection), Sofia Samper-Carro (Australian National University), Heather Janetzki (Queensland Museum), Gavin Dally (Museum and Art Gallery Northern Territory), Jude Philp (Macleay Museum), David Stemmer (South Australian Museum), Mike Letnic (University of New South Wales), Kenny Travouillon (Western Australian Museum) and Molly Hagemann (Bernice Bishop Museum). We also acknowledge the Kyoto University Primatology Research Institute’s Digital Morphology Museum (KUPRI DMM) for providing 3D-scan data, and the private collectors and institutions (KUPRI) who provided the original specimens; we are additionally grateful for Naoko Egi’s assistance in determining their affiliations. We extend thanks to Julie Sebanc-Butler for performing the preliminary FTIR analysis of the Mungo and Milkengay skeletal remains; to Fiona Petchey and Lorena Becerra-Valdivia for assistance with dating and isotopic analysis; Nicola Stern, Nathan Jankowski and Jim Bowler for expert insights into the sedimentology and stratigraphy of Lake Mungo’s southern lunette; and to Harvey Johnston and Peter Brown for first-hand information regarding the discovery and provenance of many of the Mungo dingoes. We are also grateful to Damian Morrant for access and permission to use his isotopic data from modern dingoes. This research was funded by an Australian Government Research Training Program scholarship, and the Carlyle-Greenwell Research Scholarship from the University of Sydney. Assistance with publication expenses was also provided by the Ben Sandford Cullen Scholarship from the University of Sydney and the Deputy Vice Chancellor, Research at the University of New England.
Author information
Authors and Affiliations
Consortia
Contributions
L.G.K. conceived the study under guidelines of and consultation with the WLRWHAAG. L.G.K. and M.F. designed and conducted sampling procedures for AMS and isotopic dating. L.G.K. collected morphometric data used in the study. L.G.K. and A.H-B. conducted the geometric morphometric statistical analyses and prepared corresponding figures together. L.G.K. wrote main text and supplementary information file. All authors contributed to text of Discussion, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Koungoulos, L.G., Hulme-Beaman, A., Fillios, M. et al. Phenotypic diversity in early Australian dingoes revealed by traditional and 3D geometric morphometric analysis. Sci Rep 14, 21228 (2024). https://doi.org/10.1038/s41598-024-65729-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65729-3
- Springer Nature Limited