Abstract
The systemic inflammatory response following acute ischaemic stroke remains incompletely understood. We characterised the circulating inflammatory profile in 173 acute ischaemic stroke patients by measuring 65 cytokines and chemokines in plasma. Participants were grouped based on their inflammatory response, determined by high-sensitivity C-reactive protein levels in the acute phase. We compared stroke patients’ profiles with 42 people experiencing spontaneous cervical artery dissection without stroke. Furthermore, variations in cytokine levels among stroke aetiologies were analysed. Follow-up samples were collected in a subgroup of ischaemic stroke patients at three and twelve months. Ischaemic stroke patients had elevated plasma levels of HGF and SDF-1α, and lower IL-4 levels, compared to spontaneous cervical artery dissection patients without stroke. Aetiology-subgroup analysis revealed reduced levels of nine cytokines/chemokines (HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, APRIL, SCF), and elevated levels of IL-4 and MIP-1β, in spontaneous cervical artery dissection (with or without ischaemic stroke as levels were comparable between both groups) compared to other aetiologies. The majority of cytokine/chemokine levels remained stable across the study period. Our research indicates that stroke due to large artery atherosclerosis, cardioembolism, and small vessel occlusion triggers a stronger inflammatory response than spontaneous cervical artery dissection.
Similar content being viewed by others
Introduction
Certain factors, such as the severity of the stroke and the patient's age which influence the high disability and mortality rates associated with stroke1, remain beyond our control2. However, other factors such as inflammation related to stroke, have been explored as new targets to prevent stroke-related complications and improve functional outcomes3. These concepts were adopted with the emergence of novel therapeutic targets aimed at reducing peripheral inflammation in individuals after myocardial infarction, since elevated cytokine levels have been linked to unfavourable clinical outcomes in ischaemic stroke patients4. Long-term studies have revealed systemic inflammation that emerges not only within minutes to hours after acute ischaemic stroke (AIS), but persists for several days and drives complications5,6. Although several receptor antagonists have been studied for their potential to reduce circulating inflammatory cytokine levels and improve clinical outcomes after AIS, their effectiveness remains uncertain. Large ongoing trials7 and conflicting results from smaller studies8,9, reflect our incomplete understanding of immune processes following cerebral ischaemia. Nevertheless, some ongoing trials show promise, such as the investigation of colchicine to prevent inflammation10.
C-reactive protein (CRP), a prominent acute-phase protein, is one of the most extensively studied blood markers for inflammation and is implemented in many laboratory panels used in people with AIS11. High CRP levels have been found not only to be associated with an increased risk of developing ischaemic stroke12, but also to predict functional outcome and mortality when measured at the time of admission13,14,15,16,17,18. Other prominent inflammatory markers in stroke research include interleukin (IL)-6, IL-1, IL-10, tumour necrosis factor (TNF)-α and TNF-β, as well as the chemokines monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, and chemokine ligand-519,20,21. Most of these cytokines and chemokines can be found upstream of CRP, but their effect on functional outcome is still not as sufficiently studied as the effect of CRP. Thus, more research is needed to clarify the role of these cytokines and chemokines in cerebrovascular disease, including their association with stroke severity, stroke aetiology, and functional outcomes. Also the precise impact of the post-ischaemic inflammatory response on brain recovery, especially in advanced stages, remains incompletely understood6,22.
In recent years, various smaller panels of pro- and anti-inflammatory cytokines and chemokines have been studied in patients with stroke. The aim of this study was to measure plasma levels of 65 different cytokines, chemokines, soluble surface molecules, and immune receptors using one methodology in patients with AIS to better understand the interplay between pro- and anti-inflammatory cytokines in the acute phase. Furthermore, we aimed to evaluate possible differences between patients showing a higher peripheral inflammatory response in the acute phase, as indicated by high CRP levels in the blood, and patients with normal CRP levels. We also investigated if levels of these 65 cytokines and chemokines remain stable in follow-up samples up to twelve months and examined their predictive value for functional outcome. Finally, we performed subtype-stratified analyses as only a few studies so far have assessed potential differences among various AIS aetiologies.
Methods
Samples, study population and routine laboratory diagnostics
The use of frozen plasma samples from a biobank for this study was approved by the local ethics committees of the Medical University Innsbruck, Austria (ReSect study: EK#UN5072, 325/4.1; STROKE-CARD study: EK#UN2013-0045). Written informed consent for diagnostic procedures and biological sample storage for research purposes was obtained from all patients or their legal representatives. All methods were performed in accordance with the relevant guidelines and regulations.
Samples from patients were included from these two separate cohort studies, the STROKE-CARD trial (post-stroke disease management trial investigating an unselected cohort of consecutive AIS patients treated at the Medical University of Innsbruck)23,24 and the ReSect study (cohort study of spontaneous cervical artery dissection (sCeAD) patients treated at the Medical University of Innsbruck)25,26. ReSect study participants were dichotomised as those suffering AIS due to sCeAD and those with local symptoms only (i.e. no ischaemic stroke; sCeAD-nonAIS). Immediately after the acute event during the baseline assessment, ischaemic stroke aetiology was assessed using the TOAST criteria27 and AIS patients were classified into stroke due to large artery atherosclerosis (LAA), cardioembolism (CE), small vessel occlusion (SVO) or sCeAD. Additionally, in the STROKE-CARD cohort, stroke aetiology was reassessed at the three months follow-up visit. Details of patient selection and recruitment strategies for both studies have already been published23,24,25,26, and details on exclusion and inclusion criteria for both studies (STROKE-CARD and ReSect) are listed in the Supplementary methods (Supplementary File 1). Blood samples in both studies were drawn after an overnight fast and at least 12 h of abstinence from smoking, typically at 8 am. The samples were then immediately used for routine testing. After centrifugation, plasma samples were stored in a biobank at − 80 °C within 3 h after blood sampling. Individuals with any clinical or laboratory symptoms of infection prior to blood sampling for this study were excluded. In addition, particularly for blood sampling at follow-up, individuals who reported having a current infection or a positive history of infection the weeks before were not included in the selected patient cohort analysed in the current study.
At − 80 °C stored plasma samples of 173 people after AIS and 42 samples of sCeAD-nonAIS were included. Patients after AIS were classified based on high-sensitivity CRP levels, an acute phase protein implemented in stroke diagnostics laboratory panels at the Medical University of Innsbruck, as either having CRP levels ≤ 5 mg/l or > 5 mg/l. This cut-off of 5 mg/l was determined according to the standard high-sensitivity CRP reference levels used in clinical routine diagnostics at the University Hospital of Innsbruck28. No other upstream inflammatory markers except for CRP levels were measured in Innsbruck during the emergency diagnostic work-up. The following baseline laboratory parameters were taken from the emergency blood panel determined at the time of admission, even before any acute procedure was performed: CRP, the international normalised ratio (INR) level, prothrombin-time (PT) activity, platelet count, D-dimer level, antithrombin-III (AT-III) activity, fasting glucose, and lipid levels. These laboratory parameters were also assessed at month three and month twelve follow-up visits, except for D-dimer and AT-III activity levels. Blood pressure levels above 140/90 mmHg, or above 130/85 mmHg in people with diabetes, renal impairment, or small-vessel disease, or those on antihypertensive medication, were defined as hypertension. Diabetes was defined by a HbA1c ≥ 6.5%, fasting glucose levels ≥ 126 mg/dl, or non-fasting glucose levels ≥ 200 mg/dl. Dyslipidaemia was defined by low-density lipoprotein cholesterol (LDL-C) levels > 100 mg/dl or > 70 mg/dl in high-risk patients according to guidelines at the time of the studies.
To assess the stability of cytokines, chemokines, and related molecules, as well as their change during disease recovery, some people after AIS were clinically followed up for up to 12 months after the initial event, with plasma samples taken regularly. Specifically, plasma samples from 79 people after AIS were measured at both 3 and 12 months post-stroke. Among these 79 people, 17 belonged to the AIS group with CRP levels > 5 mg/l, and 62 belonged to the group with levels ≤ 5 mg/l. Unfortunately, only baseline samples were available for the remaining individuals.
Classification of stroke severity and parameters of functional outcome after twelve months
For acute assessment of stroke severity, the National Institutes of Health Stroke Scale (NIHSS) was evaluated upon admission, along with the modified Rankin Scale (mRS) score. People were grouped based on their NIHSS scores into minor (0–5), moderate (6–15), or severe (≥ 16) stroke categories29.
Long-term functional outcomes based on the mRS were also assessed at the 12-month follow-up visit, during which plasma samples were taken. Missing mRS follow-up data in AIS patients without this 12-month follow-up visit was completed through retrospective chart review of all available electronic medical files. The collected mRS-follow-up data is addressed to as “mRS at last clinical follow-up”. Good functional outcome was defined as mRS ≤ 2.
Measurement of plasma levels of 65 cytokines, chemokines, and related molecules
Multiplex bead-based immunoassays measuring 65 cytokines, chemokines, and related molecules, were performed according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA; Immune monitoring 65-plex human ProcartaPlex panel, cat. #EPX650-10065-901). In detail the following 65 analytes were measured: a proliferation-inducing ligand (APRIL), B cell activating factor (BAFF), B lymphocyte chemoattractant (BLC), TNF receptor superfamily member 8 (CD30), CD40 ligand (CD40L), neutrophil-activating protein 78 (ENA-78), Eotaxin, Eotaxin-2, Eotaxin-3, fibroblast growth factor (FGF-2), Fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), growth related oncogene α (GROα), hepatocyte growth factor (HGF), interferon (IFN)-α, IFN-ɣ, IL-1α, IL-1β, IL-2, IL-2R, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-18, IL-20, IL-21, IL-22, IL-23, IL-27, IL-31, interferon γ-induced protein 10 kDa (IP-10), interferon-inducible T cell alpha chemoattractant (I-TAC), leukaemia inhibitory factor (LIF), MCP-1, MCP-2, MCP-3, macrophage colony-stimulating factor (M-CSF), macrophage-derived chemokine (MDC), macrophage migration inhibitory factor (MIF), monokine induced by gamma interferon (MIG), MIP-1α, MIP-1β, MIP-3α, matrix metalloproteinase-1 (MMP-1), nerve growth factor β (NGF-β), stem cell factor (SCF), stromal cell-derived factor-1α (SDF-1α), TNF-α, TNF-β, TNF-receptor (R)-2, tumour necrosis factor related apoptosis inducing ligand (TRAIL), thymic stromal lymphopoietin (TSLP), tumour necrosis factor-like weak inducer of apoptosis (TWEAK), and vascular endothelial growth factor-A (VEGF-A). At -80 °C stored AIS and sCeAD-nonAIS plasma sample aliquots were thawed on ice. Then, samples were centrifuged at 10,000 g for 10 min to pellet out lipid particulates. Afterwards, the plasma samples were immediately used. First, magnetic beads in a 96-well flat-bottom plate were washed and 25 µl of universal assay buffer along with 25 µl of undiluted plasma samples or a four-fold serial diluted standard was added into each well. The samples were incubated for 120 min at room temperature (RT) on a shaker at 500 rounds per minute, followed by washing. Next, 25 ml of detection antibody mixture was added to each well and incubated for 30 min at RT on a shaker, then washed twice. Afterwards, 50 µl of streptavidin–phycoerythrin solution was added, and the plates were placed on a shaker for another 30 min incubation at RT. Then, the plates were washed again, and beads were dissolved in 120 µl of reading buffer. After 5 min of incubation while being gently shaken at RT, fluorescence intensity was measured using Luminex MAGPIX instrument according to the manufacturer’s instructions (Software: xPonent 4.2 and ProcartaPlex Analyst 1.0). In samples exceeding the concentration of the highest standard, this concentration limit was used for further analysis. Only cytokines/chemokines with more than 10% of all baseline samples showing levels above the lowest standard were included in further analyses.
Statistical analysis
Statistical analyses were performed using GraphPad Prism-10 (GraphPad Software Inc., La Jolla, California, United States) and SPSS-27 (IBM SPSS Statistics, Armonk, NY). All figures were created by the authors using GraphPad Prism-10.
The primary aim of the study was to compare the peripheral cytokine/chemokine profile in people after AIS classified by high-sensitivity CRP levels at admission, those AIS patients with unaltered peripheral inflammatory responses at admission, and people without stroke having sCeAD with local symptoms only. For overall multiple group comparisons, the non-parametric Kruskal–Wallis test was used. P-values were corrected for multiple (n = 65) comparisons using Bonferroni’s correction. Effect size coefficients η2 were calculated from the Kruskal–Wallis statistics using the formula \(\eta 2=\frac{(H-k+1)}{(n-k)}\) (H = Kruskal–Wallis H value; k = number of groups; n = number of total observations) and classified as either small (0.01 to < 0.06), moderate (0.06 to < 0.14), or large (η2 ≥ 0.14) according to Cohen, 198830. Dunn’s multiple comparisons tests were used to compare subgroups. The necessary sample size for the Kruskal–Wallis test (for 5% α and 80% power (1-ß)) was calculated using G*Power software based on F values calculated from η2 values (F = \(\sqrt{\frac{{\eta }^{2}}{(1-{\eta }^{2})}}\)). To detect a moderate effect size of F > 0.25 (corresponding to η2 > 0.06) it was determined to be > 159 (total sample size) and to detect a large effect size of F > 0.40 (corresponding to η2 > 0.14) it was determined to be > 66 (total sample size).
Secondary aims were (i) to perform subtype-stratified analyses to assess potential differences among various AIS aetiologies (using the non-parametric Kruskal–Wallis test). The necessary sample size was calculated as described above and was determined to be > 180 (total sample size) to detect a moderate effect size of F > 0.25 (corresponding to η2 > 0.06) or > 76 (total sample size) to detect a large effect size of F > 0.40 (corresponding to η2 > 0.14). Moreover, a multivariate linear regression model was used to predict the role of clinical (age, sex, ischaemic stroke, aetiology, NIHSS, and mRS score) and laboratory (CRP) parameters on baseline plasma levels of significantly altered analytes. Cytokine levels were log10 transformed to meet the assumption of the model, and results are shown as estimates b (with standard error of mean) and standardised estimates ß (with 95% confidence intervals).
(ii) To analyse the associations of altered cytokines/chemokines with demographic, clinical, and laboratory characteristics of the acute event (using non-parametric Spearman’s rank correlation test, Mann–Whitney U test, chi-square test, or Fisher’s exact test). Due to limited statistical power and the large number of statistical comparisons, we only show effect sizes, but no p-values. Effect size coefficients R were calculated from the Mann–Whitney U statistics using to the formula \(R= \frac{z}{\sqrt{N}}\) (z = Mann–Whitney U z-value; N = total number of ranks). Calculated R and Spearman ρ effect sizes were classified as either small (-0.3 to -0.1, or 0.1 to 0.3), moderate (-0.5 to -0.3, or 0.3 to 0.5), or large (< -0.5 or > 0.5) according to Cohen, 198830. Effect sizes were then grouped by k-means clustering (Pearson’s correlation) and are shown as a heat map.
(iii) To investigate the association of baseline cytokine/chemokine levels for functional outcome (by multivariate binary logistic regression analysis).
(iv) To examine if levels of cytokines and chemokines remain stable in follow-up samples in a subgroup of AIS patients up to twelve months and to analyse their association with AIS subtypes and outcome (using the non-parametric Friedman test or Kruskal–Wallis test with Dunn’s multiple comparison tests).
Statistical significance was defined as two-sided p-value < 0.05 after correction for multiple comparisons using Bonferroni’s correction.
Ethics approval and consent to participate
The use of frozen samples from a biobank for this study was approved by the local ethics committees of the Medical University Innsbruck, Austria (ReSect study: EK#UN5072, 325/4.1, 29.03.201931; STROKE-CARD study: EK# UN2013-0045 and is registered with Clinical-Trials.gov number NCT02156778 (03.01.2014)23). All patients or their legal representatives gave written informed consent to diagnostic procedures and biological sample storage for research purposes.
Results
Baseline characteristics of study participants
Baseline characteristics of patients after AIS classified according to their baseline CRP levels and characteristics of sCeAD-nonAIS patients are presented in Table 1. Among all AIS patients, 49 (28.3%) were classified as having baseline CRP levels of > 5 mg/l, and 124 (71.7%) had CRP levels ≤ 5 mg/l. Cerebrovascular risk factors of all stroke patients grouped according to the underlying aetiology are listed in Table 2, along with Table S1 of the Supplementary file 1.
Altered cytokines/chemokines in AIS compared to sCeAD-nonAIS
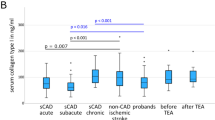
A comparison between the two stroke groups (baseline CRP levels ≤ 5 mg/l and CRP levels > 5 mg/l) and sCeAD-nonAIS revealed differences after Bonferroni’s correction for multiple comparisons for the following cytokines and chemokines: HGF (p = 0.00004, η2 = 0.085), IL-4 (p = 0.0002, η2 = 0.071), and SDF-1α (p = 0.0003, η2 = 0.066), with moderate effect sizes (Fig. 1). All other 62 cytokines/chemokines for which differences were not found between the AIS groups with different CRP levels and sCeAD-nonAIS are displayed in the Supplementary Table S2. HGF and SDF-1α plasma levels were lower in the sCeAD-nonAIS cohort compared to AIS with CRP levels ≤ 5 mg/l. In contrast, IL-4 plasma levels were higher in sCeAD-nonAIS. Moreover, only IL-4 levels differed between AIS patients with CRP levels of ≤ 5 mg/l or > 5 mg/l (Fig. 1).
Altered plasma cytokine/chemokine concentrations in people with different ischaemic stroke (AIS) aetiologies and people with spontaneous cervical artery dissection with local symptoms only (sCeAD-nonAIS). (A) Volcano plot showing η2 effect sizes versus -log10 adjusted p-values. The significance threshold for p-values adjusted by Bonferroni’s correction for 65 comparisons is indicated by the red dashed line. Levels for moderate (η2 > 0.06) and large (η2 > 0.14) effect size are indicated by dotted lines. Plasma concentrations [pg/ml] of (B) HGF, (D) IL-4, and (F) SDF-1α in people with stroke and CRP levels > 5 mg/l, n = 49 or stroke with CRP levels ≤ 5 mg/l, n = 124 and sCeAD-nonAIS, n = 42. Plasma concentrations [pg/ml] of (C) HGF, (E) IL-4, and (G) SDF-1α in people with sCeAD (with and without AIS) and patients with different mechanism leading to stroke (small vessel occlusion (SVO) n = 15; cardio embolism (CE) n = 34; large artery atherosclerosis (LAA) n = 30). sCeAD-nonAIS patients (n = 42) are indicated in yellow. Multiple comparisons were performed using Kruskal–Wallis test with Dunn’s multiple comparison correction (compared to sCeAD (*) or AIS-CRP > 5 mg/l (#): ***p < 0.001, **/##p < 0.01, *p < 0.05). CE cardioembolic stroke, HGF hepatocyte-growth-factor, IL interleukin, LAA large artery atherosclerotic stroke, SDF-1α stromal-cell-derived factor-1α, SVO small-vessel occlusive stroke.
The comparison of underlying causes for AIS showed that sCeAD was associated with lower plasma levels of HGF (p < 0.001, η2 = 0.455, large effect size) and SDF-1α (p < 0.001, η2 = 0.469, large effect size) compared to AIS due to SVO, CE, and LAA (Fig. 1). Only for IL-4 (p < 0.001, η2 = 0.151, large effect size) were higher levels found in people with sCeAD compared to other stroke aetiology groups. Levels of these cytokines were comparable between sCeAD-AIS and sCeAD-nonAIS cohorts (Supplementary Fig. S1). Since the effect sizes of these comparisons were much larger than those of the initial comparisons, we performed a multivariate linear regression analysis for HGF, IL-4, and SDF-1α adjusted for age, sex, CRP levels > 5 mg/l, NIHSS, and mRS score, AIS, and aetiology (Fig. 2 and Supplementary Tables S3–S5). Results clearly indicate that the strongest predictor of HGF, IL-4, and SDF-1α levels was aetiology, followed by age for HGF and IL-4, and CRP levels > 5 mg/l for IL-4.
Multivariate linear regression model to predict the role of clinical and laboratory parameters on baseline plasma levels of (A) HGF, (B) IL-4, and (C) SDF-1α. Results are shown as standardised estimates ß with 95% CI (confidence intervals). All important predictors are shown in red, and asterisks indicate the level of significance (***p < 0.001; **p < 0.01; *p < 0.05). HGF, IL-4, and SDF-1α levels were log10 transformed to meet the assumptions of the model. Model fit: (A) R = 0.671, R2 = 0.450, F = 18.3, p < 0.001, collinearity: VIF < 2.0, autocorrelation: R = -0.02. (B) R = 0.417, R2 = 0.174, F = 4.7, p < 0.001, collinearity: VIF < 2.0, autocorrelation: R = 0.14. (C) R = 0.595, R2 = 0.354, F = 12.3, p < 0.001, collinearity: VIF < 2.0, autocorrelation: R = 0.08. More details are provided in Tables S3–S5. AIS acute ischaemic stroke, CE cardioembolic stroke, CRP C-reactive protein, HGF hepatocyte-growth-factor, IL interleukin, LAA large artery atherosclerotic stroke, mRS modified Rankin Scale, NIHSS National Institutes of Health Stroke Scale, sCeAD spontaneous cervical artery dissection leading to stroke or local symptoms only, SDF-1α stromal-cell-derived factor-1α, SVO small vessel occlusive stroke.
Due to this result, we next compared the levels of all analytes among the different aetiologies. We detected differences after Bonferroni’s correction for the following cytokines and chemokines: HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, MIP-1β, APRIL, SCF, and IL-4 (all large effect sizes); and GROα, Eotaxin-3, IP-10, and BAFF (all moderate effect sizes). However, since the necessary sample size was determined to be > 180 (for 4 groups, 45/group) to detect a moderate effect size of F > 0.25 (corresponding to η2 > 0.06) and the sizes of three groups were smaller (LAA, n = 30; CE, n = 34; SVO, n = 15), we only considered results with a large effect size F ≥ 0.40 (corresponding to η2 ≥ 0.14). Therefore, only the results of HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, MIP-1β, APRIL, SCF, and IL-4 are statistically significant with sufficient power (Figs. 3, 4, Supplementary Table S6). Finally, we also compared the pooled LAA, CE, and SVO group with sCeAD and confirmed our findings, whereas there were no differences between the two sCeAD subgroups (sCeAD-nonAIS versus sCeAD-AIS). Increased CRP levels > 5 mg/l had no impact on levels of cytokines and chemokines (Supplementary Fig. S1).
Altered plasma cytokine/chemokine concentrations in people with different stroke aetiologies. (A) Volcano plot showing η2 effect sizes versus -log10 adjusted p-values. The significance threshold for p-values adjusted by Bonferroni’s correction for 65 comparisons is indicated by the red dashed line. Levels for moderate (η2 > 0.06) and large (η2 > 0.14) effect size are indicated by dotted lines. (B) Heatmap of altered analytes shown as standardised values (z-scores) of log10 transformed cytokine values grouped according to their aetiology. Negative z-scores (decreased levels of analytes) are shown by dark colours, positive z-scores (increased levels of analytes) are shown by light colours. The heatmap was created by the authors using GraphPad Prism-10 (GraphPad Software Inc., La Jolla, California, United States; https://www.graphpad.com). APRIL a proliferation-inducing ligand, BAFF B-cell activating factor, CD30 TNF receptor superfamily member 8, CE cardioembolic stroke, GRO-α growth regulated oncogene-α, HGF hepatocyte-growth-factor, IL interleukin, IL-2R interleukin-2 receptor, IP-10 interferon γ-induced protein 10 kDa, LAA large artery atherosclerotic stroke, MIF macrophage migration inhibitory factor, MIP-1β macrophage inflammatory protein-1β, sCeAD spontaneous cervical artery dissection leading to stroke, SCF stem cell factor, SDF-1α stromal-cell-derived factor-1α, SVO small vessel occlusive stroke, TNF-RII tumour necrosis factor receptor 2.
Altered plasma cytokine/chemokine concentrations in people with different stroke aetiologies. Plasma levels of (A) IL-2R, (B) TNF-RII, (C) CD30, (D) IL-16, (E) MIF, (F) MIP-1β, (G) APRIL, and (H) SCF were grouped based on the underlying mechanisms: spontaneous cervical artery dissection (sCeAD) with AIS (n = 94) or local symptoms only (n = 42, shown in yellow); small vessel occlusive stroke (SVO) n = 15; cardio embolic stroke (CE) n = 34; large artery atherosclerotic stroke (LAA) n = 30. Group comparisons were performed using Kruskal–Wallis test with Dunn’s multiple comparison tests (difference to sCeAD: ***p < 0.001; **p < 0.01; *p < 0.05). AIS acute ischaemic stroke, APRIL a proliferation-inducing ligand, CD30 TNF receptor superfamily member 8, IL interleukin, IL-2R interleukin 2 receptor, MIF macrophage migration inhibitory factor, MIP-1β macrophage inflammatory protein-1β, SCF stem cell factor, TNF-RII tumour necrosis factor receptor 2.
Differences in HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, MIP-1β, APRIL, SCF, and IL-4 levels according to demographic, clinical and laboratory parameters at admission
Next, we analysed the associations of these altered cytokines/chemokines (HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, MIP-1β, APRIL, SCF, IL-4) with demographic, clinical, and laboratory characteristics of the acute event in the entire study cohort. The heatmap in Fig. 5 shows the effects sizes (R and ρ) of these comparisons/correlations according to hierarchical clustering. We could identify two groups of analytes with differential associations: (i) HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, APRIL, and SCF were negatively associated with sCeAD, CRP, lipids, platelets, and female sex, and positively associated with AIS, LAA, CE, SVO, age, diabetes, dyslipidaemia, hypertension, HbA1c, atrial fibrillation, and a positive history of a transient ischaemic attack.
Heatmap of Spearman’s correlation coefficients for continuous clinical and laboratory characteristics or Mann–Whitney U test effect sizes for dichotomous characteristics in comparison to baseline plasma levels of IL-2R, HGF, TNF-RII, SDF-1α, CD30, MIF, IL-16, APRIL, SCF, MIP-1β, and IL-4. Data are depicted in colours ranging from blue to red, indicating a negative to positive effect size R or Spearman’s ρ grouped by k-means clustering. Samples from all 215 study participants (AIS and sCeAD-nonAIS) at baseline were included in this figure. The heatmap was created by the authors using GraphPad Prism-10 (GraphPad Software Inc., La Jolla, California, United States; https://www.graphpad.com). APRIL a proliferation-inducing ligand, AT-III antithrombin-III, CCA/ICA common carotid artery/internal carotid artery, CD30 TNF receptor superfamily member 8, CE cardioembolic stroke, CRP C-reactive protein, HbA1c glycated haemoglobin A1c, HDL-C high-density lipoprotein cholesterol, HGF hepatocyte-growth-factor, IL interleukin, IL-2R interleukin 2 receptor, INR international normalised ratio, LAA large artery atherosclerotic stroke, LDL-C low-density lipoprotein cholesterol, MIF macrophage migration inhibitory factor, MIP-1β macrophage inflammatory protein-1β, mRS modified Rankin Scale, NIHSS National Institutes of Health Stroke Scale, PT prothrombin time, sCeAD spontaneous cervical artery dissection, SCF stem cell factor, SDF-1α stromal-cell-derived factor-1α, SVO small vessel occlusive stroke, TIA transient ischaemic attack, TNF-RII tumour necrosis factor receptor 2.
In contrast, MIP-1β and IL-4 showed a completely different picture with a positive association with sCeAD, CRP, and many coagulation parameters. Furthermore, especially MIP-1β showed a negative association with age, CE, LAA, SVO, INR, and hypertension among many more.
Association of baseline cytokine/chemokine levels with functional outcome
We then analysed whether baseline levels of HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, MIP-1β, APRIL, SCF, and IL-4 predicted functional outcome (mRS 0–2 or mRS 3–5) in a multivariate binary logistic regression model adjusted for sex, CRP levels, NIHSS, and age at admission. Although baseline levels of HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, and APRIL were higher in AIS patients with mRS 3–5, in the multivariate model, only higher age and higher NIHSS assessed in the acute phase were associated with mRS > 2 (Supplementary Table S7).
Stability of plasma cytokine/chemokine levels after month three and month twelve of follow-up in a subgroup of AIS patients
Plasma cytokine/chemokine levels were measured in a subgroup of people with AIS at a median of 3.3 (range 2.3–4.3) months, and for the last follow-up measurement at a median of 12.6 (range 11.1–14.2) months after the acute event. This subgroup included 30 patients with stroke due to LAA, 34 with CE stroke, and 15 with SVO stroke. Clinical and laboratory characteristics at these follow-up visits are shown in Supplementary Table S8 and Fig. S2.
A decrease in plasma levels comparing baseline and both follow-up time points was observed for HGF, and these plasma levels remained stable between three-month and twelve-month follow-up time points. Similarly, TNF-RII levels decreased three months after the acute event, while plasma CD30 levels increased during follow-up. Statistical testing to assess differences between stroke aetiology groups regarding baseline and follow-up cytokine/chemokine levels was not performed due to the limited sample size.
Discussion
Our investigation into the systemic inflammatory response following AIS revealed distinct plasma concentrations of HGF, SDF-1α, and IL-4 compared to individuals with sCeAD-nonAIS. Our study highlighted unique inflammatory profiles between sCeAD and other stroke aetiologies, though no differences were found between sCeAD with or without stroke. The comparison of all 65 cytokines and chemokines among the four aetiology groups (sCeAD, LAA, CE, and SVO) revealed differences for eleven analytes. Plasma levels of HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, APRIL, and SCF were lower in sCeAD than in LAA, CE or SVO, but IL-4 and MIP-1β levels were higher in sCeAD. Elevated CRP levels were not associated with these cytokines and chemokines. In summary, our results indicate substantial differences in the inflammatory response between dissection (with or without stroke) and other stroke aetiologies (LAA, CE, and SVO).
Many of the proteins showing differences in our study play established roles in stroke or cardiovascular diseases. HGF, for instance, is recognised as an endothelium-specific growth factor with diverse beneficial functions, including anti-inflammatory, anti-fibrotic, and pro-angiogenetic properties32. It is primarily released in response to endothelial injury, leading to elevated levels observed in cardiovascular conditions such as atherosclerosis33, acute myocardial infarction34, or AIS35. Previous research has linked higher HGF levels with poor outcomes in these diseases36,37, likely due to increased endothelial release. Interestingly, our study revealed a decrease in HGF levels three months after the acute stroke event, suggesting its involvement not only in the inflammatory response to stroke but also in reflecting the response to cerebral ischaemia itself during the acute phase. In contrast to HGF, levels of most other cytokines/chemokines remained elevated up to one year, indicating a more generalised inflammatory response, as ischaemia itself would not be expected to drive inflammatory cytokine and chemokine levels to a similar extent after twelve months. This is consistent with previous findings where upregulated proteins during the acute phase of stroke remained elevated in follow-up measurements38.
SDF-1α is produced by activated astrocytes, microglia, and vascular endothelial cells. It plays a crucial role in recruiting monocytes locally to the injured brain region after ischaemic stroke39,40,41 and mobilises endothelial progenitor cells, thus promoting angiogenesis42. Elevated SDF-1α levels have been observed in individuals with cardiovascular risk factors43,44, and plasma levels have been linked to adverse cardiovascular outcomes in people with coronary artery disease45. Similarly, elevated levels of IL-2R have been reported in coronary artery atherosclerosis46 and associated with various cardiovascular risk factors, including diabetes mellitus and hypertension47, as confirmed in our study.
Members of the TNF receptor superfamily such as CD30, TNF-RII, and APRIL, expressed on various immune cells48,49 were found to be upregulated in the blood of ischaemic stroke patients in the sub-acute phase50,51,52. APRIL was shown to protect against atherosclerosis by binding to heparin-sulphate proteoglycans in mice, and decreased serum levels are associated with long-term cardiovascular mortality in individuals with atherosclerosis53. IL-16, released by activated CD8+ T cells, contributes to the inflammatory response following ischaemic stroke. It activates CD4+ T cells, monocytes, macrophages, and dendritic cells, leading to the expression of various inflammatory cytokines. This process drives secondary brain damage19,54,55,56. MIF, a pleiotropic inflammatory mediator with chemokine-like functions, promotes leukocyte migration to inflammatory sites, including atherosclerosis57. MIF is not only produced by monocytes, macrophages, B- or T-cells, but also by endothelial and epithelial cells58.
SCF, produced by bone marrow stromal cells, induces neuroproliferation, reduces infarct size, and improves functional outcome in ischaemic stroke models59,60.
Distinct findings of higher plasma IL-4 and MIP-1β levels were observed in sCeAD patients compared to other stroke aetiologies. IL-4 has an anti-inflammatory role and is a critical regulator of M2 polarization. Following its secretion by neurons in response to ischaemia, it stimulates microglial phagocytosis and enables clearance of apoptotic neurons. Consequently, it promotes long-term recovery of microglia/macrophages61. MIP-1β, a chemokine secreted by various vascular and hematopoietic cells62, is linked to atherosclerosis63. Elevated blood levels of MIP-1β are reported in people with this condition64. However, such elevations were not observed in cohorts of stroke patients21, warranting additional investigation into underlying mechanisms regarding its differential expression in distinct cardiovascular pathologies. IL-4, along with MIP-1β, was associated with distinct clinical parameters, showing mostly positive Spearman’s correlation values with platelet count, PT, AT-III, or CRP in our study. Interleukins play a unique role in megakaryopoiesis, thrombopoiesis, and platelet function, with the majority having positive effects. However, a subset such as IL-4 or IL-1α has been reported to possess inhibitory properties on megakaryocyte differentiation, thereby inhibiting platelet production65,66,67,68. This contradicts the findings of our study and warrants further investigation. This includes a more comprehensive characterization of the study participants, particularly regarding haematological disorders that may have influenced these outcomes.
Experimental studies and clinical trials using above-described cytokines and chemokines have shown promising results in ischaemia treatment. Preclinical studies on rats or mice demonstrated the positive effect of HGF on protecting blood–brain barrier integrity, reducing infarct volume, and improving functional recovery after stroke69,70,71,72. Clinical trials testing HGF in peripheral or coronary artery ischaemia proved good tolerance73,74,75. However, to our knowledge, such trials for ischaemic stroke have not been conducted yet. Other therapeutic approaches involving cytokines and chemokines have also yielded promising results, especially in animal models76,77,78,79.
In our study cohort, individuals after sCeAD, whether experiencing a stroke or only local symptoms, had the lowest levels of cytokines compared to other stroke aetiology groups during the acute phase. This is an important finding, as it distinguishes our study from previous research. While another research group examined a similar panel of cytokines and chemokines in the blood of AIS patients, they neither conducted a comparative analysis across different AIS aetiology groups nor included such a substantial cohort of patients experiencing AIS due to sCeAD, including those presenting solely with local symptoms21. However, another study, albeit with a smaller sample size of 29 patients diagnosed with sCeAD leading to AIS, reported comparable findings of decreased cytokine levels in this aetiology of AIS compared to other causes38.
Our results also indicate that only higher age and higher NIHSS score, but not levels of any cytokine/chemokine, were associated with mRS > 2 after twelve months, indicating unfavourable outcomes.
There are several limitations to this study. Firstly, it is retrospective and includes a relatively small number of AIS patients. Although the sample size was sufficient to detect a moderate effect size for the primary aim, unequal group sizes based on CRP levels and TOAST aetiologies limit the statistical power. To address this, we used Bonferroni’s correction for multiple comparisons and focused on cytokines and chemokines with large effect sizes. However, the small sample size for most subgroup analyses, especially when classifying AIS patients by mRS scores (≤ 2 or > 2), further limits statistical power. We hypothesize that more cytokine/chemokine levels might differ between groups, particularly when comparing stroke due to sCeAD and other aetiologies. Therefore, larger studies including more AIS patients and individuals with sCeAD are needed to confirm our results and establish causality between cytokine levels and clinical factors.
Secondly, the retrospective design and inclusion of stroke patients from two different trials (STROKE-CARD trial, ReSect-study) resulted in differing clinical data sets and some missing clinical information, potentially introducing reporting bias. The time span between the onset of AIS or sCeAD symptoms and blood sampling for cytokine/chemokine assessment varied and was not standardised, as blood sampling was always performed at 8 am.
Moreover, this study does not accurately reflect the natural distribution of ischaemic stroke causes. In this study, patients with sCeAD-AIS constitute the majority, whereas patients with CE, which typically represents a major aetiology in AIS, are underrepresented. Since sCeAD accounts for approximately 1–2% of stroke patients80, its prevalence in this study is disproportionate. This also affects the median age of participants, as sCeAD leading to AIS or local symptoms predominantly occurs in young and middle-aged adults, whereas AIS due to other aetiologies commonly affects older people81. The selection of a group of people with sCeAD-nonAIS might impact the altered cytokine profile observed. While some epidemiological observations suggest a link between inflammation and sCeAD, particularly following infections82,83,84, the exact underlying pathophysiological mechanisms remain unclear. Reported infections include respiratory and urinary tract infections, and gastroenteritis, typically appearing within four weeks before the sCeAD event85. Notably, these infections often present with mild symptoms and usually resolve before hospitalization86, so we speculate they may not influence the cytokine/chemokine profile observed post-sCeAD event in our study.
We categorised participants into CRP groups of ≤ 5 mg/l or > 5 mg/l. It is crucial to acknowledge that CRP, as an acute-phase protein, is a nonspecific indicator of inflammation. Elevated CRP levels can result from infections87, tissue damage associated with atherosclerosis or ischaemic stroke88, chronic diseases89, or autoimmune disorders90,91. Therefore, there is a possibility of misclassifying AIS patient into the > 5 mg/l group if elevated CRP levels did not originate from the acute stroke event.
Finally, we evaluated long-term functional outcomes at twelve months and at the last clinical follow-up visit, with a median of 5.1 years post-stroke in the > 5 mg/l CRP group and 1.4 years in the ≤ 5 mg/l CRP group using the mRS score92. Unfortunately, neuroimaging scans were not included to investigate potential associations between infarct volume and baseline cytokine/chemokine levels. Ischaemic lesion volume has been suggested as a surrogate imaging biomarker in the early clinical phase of AIS to predict functional outcomes93. Previous studies have established an independent relationship between ischaemic lesion volume and functional outcome94, with a strong correlation between ischaemic stroke infarct volume on follow-up CT and MRI scans and the mRS score95. However, these findings are influenced by factors such as ischaemic lesion location and the timing of lesion volume measurement or mRS assessment96. Furthermore, non-modifiable factors like older age, stroke severity, or pre-stroke dependency, as well as complications during hospitalization, including pneumonia, increased intracranial pressure, or cerebral oedema, affect outcomes after three months97,98. Unfortunately, we did not assess early in-hospital complications in our study cohort.
Strengths of the current study are the inclusion of well-characterised ischaemic stroke patients, with particular emphasis on a sizable cohort of sCeAD-AIS patients. Importantly, we directly compared various stroke aetiologies, enabling us to find differences in the profiles of numerous inflammation-associated proteins between AIS patients and additionally those experiencing only local symptoms of sCeAD. Moreover, the standardised blood-sampling at three and twelve months post-event enabled the acquisition of valuable long-term follow-up data on cytokine and chemokine levels. This aspect greatly contributes to our understanding of the dynamics of these inflammatory mediators in the aftermath of AIS.
Conclusions
Measuring 65 plasma cytokines and chemokines revealed that individuals with stroke due to LAA, CE, and SVO have a stronger inflammatory response than those with sCeAD, particularly for the analytes HGF, SDF-1α, IL-2R, CD30, TNF-RII, IL-16, MIF, APRIL, and SCF.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
Acute ischaemic stroke
- APRIL:
-
A proliferation-inducing ligand
- AT-III:
-
Antithrombin-III
- BAFF:
-
B-cell activation factor
- BLC:
-
B-lymphocyte chemoattractant
- CCA/ICA:
-
Common carotid artery/internal carotid artery
- CD30:
-
TNF receptor superfamily member 8
- CD40L:
-
CD40-ligand
- CE:
-
Cardioembolism
- CRP:
-
C-reactive protein
- ENA-78:
-
Epithelial neutrophil-activating peptide-78
- FGF:
-
Fibroblast-growth factor
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- GRO:
-
Growth-regulated oncogene
- HbA1c:
-
Glycated haemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- HGF:
-
Hepatocyte growth factor
- IFN:
-
Interferon
- IL:
-
Interleukin
- IL-2R:
-
Interleukin 2 receptor
- INR:
-
International normalised ratio
- IP:
-
Interferon-ɣ-induced protein
- I-TAC:
-
Interferon-inducible T-cell α-chemoattractant
- LAA:
-
Large artery atherosclerosis
- LDL-C:
-
Low density lipoprotein cholesterol
- LIF:
-
Leukaemia inhibitory factor
- MCP:
-
Monocyte chemoattractant protein
- MCRP:
-
Monocyte chemoattractant protein
- M-CSF:
-
Macrophage colony-stimulating factor
- MDC:
-
Macrophage-derived chemokine
- MIF:
-
Macrophage migration inhibitory factor
- MIG:
-
Monokine induced by interferon-ɣ
- MIP:
-
Macrophage inflammatory protein
- MMP:
-
Matrix metalloproteinase
- mRS:
-
Modified Rankin Scale
- NGF:
-
Nerve growth factor
- NIHSS:
-
National Institutes of Health Stroke Scale
- PT:
-
Prothrombin time
- sCeAD:
-
Spontaneous cervical artery dissection
- sCeAD-nonAIS:
-
Spontaneous cervical artery dissection with local symptoms only
- SCF:
-
Stem-cell factor
- SDF-1α:
-
Stromal cell-derived factor-1α
- SVO:
-
Small vessel occlusion
- TIA:
-
Transient ischaemic attack
- TNF:
-
Tumour necrosis factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TSLP:
-
Thymic stromal lymphopoietin
- TWEAK:
-
Tumour necrosis factor-like weak inducer of apoptosis
- VEGF:
-
Vascular endothelial growth factor
References
Wafa, H. A. et al. Burden of stroke in Europe: Thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke 51(8), 2418–2427 (2020).
Jabal, M. S. et al. Interpretable machine learning modeling for ischemic stroke outcome prediction. Front. Neurol. 13, 884693 (2022).
Bustamante, A. et al. Ischemic stroke outcome: A review of the influence of post-stroke complications within the different scenarios of stroke care. Eur. J. Intern. Med. 29, 9–21 (2016).
Sobowale, O. A. et al. Interleukin-1 in stroke: From bench to bedside. Stroke 47(8), 2160–2167 (2016).
Lo, E. H., Dalkara, T. & Moskowitz, M. A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4(5), 399–415 (2003).
Xu, S., Lu, J., Shao, A., Zhang, J. H. & Zhang, J. Glial cells: Role of the immune response in ischemic stroke. Front. Immunol. 11, 294 (2020).
Smith, C. J. et al. SCIL-STROKE (subcutaneous interleukin-1 receptor antagonist in ischemic stroke): A randomized controlled phase 2 trial. Stroke 49(5), 1210–1216 (2018).
Dreikorn, M. et al. Immunotherapy of experimental and human stroke with agents approved for multiple sclerosis: A systematic review. Ther. Adv. Neurol. Disord. 11, 1756286418770626 (2018).
Chavda, V., Madhwani, K. & Chaurasia, B. Stroke and immunotherapy: Potential mechanisms and its implications as immune-therapeutics. Eur. J. Neurosci. https://doi.org/10.1111/ejn.15224 (2021).
Kelly, P. et al. Colchicine for prevention of vascular inflammation in Non-CardioEmbolic stroke (CONVINCE)—Study protocol for a randomised controlled trial. Eur. Stroke J. 6(2), 222–228 (2021).
Zimmerman, M. A. et al. Diagnostic implications of C-reactive protein. Arch. Surg. 138(2), 220–224 (2003).
Zhou, Y., Han, W., Gong, D., Man, C. & Fan, Y. Hs-CRP in stroke: A meta-analysis. Clin. Chim. Acta 453, 21–27 (2016).
Bakhshayesh-Eghbali, B. et al. Ability of serum C-reactive protein and white blood cell cout in predicting acute schemic stroke. A short -term follow-up study. Caspian J. Intern. Med. 7(3), 206–210 (2016).
Gu, H.-Q. et al. Association between high-sensitivity C-reactive protein, functional disability, and stroke recurrence in patients with acute ischaemic stroke: A mediation analysis. eBioMedicine 80, 104054 (2022).
Anuk, T. et al. Prognostic implications of admission inflammatory profile in acute ischemic neurological events. Acta Neurol. Scand. 106(4), 196–199 (2002).
Di Napoli, M., Papa, F. & Bocola, V. C-reactive protein in ischemic stroke: An independent prognostic factor. Stroke 32(4), 917–924 (2001).
Cai, Z., He, W., Zhuang, F.-J. & Chen, Y. The role of high high-sensitivity C-reactive protein levels at admission on poor prognosis after acute ischemic stroke. Int. J. Neurosci. 129(5), 423–429 (2019).
VanGilder, R. L. et al. C-reactive protein and long-term ischemic stroke prognosis. J. Clin. Neurosci. 21(4), 547–553 (2014).
Zhu, H. et al. Interleukins and ischemic stroke. Front. Immunol. 13, 828447 (2022).
Pawluk, H. et al. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 15, 469–484 (2020).
Li, F. et al. Alterations of inflammatory cytokines in super-acute stroke patients and the potential pathogenesis. J. Clin. Neurosci. 99, 35–43 (2022).
Ma, Y., Yang, S., He, Q., Zhang, D. & Chang, J. The role of immune cells in post-stroke angiogenesis and neuronal remodeling: The known and the unknown. Front. Immunol. 12, 784098 (2021).
Toell, T. et al. Pragmatic trial of multifaceted intervention (STROKE-CARD care) to reduce cardiovascular risk and improve quality-of-life after ischaemic stroke and transient ischaemic attack -study protocol. BMC Neurol. 18(1), 187 (2018).
Willeit, P. et al. STROKE-CARD care to prevent cardiovascular events and improve quality of life after acute ischaemic stroke or TIA: A randomised clinical trial. EClinicalMedicine 25, 100476 (2020).
Mayer, L. et al. Local signs and symptoms in spontaneous cervical artery dissection: A single centre cohort study. J. Stroke 21(1), 112–115 (2019).
Mayer-Suess, L. et al. Extracellular matrix protein signature of recurrent spontaneous cervical artery dissection. Neurology 95(15), e2047–e2055 (2020).
Adams, H. P. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1), 35–41 (1993).
Thomas L, editor. Labor und Diagnose: Indikation und Bewertung von Laborbefunden für die medizinische Diagnostik. Studien-Ed. der 4. Aufl. Marburg: Med. Verl.-Ges (1995).
Kuster, G. W. et al. Performance of four ischemic stroke prognostic scores in a Brazilian population. Arq. Neuropsiquiatr. 74(2), 133–137 (2016).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn. (L. Erlbaum Associates, 1988).
Mayer-Suess, L. et al. Clinical characteristics and outcome in expansive compared with steno-occlusive mural hematoma in spontaneous cervical artery dissection. Int. J. Stroke 18(10), 1186–1192 (2023).
Gallo, S., Sala, V., Gatti, S. & Crepaldi, T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin. Sci. (Lond.) 129(12), 1173–1193 (2015).
Barć, P. et al. Double VEGF/HGF gene therapy in critical limb ischemia complicated by diabetes mellitus. J. Cardiovasc. Transl. Res. 14(3), 409–415 (2021).
Guo, W. et al. Supramolecular self-assembled nanofibers efficiently activate the precursor of hepatocyte growth factor for angiogenesis in myocardial infarction therapy. ACS Appl. Mater. Interfaces 13(19), 22131–22141 (2021).
Rajpathak, S. N. et al. Hepatocyte growth factor and the risk of ischemic stroke developing among postmenopausal women: Results from the Women’s Health Initiative. Stroke 41(5), 857–862 (2010).
Zhu, Z. et al. Serum hepatocyte growth factor is probably associated with 3-month prognosis of acute ischemic stroke. Stroke 49(2), 377–383 (2018).
Li, F. et al. The incremental prognostic value of hepatocyte growth factor in first-ever acute ischemic stroke: An early link between growth factor and interleukins. Front. Neurol. 12, 691886 (2021).
Stanne, T. M. et al. Longitudinal study reveals long-term proinflammatory proteomic signature after ischemic stroke across subtypes. Stroke 53(9), 2847–2858 (2022).
Hill, W. D. et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 63(1), 84–96 (2004).
Malik, M. et al. Monocyte migration and LFA-1-mediated attachment to brain microvascular endothelia is regulated by SDF-1 alpha through Lyn kinase. J. Immunol. 181(7), 4632–4637 (2008).
Bai, M., Sun, R., Cao, B., Feng, J. & Wang, J. Monocyte-related cytokines/chemokines in cerebral ischemic stroke. CNS Neurosci. Ther. 29(12), 3693–3712 (2023).
Bogoslovsky, T. et al. Stromal-derived factor-1alpha correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 42(3), 618–625 (2011).
Farouk, S. S., Rader, D. J., Reilly, M. P. & Mehta, N. N. CXCL12: A new player in coronary disease identified through human genetics. Trends Cardiovasc. Med. 20(6), 204–209 (2010).
Gao, J.-H., Yu, X.-H. & Tang, C.-K. CXC chemokine ligand 12 (CXCL12) in atherosclerosis: An underlying therapeutic target. Clin. Chim. Acta 495, 538–544 (2019).
Ghasemzadeh, N. et al. Plasma stromal cell-derived factor 1α/CXCL12 level predicts long-term adverse cardiovascular outcomes in patients with coronary artery disease. Atherosclerosis 238(1), 113–118 (2015).
Wadwa, R. P. et al. Soluble interleukin-2 receptor as a marker for progression of coronary artery calcification in type 1 diabetes. Int. J. Biochem. Cell Biol. 38(5–6), 996–1003 (2006).
Durda, P. et al. Plasma levels of soluble interleukin-2 receptor α: associations with clinical cardiovascular events and genome-wide association scan. Arterioscler. Thromb. Vasc. Biol. 35(10), 2246–2253 (2015).
van der Weyden, C. A., Pileri, S. A., Feldman, A. L., Whisstock, J. & Prince, H. M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 7(9), e603–e603 (2017).
Yeo, S. C. & Barratt, J. The contribution of a proliferation-inducing ligand (APRIL) and other TNF superfamily members in pathogenesis and progression of IgA nephropathy. Clin. Kidney J. 16(Suppl 2), ii9–ii18 (2023).
Hansen, R. B. et al. Leukocyte TNFR1 and TNFR2 expression contributes to the peripheral immune response in cases with ischemic stroke. Cells 10(4), 861 (2021).
Zhu, L. et al. Comprehensive analysis of blood-based m6A methylation in human ischemic stroke. Mol. Neurobiol. 60(2), 431–446 (2023).
Clausen, B. H. et al. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol. Commun. 8(1), 81 (2020).
Tsiantoulas, D. et al. APRIL limits atherosclerosis by binding to heparan sulfate proteoglycans. Nature 597(7874), 92–96 (2021).
Liu, X., Du, J., Zhou, Y., Shu, Q. & Li, Y. Interleukin-16 polymorphism is associated with an increased risk of ischemic stroke. Mediat. Inflamm. 2013, 564750 (2013).
Mathy, N. L. et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 100(1), 63–69 (2000).
Skundric, D. S., Cruikshank, W. W. & Drulovic, J. Role of IL-16 in CD4+ T cell-mediated regulation of relapsing multiple sclerosis. J. Neuroinflamm. 12(1), 78 (2015).
Bernhagen, J. et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13(5), 587–596 (2007).
Calandra, T. & Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 3(10), 791–800 (2003).
Zhao, L.-R. et al. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke 38(10), 2804–2811 (2007).
Zhao, L.-R., Singhal, S., Duan, W.-M., Mehta, J. & Kessler, J. A. Brain repair by hematopoietic growth factors in a rat model of stroke. Stroke 38(9), 2584–2591 (2007).
Zhao, X. et al. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci. 35(32), 11281–11291 (2015).
Bystry, R. S., Aluvihare, V., Welch, K. A., Kallikourdis, M. & Betz, A. G. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2(12), 1126–1132 (2001).
Chang, T.-T. & Chen, J.-W. Emerging role of chemokine CC motif ligand 4 related mechanisms in diabetes mellitus and cardiovascular disease: Friends or foes?. Cardiovasc. Diabetol. 15(1), 117 (2016).
Cagnin, S. et al. Reconstruction and functional analysis of altered molecular pathways in human atherosclerotic arteries. BMC Genom. 10, 13 (2009).
Catani, L. et al. Interleukin-4 downregulates nuclear factor-erythroid 2 (NF-E2) expression in primary megakaryocytes and in megakaryoblastic cell lines. Stem Cells 19(4), 339–347 (2001).
Gao, A. et al. Bone marrow endothelial cell-derived interleukin-4 contributes to thrombocytopenia in acute myeloid leukemia. Haematologica 104(10), 1950–1961 (2019).
Huang, M. et al. Interleukins in platelet biology: Unraveling the complex regulatory network. Pharmaceuticals (Basel) 17(1), 109 (2024).
Andreescu, M. The link between immune thrombocytopenia and the cytokine profile: A bridge to new therapeutical targets. Front. Hematol. 2, 1191178 (2023).
Sowa, K. et al. Impact of dental pulp stem cells overexpressing hepatocyte growth factor after cerebral ischemia/reperfusion in rats. Mol. Ther. Methods Clin. Dev. 10, 281–290 (2018).
Doeppner, T. R. et al. Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation. J. Cereb. Blood Flow Metab. 31(5), 1251–1262 (2011).
Shimamura, M. et al. Novel therapeutic strategy to treat brain ischemia: Overexpression of hepatocyte growth factor gene reduced ischemic injury without cerebral edema in rat model. Circulation 109(3), 424–431 (2004).
Tang, H. et al. Hepatocyte growth factor-modified hair follicle stem cells ameliorate cerebral ischemia/reperfusion injury in rats. Stem Cell Res. Ther. 14(1), 25 (2023).
Barć, P. et al. Two-stage gene therapy (VEGF, HGF and ANG1 plasmids) as adjunctive therapy in the treatment of critical lower limb ischemia in diabetic foot syndrome. Int. J. Environ. Res. Public Health 19(19), 12818 (2022).
Yang, Z.-J. et al. Phase I clinical trial on intracoronary administration of Ad-hHGF treating severe coronary artery disease. Mol. Biol. Rep. 36(6), 1323–1329 (2009).
Morishita, R. et al. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension 44(2), 203–209 (2004).
Li, Y. et al. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res. Ther. 9(1), 139 (2018).
Akhtar, S., Gremse, F., Kiessling, F., Weber, C. & Schober, A. CXCL12 promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arterioscler. Thromb. Vasc. Biol. 33(4), 679–686 (2013).
Qin, C. et al. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transd. Target. Ther. 7(1), 215 (2022).
Cao, Y., Yue, X., Jia, M. & Wang, J. Neuroinflammation and anti-inflammatory therapy for ischemic stroke. Heliyon 9(7), e17986 (2023).
Ranjbar, M. et al. Incidence, characteristics and prognosis of cervical artery dissection-induced ischemic stroke in central Iran. BMC Neurol. 22(1), 227 (2022).
Robertson, J. J. & Koyfman, A. Cervical artery dissections: A review. J. Emerg. Med. 51(5), 508–518 (2016).
Purdy, K., Long, R. & Jickling, G. Case report: COVID-19 infection and cervical artery dissection. Am. J. Trop. Med. Hyg. 106(3), 874–876 (2022).
Hunter, M. D. et al. Influenza-like illness is associated with increased short-term risk of cervical artery dissection. J. Stroke Cerebrovasc. Dis. 30(2), 105490 (2021).
Collamer, A. N. & Battafarano, D. A pain in the neck: Carotid artery dissection presenting as vasculitis. Milit. Med. 178(7), e851–e854 (2013).
Guillon, B. et al. Infection and the risk of spontaneous cervical artery dissection: A case-control study. Stroke 34(7), e79-81 (2003).
Li, H. et al. Unraveling the links between chronic inflammation, autoimmunity, and spontaneous cervicocranial arterial dissection. J. Clin. Med. 12(15), 5132 (2023).
Lamrous, A. et al. C-reactive protein and procalcitonin use in adults in low- and middle-income countries: a narrative review. JAC Antimicrob. Resist. 5(3), dlad057 (2023).
Kelly, P. J., Lemmens, R. & Tsivgoulis, G. Inflammation and stroke risk: A new target for prevention. Stroke 52(8), 2697–2706 (2021).
Luan, Y.-Y. & Yao, Y.-M. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 9, 1302 (2018).
Scherer, H. U., Häupl, T. & Burmester, G. R. The etiology of rheumatoid arthritis. J. Autoimmun. 110, 102400 (2020).
Aringer, M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 110, 102374 (2020).
Banks, J. L. & Marotta, C. A. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 38(3), 1091–1096 (2007).
Yoo, A. J. et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 43(5), 1323–1330 (2012).
Bucker, A. et al. Associations of ischemic lesion volume with functional outcome in patients with acute ischemic stroke: 24-hour versus 1-week imaging. Stroke 48(5), 1233–1240 (2017).
Boers, A. M. M. et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: A pooled analysis of seven randomized trials. J. Neurointerv. Surg. 10(12), 1137–1142 (2018).
Ernst, M. et al. Association of computed tomography ischemic lesion location with functional outcome in acute large vessel occlusion ischemic stroke. Stroke 48(9), 2426–2433 (2017).
Grube, M. M. et al. Influence of acute complications on outcome 3 months after ischemic stroke. PLoS One 8(9), e75719 (2013).
Balami, J. S., Chen, R.-L., Grunwald, I. Q. & Buchan, A. M. Neurological complications of acute ischaemic stroke. Lancet Neurol. 10(4), 357–371 (2011).
Acknowledgements
The authors would like to thank all participating patients and the staff of the Department of Neurology from the Medical University of Innsbruck. Furthermore, we want to thank Angus Byars, Paris Sidiropoulos, Sherman Jia, Shervin Gholizadeh, Ivana Vodopivec, Jillian Smith and Veronica Anania from F. Hoffmann-La Roche Ltd and Genentech Inc. for supporting this research and providing valuable expertise.
Funding
This study was supported by VASCage – Centre on Clinical Stroke Research (grant number: 898252). VASCage is a COMET Centre within the Competence Centers for Excellent Technologies (COMET) program and funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology, the Federal Ministry of Labor and Economy, and the federal states of Tyrol, Salzburg and Vienna. COMET is managed by the Austrian Research Promotion Agency (Österreichische Forschungsförderungsgesellschaft) in a tripartite contract with Medical University Innsbruck and Roche Austria GmbH. The ReSect study was funded by the Austrian Science Fund (#P 29514-N32).
Author information
Authors and Affiliations
Contributions
MR designed the study. MR supervised the work, analysed and interpreted data and participated in the preparation of the manuscript. AB analysed and interpreted the data, wrote the manuscript, and performed with DR all experiments. CB, LM, MK and SK recruited study participants, collected the clinical data and blood samples, and gave valuable input to improve this manuscript. All authors reviewed the manuscript critically for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that this study received funding from Roche Austria GmbH. The funder was involved in the study design and critical revision of the article for important intellectual content. AB was an employee of VASCage and has participated in meetings sponsored by or received travel funding from Novartis, Sanofi-Genzyme, Merck, Almirall, and Biogen. DR was an employee of VASCage. MK was an employee of VASCage and has received speaker’s honoraria, advisory honoraria and/or travel grants from Böhringer-Ingelheim, Sanofi, Daiichi-Sankyo, Novartis and Pfizer. SK is CEO of VASCage – Research Centre on Clinical Stroke Research. VASCage is a COMET Centre within the Competence Centers for Excellent Technologies (COMET) programme and funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology the Federal Ministry of Labour and Economy, and the federal states of Tyrol, Salzburg and Vienna. COMET is managed by the Austrian Research Promotion Agency (Österreichische Forschungsförderungsgesellschaft). FFG project number: 898252. MR has received a research support from Roche Austria. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bauer, A., Boehme, C., Mayer-Suess, L. et al. Peripheral inflammatory response in people after acute ischaemic stroke and isolated spontaneous cervical artery dissection. Sci Rep 14, 12063 (2024). https://doi.org/10.1038/s41598-024-62557-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62557-3
- Springer Nature Limited