Abstract
Membrane-associated mucins (MAMs) are proposed to play critical roles at the ocular surface; however, in vivo evidence has been lacking. Here we investigate these roles by phenotyping of a Muc4 KO mouse. Histochemical analysis for expression of the beta-galactosidase transgene replacing Muc4 revealed a spiraling ribbon pattern across the corneal epithelium, consistent with centripetal cell migration from the limbus. Depletion of Muc4 compromised transcellular barrier function, as evidenced by an increase in rose bengal staining. In addition, the corneal surface was less smooth, consistent with disruption of tear film stability. While surface cells presented with well-developed microprojections, an increase in the number of cells with fewer microprojections was observed. Moreover, an increase in skin-type keratin K10 and a decrease in transcription factor Pax6 was observed, suggesting an incipient transdifferentiation. Despite this, no evidence of inflammatory dry eye disease was apparent. In addition, Muc4 had no effect on signaling by toll-like receptor Tlr4, unlike reports for MUC1 and MUC16. Results of this study provide the first in vivo evidence for the role of MAMs in transcellular barrier function, tear film stability, apical epithelial cell architecture, and epithelial mucosal differentiation at the ocular surface.
Similar content being viewed by others
Introduction
The wet ocular surface comprises the corneal and conjunctival epithelia, and their adnexa, as well as the overlying tear film that maintains their wetness1. Wet epithelial surfaces throughout the body are protected by a layer of mucus2. This complex biological substance is critical for maintaining tissue hydration. The physicochemical properties of mucus are mainly determined by the presence of mucins, large glycoproteins that contain numerous segments of serine and threonine-rich tandem repeats of amino acids. These residues serve as sites for O-glycosylation; the resulting long, branched O-glycan chains provide mucins with water-holding properties3,4,5.
Mucins can be secreted or membrane-associated. The secreted mucins, which are produced by specialized goblet cells, can assemble into extremely large oligomeric gels via disulfide bonds3. In this form, they create a viscous mucus layer over the epithelia of the tracheobronchial, gastrointestinal, and reproductive tracts. However, at the ocular surface, they assemble into a muco-aqueous gel which imparts transparency and fluidity to the tear film6,7. This watery gel is surfaced by a layer of lipid, protecting against evaporation8. Membrane-associated mucins (MAMs) integrate into the plasma membrane and project their extracellular domains out from the apical surface of corneal and conjunctival epithelia. In this way, they form that major component of the glycocalyx that comprises the deepest tear film compartment6,9. In addition, their ectodomains (EDs) can be shed into the muco-aqueous gel of the tear film by specific cleavage near the transmembrane domain5,10.
Evidence from biophysical modeling and cell culture studies suggests that both secreted mucins and MAMs contribute to tear film stability and spreading by providing shear thinning properties to tears, reducing friction during blinks, and enhancing corneal wettability wettability7,11,12. Instability of the tear film results in dry eye, a common affliction that affects 5% to 34% of people globally13. Numerous observational studies have reported that secreted mucins, and MAMs are quantitatively or qualitatively deficient in this disease; however, their contribution to dry eye pathology remains poorly defined14,15,16.
The heavily glycosylated EDs of some MAMs are exceptionally long17,18,19. The longest in humans is MUC16 at 14,517 amino acids in length; the second longest is MUC4 at 7418 amino acids in length. In contrast, MUC1 is only 481 amino acids in length17,18. MUC4 and MUC16 are the MAMs with very long EDs expressed at the ocular surface17,18. Because of the large number of O-glycans on MAMs with very long EDs, they have been hypothesized to play a role in transcellular barrier function. Indeed, MUC16 knockdown in a cell culture model demonstrated a decrease in transcellular barrier function20, while knockdown of MUC1, a short ED MAM, did not21. Likewise, the knockdown of MUC16, but not MUC1, disrupted the actin cytoskeleton associated with tight junctions and reduced plasma membrane microprojections21. These findings suggest that MAMs with very long EDs have specialized roles that MAMs do not serve with short EDs22.
While MAM properties conferred by the O-glycan chains have received much attention, it is increasingly appreciated that MAMs also serve as cell surface receptors that sense the extracellular environment and transduce signals intracellularly. The binding of signaling proteins and phosphorylation occurs at sites in both the EDs and the cytoplasmic tails (CTs)17,18,23,24. Toll-like receptors sense danger signals and pathogen-associated molecular patterns intrinsic to microorganisms and initiate an innate immune response25. MUC1 was shown to dampen the inflammatory response after TLR5 activation by blocking its binding to MyD8826. This finding was confirmed and extended in a human corneal epithelial cell culture model, where it was found that knockdown of either MUC1 or MUC16 dampened expression of the proinflammatory cytokines TNFA, IL6 and IL8 in response to ligand-activated TLR527.
Transgenic knockout (KO) mouse lines have provided useful models for ocular surface disease28,29,30 and have made it possible to evaluate roles for specific genes (e.g.,31). There are currently three published studies on the ocular surface phenotype of MAM KO mice, two of which utilized the Muc1 KO mouse. Increased susceptibility to infection was noted in the first study32; however, the second study (which used a different genetic background) found no evidence of this or any other phenotype33. This was not due to the masking of the phenotype by compensatory upregulation of other mucin genes33.
The third study examined the phenotype of the Muc16 KO mouse34. Upregulation of inflammatory signaling and features of an ongoing repair process was observed in the ocular surface epithelia of KO mice; however, staining with the clinical dye fluorescein, which is used to measure superficial punctate keratopathy in dry eye, was unchanged. Rose bengal exclusion was not evaluated. No change in the architecture of cell surface microplicae was observed34.
Like humans, mice express both Muc4 and Muc16 at the ocular surface but with somewhat different localization patterns. Muc4 appears to substitute for Muc16 in the mouse corneal epithelium, suggesting that the Muc4 KO mouse might be more revealing of roles proposed for MAMs with very long EDs. In the present study, we investigated the role of Muc4 at the ocular surface using a Muc4 KO mouse recently created in one of our labs35.
Results
Histochemistry, gross analysis and histology
We began our investigation of an ocular surface phenotype for the Muc4 KO mouse using histochemical, gross analysis, and histological methods. Representative results are shown in Fig. 1.
Histologic analysis of Muc4 KO corneas. (A) X-gal staining on whole mount corneas showing activation of the Muc4 promoter along the corneal surface. (B, C) Representative H&E-stained cross-sectional images from WT and Muc4 KO mouse eyes showing (B) conjunctiva; scale bar = 200 µM and (C) cornea; scale bar = 100 µM; N = 5.
The Muc4 targeting approach for the mice used in this study utilized a knock-in strategy, inserting a bacterial beta-galactosidase (LacZ) transgene in the endogenous Muc4 locus, placing it under the control of the Muc4 promoter. Histochemical analysis for LacZ activity can then be used to confirm the disruption of the endogenous Muc4 gene in cells where Muc4 would normally be expressed. In the original study describing these mice, the expected beta-galactosidase activity was observed in the colon and testes, where Muc4 is expressed, while no activity was observed in the pancreas, where Muc4 is not expressed35. We performed a similar histochemical analysis of the corneal surface, comparing Muc4−/− and WT littermate mice (Fig. 1A). Blue staining indicating beta-galactosidase activity was clearly present in epithelial cells at the corneal surface of Muc4−/− mice, but absent in WT mice. The beta-galactosidase activity was observed throughout the corneal epithelium, consistent with previous qPCR expression studies33,36. However, a new finding was made possible because the 2D coronal view showed that expression was not uniform across the ocular surface. A spiraling pattern was observed, consistent with the known centripetal migration of epithelial cells from the limbus at the corneal periphery to the central cornea37. Staining was ribbon-like, with darker and lighter areas, suggesting that the level of Muc4 expression beginning around the circumference of the limbus must vary. Staining presented with increasing intensity from the periphery towards the central cornea, which was not previously reported.
The previous study from one of our labs describing development of the Muc4−/− mice found that they are viable and fertile with no obvious anatomical defects35. In an examination of the ocular surface of the Muc4−/− mouse eye via stereomicroscopy, we also found no apparent abnormalities of the corneal epithelium, conjunctival epithelia or eyelids, and no evidence of conjunctivitis or blepharitis. Hematoxylin and eosin staining of cross-sections through conjunctiva (Fig. 1B) and cornea (Fig. 1C) of Muc4−/− and WT mice also revealed no evidence of inflammation, and there were no apparent morphological differences. The conjunctival epithelium of Muc4−/− mice appeared normal and goblet cell density was similar in both WT and Muc4−/− mice (Fig. 1B). The corneal epithelium of Muc4−/− mice was intact, and the number of cell layers was the same as WT littermates (Fig. 1C). The corneal stroma of Muc4−/− mice had a typical pattern of collagen lamellae with interspersed cells, similar to their WT littermates (Fig. 1C).
Clinical staining and evaluation of smoothness
We next evaluated the ocular surface using non-invasive clinical tests. Representative results are shown in Fig. 2. First, we used fluorescein staining, which primarily measures superficial punctate keratopathy38, i.e., damage to individual epithelial cells and the tight junctions between them39,40. No significant difference in fluorescein staining was observed in Muc4−/− mice as compared to WT littermates (Fig. 2A).
Next, we used rose bengal staining, which distinguishes the disruption of the mucosal glycocalyx in cultured cells20. In contrast to fluorescein staining, rose bengal staining was significantly elevated in Muc4−/− mice, with a punctate pattern indicative of individual cells and cell cluster staining (Fig. 2B). Finally, we evaluated the smoothness of the corneal surface. This method has been used to evaluate tear film fluidity41. To this end, we examined the reflection of a ring of light on the cornea under a stereo microscope. Corneal smoothness was more frequently disrupted in Muc4−/− mice corneas as compared to their WT littermates (Fig. 2C).
Scanning electron microscopy and morphometric analysis
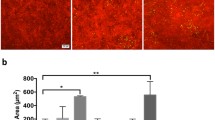
We used scanning electron microscopy (SEM) to compare the surface architecture of Muc4−/− and WT mouse eyes. Representative results are shown in Fig. 3. Much as in humans21, the apical cell layer of the mouse corneal epithelium presents plasma membrane microprojections42, as we show in the cross-sectional drawing (Fig. 3A). When viewed at high magnification, microprojections of normal appearance were found in both WT and Muc4−/− mice, although with a variation in density apparent on individual cells (Fig. 3B). An image only from a Muc4−/− mice is shown here, since WT mice looked identical. However, when the ocular surface was viewed at a lower magnification, it became apparent that there were more darker cells with lower microprojection density in Muc4−/− mice (Fig. 3C). Examples can be found in Fig. 3D, with bright cells showing high density (Fig. 3D, black asterisk), grey cells showing reduced density (Fig. 3D, arrow) and dark cells being completely smooth (Fig. 3D, white asterisks).
Ultrastructure analysis with scanning electron microscopy. (A) Schematic depicting the situation of membrane modifications in apical corneal epithelial cells. (B) Detail of the surface of three apical epithelial cells at high magnification, showing normally formed microplicae. This image was from a Muc4 KO cornea, but WT corneas appeared identical. (C) Representative, low magnification images of the whole corneal surface in WT and Muc4 KO mice. Detail of representative areas, evidencing the higher presence of darker cells in Muc4 KO mice. (D) High magnification image of the Muc4 KO mouse ocular surface showing the three different types of cells observed considering microplicae density: high microplicae density (black asterisk), reduced microplicae (arrow) and no microplicae (white asterisk). The ocular surface of WT mice looked similar. (E) Quantification of the number of “no microplicae” and “reduced microplicae” cells per field in WT and Muc4 KO mice. The data are presented as mean ± standard deviation. N = 4; *P < 0.05.
We did a morphometric analysis to compare the number of bright, gray, and dark cells (smooth cells) per 1000 × field in Muc4−/− and WT mice. While we observed a trend toward an increased number of smooth cells in the KO mice, this difference was not statistically significant. However, we found a significant increase in the number of cells with a low density of microplicae (gray cells) in Muc4−/− corneas (Fig. 3E).
Cornification, inflammatory, and transdifferentiation markers
Dry eye disease is characterized by upregulation of pro-inflammatory cytokines and markers of cornification41. In severe dry eye disease subtypes like Stevens-Johnson syndrome, ocular cicatricial pemphigoid, and Sjögren’s syndrome, the wet mucosal epithelium can transdifferentiate to a keratinized epidermal-type phenotype43,44. We compared the expression of pro-inflammatory, cornification and epidermal transdifferentiation markers in Muc4−/− mice to WT littermates by qPCR. Representative results are shown in Fig. 4. We observed no significant difference in the expression of genes encoding cytokines Tnfa and Il1a between the two groups. Expression of Il1b was significantly decreased in the cornea of Muc4−/− mice as compared to WT littermates. Consistent with this, we observed no change in the expression of the cornified Sprr2h, and Sprr1b was undetectable in both Muc4−/− and WT mice. Similarly, the cornea-specific keratin Krt12 expression was unchanged in Muc4−/− mice compared to WT littermates. Interestingly however, expression of Krt10, an epidermal keratin, was significantly increased, while Pax6, a transcription factor that regulates corneal epithelial differentiation, was significantly decreased in both the cornea and conjunctiva of Muc4−/− mice.
Gene expression analysis of the Muc4 KO mouse ocular surface. Expression of mucin genes, epithelial differentiation markers and inflammatory markers in corneal epithelium and whole conjunctivas from WT and Muc4 KO mice. Relative gene expression was calculated with the 2 − ΔΔCt method, using the levels of Rpl9 expression as housekeeping and the expression in WT tissue as the calibrator. The data are presented as mean ± standard deviation. N = 6; *P < 0.05; **P < 0.01.
One of our labs previously showed that mice lacking Muc4 upregulate other mucin genes in the colon epithelium when challenged with dextran sodium sulfate35. Since this can compensate for the effects of Muc4 knockout, altering the phenotype, we investigated whether it also occurs at the ocular surface. However, qPCR analysis revealed no change in Muc1 expression in the cornea or conjunctiva of Muc4−/− mice compared to WT littermates (Fig. 4). Similarly, Muc16 and Muc5ac expression levels were unchanged in the conjunctiva and remained undetectable in the cornea.
Challenge with lipopolysaccaride (LPS)
We tested the ability of Muc4 to interfere with TLR signaling by removing eyes from Muc4−/− and WT mice to organ culture and exposing to LPS, an agonist of Tlr4. Representative results are shown in Fig. 5. No difference in Tlr4 expression was found in the corneal epithelial cells of unchallenged Muc4−/− mice compared to WT littermates. Exposure to 1 µg/ml LPS for 4 h significantly increased the expression of Tnfa in the corneal epithelium in both Muc4−/− and WT mice. There were no significant differences in the Tnfa expression increase between the LPS-treated Muc4−/− and WT eyes.
WT and Muc4 KO eyes ex vivo exposure to LPS. WT and Muc4 KO eyes were exposed to 1 μg/ml LPS for 4 h at 37 °C. Relative gene expression of Tlr4 and Tnfa in the corneal epithelium was calculated with the 2 − ΔΔCt method, using the levels of Rpl9 expression as housekeeping and the expression in untreated-WT tissue as the calibrator. The data are presented as mean ± standard deviation. N = 9; **P < 0.01; ***P < 0.001.
Discussion
Based on biophysical, cell/organ culture, and observational evidence, MAMs have been proposed to play critical roles at the ocular surface in tear film stability7,11, transcellular barrier function20,21, apical epithelial cell architecture21, dry eye pathology14 and dampening of the immune response27. Here we investigated these hypotheses by evaluating the phenotype of transgenic Muc4 KO mice. Loss of Muc4 at the ocular surface compromised transcellular barrier function, and we also found evidence of tear film disruption. In addition, loss of Muc4 altered the architecture of the apical epithelial cell layer, as evidenced by an increase in cells with fewer microplicae. We also found evidence of an incipient transdifferentiation of the corneal epithelium to an epidermal phenotype. These results provide the first in vivo evidence supporting several of the long-standing hypotheses cited above. However, our findings did not support observational studies linking loss of MAMs to dry eye pathology. Moreover, the loss of Muc4 did not dampen the immune response mediated by tlr5.
The targeting approach for the Muc4 KO mice used in this study employed a knock-in strategy by insertion of a bacterial beta-galactosidase (LacZ) transgene in the endogenous Muc4 locus, placing it under the control of the Muc4 promoter35. Histochemical analysis of Muc4 promoter activity revealed a spiraling ribbon pattern, consistent with the known centripetal migration of epithelial cells to the central cornea from the limbus. This expression pattern is similar to that shown for genes that regulate cell fate in the cornea (e.g.,45). While in situ hybridization has been used to visualize MAMs expression at the ocular surface, and results of this study are consistent with previous findings for Muc4 expression33,36, this is the first time to our knowledge, that MAM gene expression has been visualized in a 2D coronal view, providing new information.
It has been hypothesized that MAMs with very long EDs have specialized roles that MAMs do not serve with short EDs22. The clustering of O-linked oligosaccharide chains within their tandem repeats creates steric interactions between carbohydrates and peptides, inducing the peptide core to adopt a stiff and extended conformation. This results in projection well above the cell surface, far beyond other membrane-associated proteins46. The extracellular domain of human MUC4 is predicted to extend > 2 um above the cell surface in the apical region47. Thus, MAMs with very long EDs are positioned to shield and protect the cell surface and also create a transcellular barrier20,21.
MUC4/Muc4 and MUC16/Muc16 are the MAMs with very long EDs expressed at the ocular surface of humans and mice17,18. In humans, MUC16 mRNA has been reported to be expressed evenly across the corneal and conjunctival epithelia48,49. However, in mice, Muc16 expression has been identified only in the conjunctival epithelium34. MUC4 mRNA is most abundant in the human conjunctiva, with an attenuation in expression from the corneal periphery to the central corneal50,51. Muc4 is expressed in both mouse conjunctiva and cornea36. Thus, Muc4 appears to substitute for MUC16 in the mouse corneal epithelium.
MAM transcellular barrier function is thought to be dependent on a very long and heavily glycosylated ED, which excludes small molecules20. Knockdown of MUC16 in a human cell culture model resulted in transcellular barrier disruption, as evidenced by increased rose bengal penetrance20,21. In contrast, knockdown of MUC1, a short ED MAM, decreased rose bengal penetrance, perhaps because its interspersion with MUC16 creates spaces in the barrier21. Our finding of transcellular barrier disruption in Muc4 KO mice provides the first in vivo support for this MAM role.
While Muc4 may substitute for Muc16 for some functions, this may not always be the case. Thus while both MUC4/Muc4 and MUC16/Muc16 share the feature of very long EDs, and both have cleavage sites for shedding into the tear film, the overall modular structures of their EDs are quite different17. For example, the ED of MUC4/Muc4 has three EGF-like motifs located distal to the cleavage site, which is not present in MUC16/Muc1652. Rat Muc4 was shown to interact with EGFR family member ERBB2 via the EGF-like motif closest to its transmembrane domain, resulting in phosphorylation and downstream signaling53. The EGF-like motifs are not found in the MUC16/Muc16 ED. Similarly, while both CTs are short, the few identified motifs affecting intracellular signaling differ in the CTs of the two MAMs17. Thus, Muc4 substitution for MUC16 in the corneal epithelium of mice may have functional significance in some cases.
Scanning electron microscopy of the mammalian corneal surface has revealed a contiguous mosaic of polygonal cell shapes with a range of sizes, each having a light, medium, or dark appearance42,54,55. Lighter cells have a greater density of microprojections, to which MAMs localize, while the darkest cells are entirely smooth. These differences are thought to reflect cell maturation that starts when a cell reaches the ocular surface and ends when it is desquamated42,54,55. It has been proposed that cells with more microprojections are younger cells, which gradually flatten as they mature36,51,52. When viewed by transmission electron microscopy (TEM), the shades are reversed, with the cytoplasm of light cells being electron dense, consistent with a greater metabolic and synthetic activity, while dark cells appear to have reduced metabolic activity, consistent with the idea that they are more mature42. Here, we observed the typical pattern of light, medium and dark cells at the corneal surface of both WT and Muc4 KO mice. As in the Muc16 KO mouse, well-developed microprojections were apparent in both genotypes34. However, morphometric analysis revealed that loss of Muc4 results in more cells with reduced microprojection density. The shift to more cells with reduced microprojection density was not observed in the Muc16 KO mouse34, but since Muc16 is primarily localized to the conjunctival epithelium in mice17, an effect on the microprojections would be precluded. Interestingly, knockdown of MUC16 in a human corneal epithelial cell culture model resulted larger, more spread cells with reduced cell surface microprojections21. Pull-down experiments suggested that a polybasic amino acid stretch at the proximal end of the MUC16 CT interacts with ezrin/radixin/moesin (ERM) family actin-binding proteins20, a family known to contribute to the formation of microprojections56,57. However, MUC4/Muc4 CT lacks the ERM actin-binding motif17. Moreover, our observation was not a loss of microprojections overall, but a shift towards more cells with reduced microprojections, a somewhat different effect that suggests a different mechanism.
It seems possible that the shift towards more cells with reduced microprojections could be related to shear stress. Corneal epithelial cells are constantly exposed to shear stress due to blinking. The apical surface of differentiated human corneal epithelial cells expressing MUC16 was shown to be more antiadhesive than undifferentiated cells lacking MUC16 and abrogation of mucin O-glycosylation in differentiated cultures resulted in increased adhesion58. Thus a reduction in Muc4 could reduce the lubrication and increase the shear stress caused by normal blinking. Under flow-induced shear stress, cells were larger and more spread as compared to static monolayer controls59. MAMs and the microprojections to which they localize are thought to help stabilize the tear film1. Thus the loss of Muc4 and its effect on the overall density of microprojections across the ocular surface is consistent with our observation of reduced tear film stability in Muc4 KO mice.
While expression of the corneal epithelial keratin marker K12 was unchanged in this study, we found an increase in the epidermal-type keratin K10 and a decrease in the eye-specific transcription factor Pax6. This apparent incipient keratinization in mice lacking Muc4 may be caused by the resulting reduced tear film stability and increased shear stress. K10 increase is one of the first steps in epidermal cornification: the keratins K1 and K10 form scaffolds where the cornifins will bind to form the cornified envelope60. Keratinization and cornification are hallmarks of squamous metaplasia that occurs at the ocular surface due to the desiccation and inflammation of dry eye43,61,62. Numerous observational studies have reported that MAMs are quantitatively or qualitatively deficient in dry eye disease14,15,16 and severe dry eye can result in transdifferentiation of mucosal epithelial cells to a skin phenotype63. Interestingly, we observed no other hallmarks of dry eye, including epithelial cell damage as visualized by fluorescein staining, goblet cell loss, or increased expression of inflammatory cytokine and cornification markers. This suggests the intriguing hypothesis that Muc4 is required to maintain mucosal epithelial differentiation, over and above any role in inflammatory diseases such as dry eye.
Not only did we observe no increase in expression of genes encoding inflammatory cytokines in Muc4 KO mice as compared to WT littermates, expression of the gene encoding the inflammatory cytokine Il1b was reduced. This is consistent with previous findings from one of our labs using the dextran sodium sulfate (DSS)-induced colitis model, in which Muc4 KO mice displayed reduced infiltration of inflammatory cells along with a reduction in mRNA encoding inflammatory cytokines in the inflamed colon mucosa as compared with WT littermates35. Compensatory upregulation of Muc2 and Muc3 under basal and DSS treatment conditions partly factored into this phenotype. Significantly, we did not observe compensatory upregulation of secreted mucin genes or MAMs at the ocular surface in the current study. Increased inflammation was reported at the ocular surface of the Muc16 KO mice, however, as in this study, no other signs of dry eye disease were observed34.
A limitation of the current study is that we examined only very young mice. It is intriguing also that inflammation was not seen in the KO mouse at baseline given that there appear to be epithelial defects. Perhaps dry eye disease might take more time to develop in MAM KO mice and examination of older mice might reveal disease signs. Testing these mice in a desiccating environment as well as their response to corneal debridement or wounding might also reveal fundamental roles for Muc4 in corneal health. Additional markers of keratinization and dry eye, evaluated not only by qPCR markers, but also by immunoblotting, ELISA and immunohistochemistry would be needed to thoroughly test the mucosal maintenance hypothesis.
MUC1/Muc1 and MUC16/Muc16 are known to inhibit the TLR response to challenge27,64. However, we found no difference in response to challenge with the Tlr4 agonist LPS in Muc4 KO mice or WT littermates. The differential contributions of the different MAMs to TLR activity and general inflammation suggest that changes in the proportions of these mucins can lead to very different responses to noxious stimuli and even allergens, some known to activate TLRs in ocular epithelial cells.
In conclusion, the results of this study provide the first in vivo evidence for several proposed MAM roles at the ocular surface. First, it is demonstrated that loss of Muc4 compromises transcellular barrier function. Determining the basic mechanisms that create and sustain the mucin transcellular barrier is relevant not only for addressing the negative clinical consequences of its alteration but also for improving drug delivery, as mucins are a significant impediment to the delivery of topical drugs in the eye65,66. Second, our results support the findings of biophysical studies on the requirement of MAMs for tear film stability. Third, we report effects of Muc4 loss on apical epithelial cell architecture, which may be due to the anti-adhesive role of MAMs, previously proposed based on in vitro evidence. Fourth, we obtain evidence that loss of Muc4 results in incipient keratinization, suggesting the hypothesis that Muc4 is needed to maintain mucosal differentiation at the ocular surface. Surprisingly, we found no evidence that this is accompanied by other signs of dry eye, challenging the generally accepted paradigm. Follow-up work is necessary to fully test the tentative conclusions of this fourth set of findings. Finally, we show that not every MAM can suppress the immune response through toll-like receptors.
Materials and methods
Animals
All animal experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and to the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The study was in compliance with ARRIVE guidelines. The breeding and animal procedures were approved by the IACUC of Tufts University.
This study made use of Muc4 KO mice, previously generated by targeted disruption in the Batra laboratory at the University of Nebraska Medical Center (Omaha, NE)35. Homozygous Muc4−/− mice were shown to be viable and fertile, with no apparent defects. qPCR using primers to the 3ʹ region of the transcript confirmed the lack of Muc4 expression in normal colon and lungs of Muc4−/− mice, comparing to Muc4+/+ mice that express this gene (positive control), and the lack of pancreatic expression in both Muc4−/− and Muc4+/+ mice (negative control).
Heterozygous Muc4+/− males on the C57Bl/6 background were imported to Tufts Medical Center (Boston, MA), then back-crossed with C57Bl/6 females to generate sufficient heterozygotes to expand the colony. Heterozygotes were then crossed to generate sufficient homozygous KO mice (Muc4−/−) and WT littermates (Muc4+/+) for experiments. Mice were housed on a 12-h light–dark cycle with food and water available ad libitum. Eight- to twelve-week-old mice, with an approximately equal male/female proportion, were used for all experiments.
Histochemistry
For histochemical localization of beta-galactosidase activity, a kit was utilized following the manufacturer's instructions (Abcam, Cambridge, UK). The whole eyes were carefully collected and immediately placed in Fixative Solution for one hour at room temperature. Then, the corneas were gently separated from the rest of the eye and washed twice in PBS before being incubated in freshly prepared Staining Solution overnight at 37 °C. After the overnight incubation, the corneas were thoroughly washed in PBS, mounted, and observed under a microscope.
Histology
Wild type and homozygous Muc4 KO mice (5 per group) were euthanized and the eyes with eyelids were collected and fixed in 4% paraformaldehyde for 4 h at room temperature. The fixed eyes were then cryoprotected in 30% sucrose and frozen in OCT®. Sections of 10 μm thickness were obtained using a cryostat. Hematoxylin & eosin staining (Vector) was performed on the sections and evaluated under a microscope.
Clinical staining
For the staining of the ocular surface, mice were anesthetized using an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). The eyes were then observed and photographed using a Phoenix Micron IV with a slit-lamp attachment (Phoenix Research Laboratories, Pleasanton, CA) using white and cobalt blue light as needed. For fluorescein staining, a single drop of 0.35% fluorescein was carefully instilled onto the ocular surface and, after 2 min, the excess of dye was washed with phosphate-buffered saline (PBS). Similarly, for rose bengal staining, one drop of 1% rose bengal was applied and allowed to stand for 30 s before being washed away with PBS. A modified van Bijserveld scoring system was used to assess the degree of staining. The total score went from 0 to 9, with the cornea divided in 3 areas, and each area scored from 0 to 3, with 0 indicating no staining, 1 indicating sparsely scattered staining, 2 indicating densely scattered or scattered plaques, and 3 indicating confluent or diffuse staining or diffuse plaques.
Evaluation of corneal smoothness
To evaluate the smoothness of the eyes, we used a previously described method that involved examining the reflection of a white ring under stereomicroscopy41. The eyes were imaged immediately after euthanasia to minimize the effects of post-mortem changes. The reflection of the ring was scored on a scale of 0 to 5, with a score of 0 indicating no distortion and a score of 5 indicating complete distortion or loss of the ring reflection. Scores of 1, 2, 3, and 4 represented increasing levels of distortion in one quarter, half, three-quarters, and all quarters of the ring, respectively. Smoothness was scored considering the alteration of a ring light reflection, as has been described before41. Immediately after euthanasia, images of the eyes reflecting the light of a white ring of a stereomicroscope were taken. The images were scored from 0 to 5, with the scoring meaning (0) no distortion of the ring, (1) distortion in one quarter of the ring, (2) distortion in half of the ring, (3) distortion in three quarters, (4) distortion in the four quarters and (5) when no ring could be recognized.
Scanning electron microscopy
Eyes from four Muc4−/− mice and four Muc4+/+ littermates were collected and fixed in 1/2 strength Karnovsky fixative, dehydrated through ethanol series, and then subjected to critical point drying with a SamDri-795 critical point dryer (Tousimis Research Corporation, Rockville, MD). The dried eyes were coated with chromium using an Ion Beam Coater 610 (Gatan Corp. Pleasanton, CA). Consequently, eyes were observed and photographed under a scanning electron microscope. For analysis, five different fields were randomly selected from each cornea and photographed at 1000 × magnification. The number of cells per field with no microplicae or reduced microplicae were counted.
RNA isolation and quantitative polymerase chain reaction (qPCR)
The conjunctiva was collected from both eyes using forceps and micro-scissors. The cornea was removed to collect the epithelium by scrapping it with a blade after incubating it in 20 mM EDTA for 10 min.
RNA was isolated using the GeneJET RNA Purification Kit (Thermo Fisher Scientific) following the manufacturer's instructions. The corneal epithelia were homogenized by 15 s of vortexing, while conjunctivas were passed through a syringe attached to a 20-G needle. To remove DNA contamination, PureLink® DNase Set (Invitrogen, Carlsbad, CA) was used on columns. First-strand cDNA was synthesized using the High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA), following the manufacturer's instructions.
The RT-qPCR reaction was carried out using SYBR® Green reagent (iTaq Universal SYBR Green Supermix, Bio-Rad, Hercules, CA) with specific primers (Table 1). The following parameters were used: 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C and 30 s at 60 °C. All samples were normalized to RNA levels of Rpl9 gene as the housekeeping (Table 1). The comparative CT method was used for relative quantitation, selecting the relative amount in WT mice as the calibrator.
LPS exposure
Eyes of Muc4+/+ and Muc4−/− mice were enucleated and incubated in keratinocyte serum-free medium (Gibco-Thermo Fisher Scientific, Waltham, Massachusetts, USA) containing 1 μg/ml LPS (Sigma-Aldrich, St Louis, MO, USA) for 4 h at 37 °C. The corneal epithelia were then collected as explained above and RNA was extracted. The relative gene expression of Tlr4 and Tnfa in the corneal epithelium was calculated by RT-qPCR, using Rpl9 as the housekeeping gene for comparison and WT-untreated eyes as the calibrator.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Student t-test or Mann–Whitney U test were used attending to normality of the data dis-tribution as determined by using the D’Agostino & Pearson normality test. Analysis of Variance (ANOVA) with Bonferroni's post-hoc test was applied for comparison of multiple samples. P value < 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Gipson, I. K. The ocular surface: The challenge to enable and protect vision: the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 48(4390), 4391–4398. https://doi.org/10.1167/iovs.07-0770 (2007).
McShane, A. et al. Mucus. Curr. Biol. 31, R938–R945. https://doi.org/10.1016/j.cub.2021.06.093 (2021).
Hollingsworth, M. A. & Swanson, B. J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60. https://doi.org/10.1038/nrc1251 (2004).
Cheng, P. W. & Radhakrishnan, P. Mucin O-glycan branching enzymes: Structure, function, and gene regulation. Adv. Exp. Med. Biol. 705, 465–492. https://doi.org/10.1007/978-1-4419-7877-6_25 (2011).
Argueso, P. & Gipson, I. K. Epithelial mucins of the ocular surface: Structure, biosynthesis and function. Exp. Eye Res. 73, 281–289. https://doi.org/10.1006/exer.2001.1045 (2001).
Willcox, M. D. P. et al. TFOS DEWS II tear film report. Ocul. Surf. 15, 366–403. https://doi.org/10.1016/j.jtos.2017.03.006 (2017).
Georgiev, G. A., Eftimov, P. & Yokoi, N. Contribution of mucins towards the physical properties of the tear film: A modern update. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20246132 (2019).
Argueso, P. Human ocular mucins: The endowed guardians of sight. Adv. Drug Deliv. Rev. 180, 114074. https://doi.org/10.1016/j.addr.2021.114074 (2022).
Dilly, P. N. Structure and function of the tear film. Adv. Exp. Med. Biol. 350, 239–247. https://doi.org/10.1007/978-1-4615-2417-5_41 (1994).
Govindarajan, B. et al. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS ONE 7, e32418. https://doi.org/10.1371/journal.pone.0032418 (2012).
Cui, K. W., Myung, D. J. & Fuller, G. G. Tear film stability as a function of tunable mucin concentration attached to supported lipid bilayers. J. Phys. Chem. B 126, 6338–6344. https://doi.org/10.1021/acs.jpcb.2c04154 (2022).
Ablamowicz, A. F. & Nichols, J. J. Ocular surface membrane-associated mucins. Ocul. Surf. 14, 331–341. https://doi.org/10.1016/j.jtos.2016.03.003 (2016).
Stapleton, F. et al. TFOS DEWS II epidemiology report. Ocul. Surf. 15, 334–365. https://doi.org/10.1016/j.jtos.2017.05.003 (2017).
Martinez-Carrasco, R., Argueso, P. & Fini, M. E. Dynasore protects ocular surface mucosal epithelia subjected to oxidative stress by maintaining UPR and calcium homeostasis. Free Radic. Biol. Med. 160, 57–66. https://doi.org/10.1016/j.freeradbiomed.2020.07.002 (2020).
Baudouin, C. et al. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. https://doi.org/10.1016/j.preteyeres.2018.11.007 (2018).
Berry, M., Pult, H., Purslow, C. & Murphy, P. J. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom. Vis. Sci. 85, E930-938. https://doi.org/10.1097/OPX.0b013e318188896b (2008).
Fini, M. E. et al. Membrane-associated mucins of the ocular surface: New genes, new protein functions and new biological roles in human and mouse. Progress Retin. Eye Res. 75, 100777. https://doi.org/10.1016/j.preteyeres.2019.100777 (2020).
Martinez-Carrasco, R., Argueso, P. & Fini, M. E. Membrane-associated mucins of the human ocular surface in health and disease. Ocul. Surf. 21, 313–330. https://doi.org/10.1016/j.jtos.2021.03.003 (2021).
Hattrup, C. L. & Gendler, S. J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457. https://doi.org/10.1146/annurev.physiol.70.113006.100659 (2008).
Blalock, T. D. et al. Functions of MUC16 in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 48, 4509–4518. https://doi.org/10.1167/iovs.07-0430 (2007).
Gipson, I. K., Spurr-Michaud, S., Tisdale, A. & Menon, B. B. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS ONE 9, e100393. https://doi.org/10.1371/journal.pone.0100393 (2014).
Gipson, I. K. in International Conference on Eye Research, 21st Biennial Meeting, Hyatt Regency San Francisco at Embarcadero (2014).
Moniaux, N., Escande, F., Porchet, N., Aubert, J. P. & Batra, S. K. Structural organization and classification of the human mucin genes. Front. Biosci. 6, D1192-1206 (2001).
Govindarajan, B. & Gipson, I. K. Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res. 90, 655–663. https://doi.org/10.1016/j.exer.2010.02.014 (2010).
Basu, S. & Fenton, M. J. Toll-like receptors: Function and roles in lung disease. Am. J. Physiol. 286, L887-892. https://doi.org/10.1152/ajplung.00323.2003 (2004).
Kato, K. et al. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. J. Immunol. 188, 2014–2022. https://doi.org/10.4049/jimmunol.1102405 (2012).
Menon, B. B., Kaiser-Marko, C., Spurr-Michaud, S., Tisdale, A. S. & Gipson, I. K. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol. 8, 1000–1008. https://doi.org/10.1038/mi.2014.127 (2015).
Stern, M. E. & Pflugfelder, S. C. What we have learned from animal models of dry eye. Int. Ophthalmol. Clin. 57, 109–118. https://doi.org/10.1097/IIO.0000000000000169 (2017).
Zhu, J. et al. Application of animal models in interpreting dry eye disease. Front. Med. 9, 830592. https://doi.org/10.3389/fmed.2022.830592 (2022).
Qin, D. Y., Wang, L. X. & Deng, Y. P. Transgenic dry eye mouse models: Powerful tools to study dry eye disease. Int. J. Ophthalmol. 15, 635–645. https://doi.org/10.18240/ijo.2022.04.18 (2022).
Pflugfelder, S. C. et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am. J. Pathol. 166, 61–71. https://doi.org/10.1016/S0002-9440(10)62232-8 (2005).
Kardon, R. et al. Bacterial conjunctivitis in Muc1 null mice. Invest. Ophthalmol. Vis. Sci. 40, 1328–1335 (1999).
Danjo, Y., Hazlett, L. D. & Gipson, I. K. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Invest. Ophthalmol. Vis. Sci. 41, 4080–4084 (2000).
Shirai, K. et al. Effects of the loss of conjunctival Muc16 on corneal epithelium and stroma in mice. Invest. Ophthalmol. Vis. Sci. 55, 3626–3637. https://doi.org/10.1167/iovs.13-12955 (2014).
Das, S. et al. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene 35, 2645–2654. https://doi.org/10.1038/onc.2015.327 (2016).
Lange, C. et al. Mucin gene expression is not regulated by estrogen and/or progesterone in the ocular surface epithelia of mice. Exp. Eye Res. 77, 59–68 (2003).
Thoft, R. A. & Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest. Ophthalmol. Vis. Sci. 24, 1442–1443 (1983).
Bron, A. J., Argueso, P., Irkec, M. & Bright, F. V. Clinical staining of the ocular surface: Mechanisms and interpretations. Prog. Retin. Eye Res. 44, 36–61. https://doi.org/10.1016/j.preteyeres.2014.10.001 (2015).
Bandamwar, K. L., Papas, E. B. & Garrett, Q. Fluorescein staining and physiological state of corneal epithelial cells. Contact Lens Anterior Eye 37, 213–223. https://doi.org/10.1016/j.clae.2013.11.003 (2014).
Srinivas, S. P. et al. Corneal epithelial permeability to fluorescein in humans by a multi-drop method. PLoS ONE 13, e0198831. https://doi.org/10.1371/journal.pone.0198831 (2018).
De Paiva, C. S. et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest. Ophthalmol. Vis. Sci. 47, 2847–2856. https://doi.org/10.1167/iovs.05-1281 (2006).
Hazlett, L. D., Spann, B., Wells, P. & Berk, R. S. Desquamation of the corneal epithelium in the immature mouse: A scanning and transmission microscopy study. Exp. Eye Res. 31, 21–30 (1980).
Nakamura, T. et al. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest. Ophthalmol. Vis. Sci. 42, 549–556 (2001).
McNamara, N. A., Gallup, M. & Porco, T. C. Establishing PAX6 as a biomarker to detect early loss of ocular phenotype in human patients with Sjogren’s syndrome. Invest. Ophthalmol. Vis. Sci. 55, 7079–7084. https://doi.org/10.1167/iovs.14-14828 (2014).
Di Girolamo, N. et al. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells 33, 157–169. https://doi.org/10.1002/stem.1769 (2015).
Jentoft, N. Why are proteins O-glycosylated?. Trends Biochem. Sci. 15, 291–294 (1990).
Chaturvedi, P., Singh, A. P. & Batra, S. K. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 22, 966–981. https://doi.org/10.1096/fj.07-9673rev (2008).
Gipson, I. K. In situ hybridization techniques for localizing mucin mRNA. Methods Mol. Biol. 125, 323–336. https://doi.org/10.1385/1-59259-048-9:323 (2000).
Argueso, P., Spurr-Michaud, S., Russo, C. L., Tisdale, A. & Gipson, I. K. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest. Ophthalmol. Vis. Sci. 44, 2487–2495 (2003).
Inatomi, T. et al. Expression of secretory mucin genes by human conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 37, 1684–1692 (1996).
Pflugfelder, S. C. et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest. Ophthalmol. Vis. Sci. 41, 1316–1326 (2000).
Hanson, R. L. & Hollingsworth, M. A. Functional consequences of differential O-glycosylation of MUC1, MUC4, and MUC16 (downstream effects on signaling). Biomolecules https://doi.org/10.3390/biom6030034 (2016).
Jepson, S. et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene 21, 7524–7532. https://doi.org/10.1038/sj.onc.1205970 (2002).
Pfister, R. R. The normal surface of corneal epithelium: A scanning electron microscopic study. Invest. Ophthalmol. 12, 654–668 (1973).
Doughty, M. J. Quantitative analysis of ring-shaped (crater-like) features at the tear film-epithelial interface of the rabbit cornea as assessed by scanning electron microscopy. Curr. Eye Res. 31, 999–1010. https://doi.org/10.1080/02713680601001103 (2006).
Bonilha, V. L., Finnemann, S. C. & Rodriguez-Boulan, E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J. Cell Biol. 147, 1533–1548. https://doi.org/10.1083/jcb.147.7.1533 (1999).
Yonemura, S., Tsukita, S. & Tsukita, S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J. Cell Biol. 145, 1497–1509. https://doi.org/10.1083/jcb.145.7.1497 (1999).
Sumiyoshi, M. et al. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 49, 197–203. https://doi.org/10.1167/iovs.07-1038 (2008).
Hampel, U., Garreis, F., Burgemeister, F., Essel, N. & Paulsen, F. Effect of intermittent shear stress on corneal epithelial cells using an in vitro flow culture model. Ocul. Surf. 16, 341–351. https://doi.org/10.1016/j.jtos.2018.04.005 (2018).
Bragulla, H. H. & Homberger, D. G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 214, 516–559. https://doi.org/10.1111/j.1469-7580.2009.01066.x (2009).
Chen, Y. T. et al. Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol. Vis. 15, 563–576 (2009).
Li, W. et al. Air exposure induced squamous metaplasia of human limbal epithelium. Invest. Ophthalmol. Vis. Sci. 49, 154–162. https://doi.org/10.1167/iovs.07-0883 (2008).
Kinoshita, S., Nakamura, T. & Nishida, K. Pathological keratinization of ocular surface epithelium. Adv. Exp. Med. Biol. 506, 641–646. https://doi.org/10.1007/978-1-4615-0717-8_90 (2002).
Ueno, K. et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am. J. Respir. Cell Mol. Biol. 38, 263–268. https://doi.org/10.1165/rcmb.2007-0336RC (2008).
Popov, A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J. Ocul. Pharmacol. Ther. 36, 366–375. https://doi.org/10.1089/jop.2020.0022 (2020).
Leal, J., Smyth, H. D. C. & Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 532, 555–572. https://doi.org/10.1016/j.ijpharm.2017.09.018 (2017).
Acknowledgements
The authors gratefully acknowledge Stefanie Gavett, Jocelyn Munguia and Quinn Goble of the Tufts Comparative Medicine Services Rodent Breeding Service for management of the mouse breeding colony, and Philip Seifert for scanning electron microscopy assistance from the Morphology Core of the Schepens Eye Research Institute (Boston, MA). The authors acknowledge support from NIH Grants R01EY026479 (MEF), R01EY026147 (PA), P30EY003790 (PA), R01CA273319 (SKB), U01CA200466 (SKB) and P01CA217798 (SKB); the Massachusetts Lions Eye Research Fund (Tufts Medical Center) and a challenge grant from Research to Prevent Blindness (Tufts Medical Center).
Author information
Authors and Affiliations
Contributions
Participated in research design: R.M.-C., M.E.F., P.A. Conducted experiments: R.M.-C. Provision of mice, advice on breeding, colony management: S.R., S.K.B., R.M.-C. Performed data analysis: R.M.-C. Wrote or contributed to the writing of the manuscript: R.M.-C., M.E.F., P.A., S.K.B., S.R.
Corresponding author
Ethics declarations
Competing interests
Dr. Batra is a Co-founder of Sanguine Diagnostics and Therapeutics, Inc and all other authors declare that they do not have any competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinez-Carrasco, R., Rachagani, S., Batra, S.K. et al. Roles unveiled for membrane-associated mucins at the ocular surface using a Muc4 knockout mouse model. Sci Rep 13, 13558 (2023). https://doi.org/10.1038/s41598-023-40491-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40491-0
- Springer Nature Limited
This article is cited by
-
MUC4 O-GlcNAcylation Regulates the Epithelial Phenotype in Conjunctival Epithelial Cells
Biochemical Genetics (2024)