Abstract
The family Acuariidae is a speciose group of parasitic nematodes, infecting mostly birds as definitive hosts. This study focused on the characterization of two species of acuariids, collected in two different species of piscivorous birds, the European great cormorant Phalacrocorax carbo sinensis from Italy, and the pygmy cormorant Microcarbo pygmaeus from Israel. Parasites were analyzed using light and scanning electron microscopy and by amplification and sequencing of the 28S rDNA. The results of morphological and molecular analyses showed that Ph. carbo sinensis was infected by the acuariid Syncuaria squamata (12 females) and Cosmocephalus obvelatus (1 female), whereas M. pygmaeus was infected by C. obvelatus (2 males, 12 females). The present results provide new data on the distribution of acuariid parasites of piscivorous birds, the first report of Acuariidae in Israel, and the first molecular data on S. squamata and C. obvelatus, which will be useful in future epidemiological and phylogenetic studies of these widely distributed, but less molecularly studied parasites.
Similar content being viewed by others
Introduction
The family Acuariidae Railliet, Henry and Sisoff, 1912 (Chromadorea: Rhabditida) comprises more than 300 species of parasitic nematodes, which are mostly found in aquatic and terrestrial birds1,2,3, except for few genera that are specific parasites of mammals2,4,5.
Members of this group have a complex life cycle; in aquatic species, birds are definitive hosts, while different arthropods and fish are intermediate and paratenic hosts, respectively. For some of these aquatic species, details of the life cycle have been demonstrated through either experimental studies or field observations5,6. Acuariid larvae have been reported in different groups of arthropod hosts (Wong & Anderson7, and references therein), including brine shrimps8, crabs9, ostracods10,11 and dragonfly larvae5, nevertheless, for the majority of acuariid species, the identity of intermediate/paratenic hosts is currently unknown. In the bird host, acuariids parasitize the proventriculus, under the koilin of the gizzard and, rarely oesophagus and intestine. Some species can be highly pathogenic and even cause mortality in massive infections2.
The morphology of anterior cuticular ornamentations is the most important diagnostic feature for distinguishing genera within Acuariidae in addition to the morphology of deirids and the number of precloacal papillae in adult males3,12.
Several species show restricted host and geographic distribution, while others have been reported in a wide range of bird species and appear to have cosmopolitan distribution. However, a number of these records refers to dated literature, with often incomplete morphological descriptions. The increasing use of molecular data in taxonomic studies may help understand diversity patterns among generalist and cosmopolitan acuariid species, although to date few representatives of this large family have been genetically characterized.
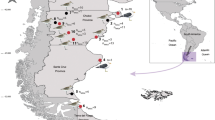
The present study was focused on the identification of acuariid parasites from two cormorant species, the European great cormorant Phalacrocorax carbo sinensis (Blumenbach, 1798) sampled in Italy, and the pygmy cormorant Microcarbo pygmaeus (Pallas, 1773) collected in Israel, providing morphological and morphometric descriptions by light and scanning electron microscopy and sequence data of the 28S rDNA.
Materials and methods
Nematodes were extracted from the intestine of 1 Ph. carbo sinensis found dead (Emilia Romagna, Italy in 2023), and from the gastric mucosa of 3 Microcarbo pygmaeus (2 from Mevo Hama and 1 from Kfar Ruppin, Israel in 2021) shot and processed fresh. For the birds from Israel all experimental protocols were approved under permit 2020/42686 from the Israel Nature and Parks Authority; all methods were carried out in accordance with relevant guidelines and regulations; all methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). In both cases the worms were carefully washed in saline and cleaned with a small brush, then preserved in 70% ethanol for downstream analyses. Few specimens from each locality were also preserved in 10% neutral buffered formalin for SEM studies.
The nematodes were observed under a dissection microscope to first evaluate gross morphology and measure the total length (TL), then under a light microscope (Leica Microsystems, Wetzlar, Germany) with the aid of a digital Nikon DS-Fi1 camera and image-acquisition software (Nikon Nis-Elements D3.0). A section of each worm was removed for DNA extraction (central 5 mm, devoided of taxonomically informative features). Anterior and posterior portions were then clarified in Amman’s lactophenol to measure internal structures by light microscope. Species identification was carried out following1,13,14.
For SEM, anterior and posterior portions of the worms were dehydrated through a graded ethanol series, subjected to critical point drying, sputter-coated with gold palladium, and observed using a Phenom XL G2 Desktop SEM (Thermo Fisher Scientific, Eindhoven, The Netherlands) operating at 5 kV.
For the molecular analysis, the genomic DNA from 20 specimens (13 from Italy and 7 from Israel) was extracted using PureLink® Genomic DNA Kit (Life Technologies, Carlsbad, California) following the manufacturer’s instructions. The amplification of the D1–D3 variable region of the 28S rDNA was performed with primers U178_f (5′-GCACCCGCTGAAYTTAAG-3′) and L1642_r (5′-CCAGCGCCATCCATTTTCA-3′)15. The thermal cycler program (Tpersonal, Biometra) was 40 cycles of 30 s at 94 °C, 30 s at 52 °C and 2 min at 72 °C, preceded by a denaturation step at 94 °C for 2 min and followed by an extended elongation step at 72 °C for 10 min. The PCR products were electrophoresed on 1% agarose gel stained with SYBR® Safe DNA Gel Stain (Thermo Fisher Scientific, Carlsbad, California) in 0.5X TBE. By NucleoSpin Gel and PCR Cleanup (Mackerey-Nagel, Düren, Germany) the amplicons were purified and sequenced with an ABI 3730 DNA analyzer (StarSEQ Mainz, Germany). The DNA trace files were assembled with ContigExpress (VectorNTI Advance 11 software, Invitrogen, Carlsbad, California) and the consensus sequences were compared with previously published data by BLAST tools (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple sequences alignments were constructed using BioEdit 7.2.5 together with some of the sequences reported by Mutafchiev et al.3. Pairwise distance, and maximum likelihood (ML) tree (GTR + G, bootstrap of 1,000 replicates) were obtained by MEGA 7.
Results
A total of 13 acuariid nematodes (all females) were recovered from Ph. carbo sinensis and morphologically identified as Syncuaria squamata (Linstow, 1883) except one identified as Cosmocephalus obvelatus (Creplin, 1825) Seurat, 1919, while 14 acuariids (2 males, 12 females) were found in M. pygmaeus (intensity ranging from 1 to 10) and morphologically identified as C. obvelatus. Morphometric data of the specimens analyzed are reported in Tables 1 and 2 and are expressed in micrometres (µm), unless otherwise stated.
Morphological description
Syncuaria squamata (von Linstow, 1883) Wong, Anderson & Bartlett, 1986
Adult female morphology: oral opening slit-like, surrounded by two pseudolabia, each with a single amphid and a pair of papillae, and two bifid interlabia (Fig. 1a,b). Narrow cordons, originating dorsally and ventrally to pseudolabia, anastomising laterally, formed by serrated, crescent-shaped, cuticular plates (Fig. 1c–d). Deirids tricuspid (Fig. 1d). Nerve ring surrounding anterior part of muscular oesophagus. Lateral alae well developed (Fig. 1e–f) tapering gradually to posterior end. Phasmids small, subterminal. Vulva simple, near tail end (Fig. 1g). Reproductive system monodelphic. Tail conical, with rounded tip (Fig. 1g). Eggs oval, embryonated, covered with small protuberances (Fig. 1h).
Female specimen of Syncuaria squamata from Phalacrocorax carbo sinensis, light microscopy and SEM micrographs. (a) Anterior end, sublateral view; cephalic papillae (cp) (scale bar = 30 µm). (b) Apical view of anterior end showing slit-like oral opening surrounded by two pseudolabia and two bifid interlabia (scale bar = 50 µm). (c) Dorsoventral view of anterior end (scale bar = 100 µm). (d) Detail of cuticular plates (cp) of cordons and tricuspid deirid (de) (scale bar = 50 µm). (e) Lateral alae (la) in anterior part of body (scale bar = 300 µm). (f) Detail of lateral alae immediately posterior to cordons (scale bar = 80 µm). (g) Posterior end showing the position of vulva (v) and anus (a) (scale bar = 100 µm). (h) Eggs (scale bar = 50 µm) (inset: detail of one egg at higher magnification. Scale bar = 10 µm).
Cosmocephalus obvelatus (Creplin, 1825) Seurat, 1919
Adult general morphology: oral opening slit-like, surrounded by two pseudolabia, each with a single amphid and a pair of papillae, and two bifid interlabia (Fig. 2a); cordons originating dorsally and ventrally to pseudolabia, anastomising laterally, with scalloped inner borders (Fig. 2b). Anterior loops of cordons do not exceed one quarter of the length of cordons. Deirids bicuspid (Fig. 2c). Lateral alae well developed, extending from level posterior to deirids (Fig. 2c) to around middle of body. Excretory pore posterior to deirids (Fig. 2d). Nerve ring surrounding anterior part of muscular oesophagus, approximately at level of deirids. Phasmids subterminal.
Cosmocephalus obvelatus from Microcarbo pygmaeus, light microscopy and SEM micrographs. (a) Apical view of anterior end, cephalic papillae (cp) and amphid (am) (scale bar = 30 µm). (b) Lateral view of anterior end showing the appearance of cordons (scale bar = 200 µm). (c) Bicuspid deirid (d) and detail of lateral ala (la) (scale bar = 50 µm). (d) Anterior portion of muscular oesophagus with nerve ring and excretory pore (arrow) (scale bar = 10 µm). (e) Posterior end of male showing caudal papillae (p) (scale bar = 100 µm). (f) Posterior end of male showing the appearance of left (lsp) and right (rsp) spicules (scale bar = 100 µm). (g) Posterior end of female showing characteristic button-like tip (scale bar = 50 µm) (inset: SEM micrograph. Scale bar = 15 µm). (h) Eggs (scale bar = 50 µm) (inset: detail of eggs at higher magnification. Scale bar = 10 µm).
Adult male: Tail with ventral cuticular ridge, anterior to cloaca; ten pairs of caudal papillae, 4 pre-cloacal and 5 post-cloacal, and a single precloacal papilla, slightly anterior to cloacal opening (Fig. 2e). Spicules unequal: left spicule longer and narrower, with small projection on its distal end; right spicule shorter, and wider (Fig. 2f).
Adult female: Vulva around middle of body; lateral alae extending to level of vulva; uterus didelphic; tail conical, with characteristic button-like tip (Fig. 2g). Eggs oval, embryonated (Fig. 2h).
Molecular analyses
All 13 specimens from Italy were successfully amplified. A BLAST search revealed that 12 samples exhibited 98.6% similarity with Syncuaria sagittata (Rudolphi, 1809) (MT086856) and Decorataria decorata (Cram, 1927) (MT0868423), while one specimen showed 99.4% similarity (33% coverage) with Cosmocephalus jaenschi Johnston and Mawson, 1941 (OP45579616).
Among the 7 specimens collected in Israel, only one gave a readable pherograms, probably due to preservation issues; the similarity was 99.4% with C. jaenschi (OP45579616). The p-distance analysis revealed that all the Syncuaria sequences obtained in this study were identical to each other and showed a 0.8% distance from S. sagittata. This confirms that they do not belong to the latter species, as observed through morphological identification, but rather to S. squamata, for which no sequences are available in GenBank. In fact, in the Maximum Likelihood (ML) tree (Fig. 3), our sequences form a well-supported cluster, distinctly separated from the cluster (98% bootstrap) formed by S. sagittata and D. decorata.
Concerning the Cosmocephalus sp., the two specimens (one from Italy and one from Israel) were identical to each other with a p-distance of 1.6% with C. jaenschi (OP45579516) but 0% with OP455796 (C. jaenschi16). These results coupled with the morphological observation allowed to state that our species was not C. jaenschi but C. obvelatus (no sequences available in GB) and move the specimen from sprat of Bennett et al.16 in the latter species; the ML tree confirmed this observation (100% bootstrap) (Fig. 3).
Discussion
The genus Syncuaria Gilbert, 1927 comprises eight species parasitizing members of the Ciconiiformes and one species specific of grebes17. The genera Skrjabinocara Kurashvili, 1940 and Decorataria Sobolev, 1949 have been considered as synonyms of Syncuaria13,18,19, although other authors have retained the validity of the three genera2,3,20.
The species S. squamata has been reported from the proventriculus and gizzard of different cormorant species (Ph. Carbo (Linnaeus), Ph. auritus (Lesson, 1831), Ph. brasilianus (Gmelin, 1789) and M. pygmaeus) and from Accipitriformes1,21. It was originally described as Filaria squamata by Linstow22, then redescribed as Skrjabinocara squamata by Kurochkin10, and finally assigned to the genus Syncuaria by Wong et al.13. This species is widely distributed and reported from different areas of Europe, Asia, and America1,23.
In the definitive host, prevalence and intensity values can be highly variable: Kanarek and Rolbiecki24 found that S. squamata infection was heavier in the immature birds (84.6%, 40.9 worms, and 1–120 worms) than in the adults (3.7%, 1 worm). These differences may suggest either a possible acquired resistance or an ontogenetic dietary shift of the cormorants. Moravec and Scholz23 found prevalence values of 15% and 20% and intensity values of 1–12 (mean = 4) and 1–4 (mean = 2) in Ph. carbo sinensis from South Bohemia and South Moravia, respectively. In Italy, S. squamata has already been reported from great cormorants with a prevalence around 20% and intensity values ranging from 1 to 1025. In Ph. carbo sinensis examined during the present investigation, intensity of S. squamata was 14. This overall low intensity may be related to possible mechanisms of competitive interactions with other species of parasitic helminths, as suggested by Dezfuli et al.25; in fact, in the same Ph. carbo sinensis analyzed in the present work, high intensity values of the anisakid nematodes Contracaecum rudolphii A and Contracaecum rudolphii B were also observed26; the same M. pygmaeus analyzed in the present work, were infected also by Amirthalingamia macracantha (Joyeux and Baer 1935) (Cestoda)27.
Interestingly, in our study only female specimens were recovered. This finding is in accordance with the observation of Monteiro et al.21, who found only one male out of 9 S. squamata recovered from Ph. brasilianus. Morphological features, as observed in light and scanning electron microscopy, and morphometry (Table 1) are in accordance with previous studies1,13,21,23,24,28, although variability in the shape of deirids (both bifid and trifid) has been reported by some of these authors21,23. The only Syncuaria available in GenBank is the species S. sagittata, which in the ML tree is clearly separated from S. squamata described in the present study; the two species also show different morphological features. Particularly, S. sagittata was previously known as Desportesius sagittatus (Rudolphi, 1809)13,29 and was subsequently assigned to the genus Syncuaria based on phylogenetic analysis3 (Mutafchiev et al. 2020); this species is found in the gizzard of different birds (Ardea purpurea Linnaeus, 1766, Ciconia nigra Linnaeus, 1758, Nycticorax nycticorax Linnaeus, 1758)1 and is clearly distinguishable from S. squamata by its overall smaller size and by the morphology of anterior end, characterized by recurrent cordons reaching approximately half of the muscular oesophagus30. Therefore, our molecular data further support the separation between these two congeneric species.
The genus Cosmocephalus includes seven species14, all parasitic in aquatic birds. Particularly, the species C. obvelatus shows a cosmopolitan distribution and low specificity, being reported from a wide range of bird species1,7, including several species of cormorants (Phalacrocoracidae). In other groups of bird parasites (e.g. in Anisakidae of the genus Contracaecum Railliet & Henry, 1912), the use of molecular data has helped to reveal hidden genetic diversity26; however, as mentioned earlier, few sequence data of acuariids are available in public databases: for the genus Cosmocephalus, such data are available only for the species C. jaenschi. Nevertheless, the wide distribution of this species has also been associated to its ecology and to the wide-ranging ability of its hosts7,31.
Cosmocephalus obvelatus was previously reported in Ph. carbo sinensis from the European continent32,33 and in M. pygmaeus1; our study reports for the first time the occurrence of this nematode species in birds from Italy and Israel. Unidentified Cosmocephalus sp. was also reported in M. pygmaeus from Indonesia34 however morphological details provided are insufficient to confirm its specific identity. Another congeneric species reported from M. pygmaeus is C. jaenschi, which differed from C. obvelatus mainly for having tricuspid deirids (but bicuspid deirids have also been occasionally described, see Presswell and Bennett35 and different spicule morphometry. In particular, the size range of spicules from our specimens overlaps with that reported in recent detailed redescription of C. obvelatus14,31 and in older descriptions from Europe1,36, while it differs from the size range reported for C. jaenschi14,35. Both species are described as having an appendage on the distal end of the left spicule; however, in C. jaenschi this appendage appears more prominent than in C. obvelatus14. Furthermore, in our male specimens, the proximal pair of precloacal papillae lies slightly external to the distribution of the other precloacal papillae, a feature already reported for C. obvelatus31.
Interestingly, C. jaenschi has been reported mostly, although not exclusively, from the southern hemisphere16,35,37,38,39. Indeed, in the current study, the phylogenetic analysis revealed that the larval specimen identified as C. jaenschi (OP455796) by Bennet et al.16 in Sprattus antipodum (Hector, 1872) from New Zealand actually belongs to the species C. obvelatus. This conclusion is supported by a p-distance of 0% and a bootstrap value of 100%.
At present, morphological information on larval stages of most acuariid species is insufficient to allow their identification to the species level, further supporting the possibility of a misidentification.
The morphology of male and female C. obvelatus from our study are in accordance with other light and scanning electron microscopy studies of specimens from different hosts7,14,31,40,41,42.
This paper provides new data on the distribution of acuariid parasites of piscivorous birds, particularly in areas with no published information such as Israel. In addition, we provide the first genetic data of the species S. squamata and C. obvelatus, of possible usefulness for future epidemiological and phylogenetic studies on these widely distributed parasites.
Data availability
The DNA sequences generated in this study have been deposited on the public database GenBank under Accession Numbers OR944168-79 (Syncuaria squamata) and OR944180-81 (Cosmocephalus obvelatus).
References
Baruš, V., Sergeeva, T. P., Sonin, M. D. & Ryzhikov, K. M. Helminths of fish-eating birds of the Palaearctic Region: Nematoda. In Helminths of fish-eating birds (eds Ryšavý, B. & Ryzhikov, K. M.) (Springer, Dordrecht, 1978).
Bain, O., Mutafchiev, Y. & Junker, K. Order Spirurida. In Handbook of Zoology: Gastrotricha, Cycloneuralia and Gnathifera Vol. 2 (ed. Schmidt-Rhaesa, S.) 661–732 (Berlin, De Gruyter, 2014).
Mutafchiev, Y., Georgiev, B. B. & Mariaux, J. A 28S rDNA-based phylogeny of the nematode family Acuariidae (Spirurida) parasitic in vertebrates. Zool. Scr. 49, 641–657 (2020).
Beveridge, I. & Barker, I. K. Acuariid, capillariid and hymenolepidid parasites of the dasyurid marsupial Antechinus stuartii Macleay, 1841, from southeastern Australia. J. Helminthol. 49, 211–227 (1975).
Anderson, R. C. Nematode parasites of vertebrates: Their development and transmission 2nd edn. (CABI Publishing, 2000).
Moravec, F. & Scholz, T. Observations on the development of Syncuaria squamata (Nematoda: Acuariidae), a parasite of cormorants, in the intermediate and paratenic hosts. Folia Parasitol. 41, 183–183 (1994).
Wong, P. L. & Anderson, R. C. The transmission and development of Cosmocephalus obvelatus (Nematoda: Acuaroidea) of gulls (Laridae). Can. J. Zool. 60, 1426–1440 (1982).
Redón, S. et al. Helminth parasites of Artemia franciscana (Crustacea: Branchiopoda) in the Great Salt Lake, Utah: First data from the native range of this invader of European wetlands. Folia Parasitol. 62, 030 (2015).
Moravec, F., Fredensborg, B. L., Latham, A. D. M. & Poulin, R. Larval Spirurida (Nematoda) from the crab Macrophthalmus hirtipes in New Zealand. Folia Parasitol 50, 109–114 (2003).
Kurochkin, Y. V. Study of nematodes of the genus Skrjabinocara Kurashvili, 1941. Tr. Astrakh. Gos. Zapov 4, 325–336 (1958).
Wong, P. L. & Anderson, R. C. Development of Syncuaria squamata (Linstow, 1883) (Nematoda: Acuarioidea) in ostracods (Ostracoda) and double-crested cormorants (Phalacrocorax auritus auritus). Can. J. Zool. 65, 2524–2531 (1987).
Baruš, V. & Majumdar, G. Scanning electron microscopic studies on the cordon structures of seven acuariid genera (Nematoda: Acuariidae). Folia Parasitol. 22, 125–131 (1975).
Wong, P. L., Anderson, R. C. & Bartlett, C. M. Revision of the genus Syncuaria Gilbert, 1927 (Nematoda: Acuarioidea). Can. J. Zool. 64, 1186–1196 (1986).
Mutafchiev, Y., Halajian, A. & Georgiev, B. B. Two new nematode species of the genus Cosmocephalus Molin, 1858 (Spirurida: Acuariidae), with an amended generic diagnosis and an identification key to Cosmocephalus spp. Zootaxa 2349, 1–20 (2010).
Lockyer, A. E., Olson, P. D. & Littlewood, D. T. J. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): Implications and a review of the cercomer theory. Biol. J. Linn. Soc. 78, 155–171 (2003).
Bennett, J., Poulin, R. & Presswell, B. Large-scale genetic investigation of nematode diversity and their phylogenetic patterns in New Zealand’s marine animals. Parasitol. 149, 1794–1809 (2022).
Mutafchiev, Y. & Georgiev, B. B. A new acuariid nematode, Syncuaria mackoi n. sp (Spirurida), from Ciconia nigra (L.) (Ciconiiformes: Ciconiidae) in Europe. Sys. Parasitol. 70, 71–79 (2008).
Digiani, M. C. First report of the genus Syncuaria (Nematoda: Acuariidae) in Argentina, with description of a new species. Folia Parasitol. 46, 139–144 (1999).
Zhang, L., Brooks, D. R. & Causey, D. A new species of Syncuaria Gilbert, 1927 (Nematoda: Acuarioidea: Acuariidae) in the wood stork, Mycteria americana L. (Aves: Ciconiiformes: Ciconiidae) from the Area de Conservacion Guanacaste. Costa Rica. J. Parasitol. 89, 1034–1038 (2003).
Smogorzhevskaya, L. A. Nematodes Part 3. In Acuarioidea Vol. 32 (ed. Sharpilo, V. P.) 189 (Naukova Dumka, Fauna Ukrainy Kiev, 1990).
Monteiro, C. M., Amato, J. F. & Amato, S. B. Primeiro registro de Syncuaria squamata (Linstow) (Nematoda, Acuariidae) em biguás, Phalacrocorax brasilianus (Gmelin) (Aves, Phalacrocoracidae) no Brasil. Rev. Bras. Zool. 23, 1268–1272 (2006).
Linstow, O. Nematoden, Trematoden und Acanthocephalen, gesammelt von Prof. Fedtschenko in Turkestan. Archiv für Naturgeschichte 49, 274–314 (1883).
Moravec, F. & Scholz, T. Helminth parasites of the lesser great cormorant Phalacrocorax carbo sinensis from two nesting regions in the Czech Republic. Folia Parasitol. 63, 022 (2016).
Kanarek, G. & Rolbiecki, L. Syncuaria squamata (Linstow, 1883) (Nematoda: Acuariidae) in the great cormorant [Phalacrocorax carbo sinensis (Blumenbach, 1798) in Northern Poland. Helminthologia 43, 33–36 (2006).
Dezfuli, B. S., Volponi, S., Beltrami, I. & Poulin, R. Intra- and interspecific density-dependent effects on growth in helminth parasites of the cormorant, Phalacrocorax carbo sinensis. Parasitol. 124, 537–544 (2002).
Caffara, M. et al. Molecular and morphological studies on Contracaecum rudolphii A and C. rudolphii B in great cormorants (Phalacrocorax carbo sinensis) from Italy and Israel. Parasitol. 150, 1040–1051 (2023).
Davidovich, N., Yasur-Landau, D., Behar, A., Pretto, T. & Scholz, T. Invasive parasites and global change: Evidence for the recent and rapid spillover of a potential pathogen of tilapias with a complex, three-host life cycle. Heliyon 9, e18831 (2023).
Moravec, F. First record of the nematode Syncuaria squamata (Linstow, 1883) from common cormorants (Phalacrocorax carbo (L.)) in Czechoslovakia. Folia Parasitol. 37, 365–366 (1990).
Skrjabin, K. I., Sobolev, A.A. & Ivashkin, V. M. Spirurata of animals and man and the diseases caused by them. Part 3. Acuarioidea in Osnovy Nematologii (ed. Skrjabin K. I.) 14, 572 (Moscow: Nauka (in Russian) 1965).
Wong, P. L. & Anderson, R. C. Revision of the genus Desportesius Chabaud and Campana, 1949 (Nematoda: Acuarioidea) mainly from the gizzard of ciconiform birds. Can. J. Zool. 64, 2520–2530 (1986).
Diaz, J. I., Navone, G. T. & Cremonte, F. New host and distribution records of Cosmocephalus obvelatus (Creplin, 1925) (Nematoda: Acuariidae), with morphometric comparisons. Comp. Parasitol. 68, 277–282 (2001).
Švažas, S. et al. The role of Great Cormorant (Phalacrocorax carbo sinensis) for fish stock and dispersal of helminthes parasites in the Curonian Lagoon area. Vet. Zootech-Lith. 55, 79–87 (2011).
Stocka, I., Dziekońska-Rynko, J., Mierzejewska, K., Stańczak, K. & Wziątek, B. Occurrence of Nematodes in the alimentary tract of great cormorants [(Linnaeus, 1758)] in colonies located in the upper and lower Vistula River. Oceanol. Hydrobiol. Stud. 46, 168–173 (2017).
Purwaningsih, E. Nematodes of birds from Indonesia. Penyakit Hewan 25, 29–33 (1993).
Presswell, B. & Bennett, J. Helminth parasites of shags (Phalacrocoracidae) from the Otago region of southern New Zealand. J. Helminthol. 95, e9 (2021).
Cram, E. B. Bird parasites of the suborders Strongylata, Ascaridata and Spirurata. Bull. U.S. Natl. Mus. 140, 1–465 (1927).
Johnston, T. H. & Mawson, P. M. Additional nematodes from Australian birds. Trans. R. Soc. S. Aust. 65, 254–262 (1941).
Johnston, T. H. & Mawson, P. M. Remarks on some parasitic nematodes from Australia and New Zealand. Trans. R. Soc. S. Aust. 68, 60–66 (1944).
Johnston, T. H. & Mawson, P. M. Parasitic nematodes. British, Australian and New Zealand Antarctic Research Expedition 1929–1931, Report series B 5, 73–159 (1945).
Azuma, H., Okamoto, M., Ohbayashi, M., Nishine, Y. & Mukai, T. Cosmocephalus obvelatus (Creplin, 1825) (Nematoda: Acuariidae) collected from the esophagus of rockhopper penguin, Eudyptes crestatus. Jpn. J. Vet. Res. 36, 73–77 (1988).
Frantová, D. On the morphology and surface ultrastructure of some parasitic nematodes (Nematoda) of birds (Aves). Acta Soc. Zool. Bohem. 66, 85–97 (2002).
Kim, S. M., Park, B. K., Jung, B. D. & Kim, H. C. First record of Cosmocephalus obvelatus (Acuariidae) in common gulls (Larus canus) from Gangneung, Korea. Korean J. Parasitol. 53, 101–104 (2015).
Anderson, R. C. & Wong, P. L. Redescription of Cosmocephalus obvelatus (Creplin, 1825) (Nematoda: Acuarioidea) from Larus delawarensis Ord (Laridae). Can. J. Zool. 59, 1897–1902 (1981).
Acknowledgements
We thank the Israel Nature and Parks Authority (INPA) for permission to collect wild piscivorous birds. This study was supported by the Israeli Veterinary Services and by the University of Bologna, Italy. Scanning Electron Microscopy acquired thanks to the Department of Excellence Project 2018-2022 funded by the Italian Ministry of Education, Universities and Research.
Author information
Authors and Affiliations
Contributions
M.C. and P.T. wrote the main manuscript text, carried out all the analyses and prepared all the figures. A.C. and V.L. provided the technical support for S.E.M. and molecular analyses. N.D. carried out the sampling and necropsies. A.G. and M.L.F. revised the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tedesco, P., Caffara, M., Davidovich, N. et al. Morphological and molecular data on acuariid nematodes in European great cormorants (Phalacrocorax carbo sinensis) and pygmy cormorants (Microcarbo pygmaeus). Sci Rep 14, 13712 (2024). https://doi.org/10.1038/s41598-024-64678-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64678-1
- Springer Nature Limited