Abstract
In this cross-sectional study, we assessed the physical fitness levels of active community-dwelling older adults. Moreover, we investigated the correlation of their (stratified by age and sex) fitness levels with handgrip strength (HGS). Comprehensive physical fitness tests, including sarcopenia screening, were conducted with a total of 2,130 older adults residing in a rural area of Taiwan. The 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of age- and sex-specific physical fitness levels were determined. Furthermore, we identified the key parameters for assessing the physical fitness of older adults and performed stepwise multiple linear regression analysis. Both men and women exhibited age-related decreases in all aspects of functional fitness, a trend indicating that older adults in Taiwan may lose their independence in the future. Furthermore, the regression analysis revealed that HGS was positively correlated with sex, body mass index, and the results of 30-s arm curl and back scratch tests but negatively correlated with age and the result of 8-foot up-and-go test. Our reference values for physical fitness may help assess the fitness levels of active community-dwelling older adults and design community-based health programs to prevent the early loss of independence in community-dwelling older adults in Taiwan.
Similar content being viewed by others
Introduction
Community-dwelling older adults experience several health challenges, such as frailty, osteoporosis, sarcopenia, and various musculoskeletal ailments1,2,3. In a study including a total of 740 community-dwelling older adults, 7.4% of the participants had frailty and 49.7% had prefrailty1. In the aforementioned study, approximately 33.3% frail adults had deficits in their activities of daily living and approximately 80% had various comorbidities1. In Taiwan, frailty and prefrailty have been associated with recent falls, hip fractures, and osteoporosis in community-dwelling adults aged ≥ 50 years4. Moreover, patients with musculoskeletal disorders experience higher levels of muscular deconditioning than do healthy individuals5,6 and are less likely to meet the required physical activity standards. A 3-year-long prospective cohort study including community-dwelling older adults in Taiwan revealed that 11.0% of the participants exhibited the gradual impairment of their activities of daily living throughout the study period; age was found to be the most relevant predictive factor for chronic disability7. In addition to medical treatment, the importance of preventing and delaying disability cannot be overemphasized. In 2015, a program based on the National Ten-Year Long-Term Care Plan 2.0 was launched in Taiwan to prevent and delay disability in older adults. To maintain their fitness level, older adults can participate in various health promotion activities within their communities, such as exercise or dance programs at long-term community care centers. Thus, strategies to evaluate the physical status of older adults and the efficacy of community-based health promotion programs must be explored.
Physical fitness is defined as the capacity to perform daily physical activities safely and independently without fatigue8,9. The predominant forms of physical fitness are health-related physical fitness and skill-related physical fitness. The former is associated with everyday activities, whereas the latter is associated with athletic activities. Rikli and Jones designed a functional fitness assessment tool for community-dwelling older adults; the validated tool comprises multiple simple but effective tests to evaluate the muscle strength, flexibility, aerobic endurance, agility, and dynamic balance of older adults through daily activities10. Suitable physical fitness tests can be used to objectively evaluate physical function in older adults who participate in community activities to maintain or promote health11. In addition, these tests are widely available, less time-consuming, and convenient and require no special equipment, all of which contribute to the clinical practicality of these tests12. Notwithstanding the low precision and specificity compared with advanced laboratory-based assessments, the tests used to assess physical performance in older adults may help evaluate muscular strength and endurance13,14,15,16, flexibility17, cardiorespiratory endurance18,19,20,21, static and dynamic balance.
Reference values for physical fitness are required to improve the interpretability and utility of physical fitness test results in a clinical setting. Although reference values and equations for physical fitness tests, such as grip strength22,23,24,25,26 and the 30-s chair stand test27, have been determined previously, these values were determined by considering the results of various trials and merely one aspect of physical fitness. Reference values for the physical performance levels of older adults have been reported in studies conducted in the United States27, Brazil28, Spain29, India30, Nepal31, and Poland32. Although studies conducted in Taiwan have reported ratings to assess the physical performance levels of older adults33,34, the studies did not explore the active community-dwellings or considered all aspects of physical fitness or the screening for sarcopenia or osteoporosis. To design tailored programs for active community-dwelling older adults on the basis of their physical fitness level and overall health status, all aspects of physical fitness must be assessed in addition to percentile distribution to help older adults compare their functional status with relevant standards. Furthermore, because the aforementioned study was conducted > 10 years ago33, updated reference values for physical fitness must be determined, particularly for active community-dwelling older adults.
Handgrip strength (HGS), a common physical fitness test, is convenient for use in community-based assessments. The decline in HGS is reportedly associated with physical limitations in older adults aged ≥ 60 years35. A pooled analysis including a total of 6,426 community-dwelling older adults indicated that low HGS is associated with mortality36. Thus, HGS can be regarded as a clinically important indicator of the health status of older adults. Therefore, to simplify the assessment work, HGS test can be used as a single test to efficiently determine the physical fitness levels of community-dwelling older adults and obtain objective data. Hence, in the present study, we investigated the possible correlation of HGS with various parameters of physical fitness.
With the aging of community-dwelling older adults, the annual health expenditure of the government increases every year. Thus, various community-based health promotion programs have been launched for maintaining physical fitness levels and delaying disability in community-dwelling older adults. For clinical purposes, a comprehensive survey encompassing all aspects of physical fitness must be conducted to determine the reference values for the physical fitness levels of older men and women. Thus, in the present cross-sectional study, we sought to determine age- and sex-specific reference values for the physical fitness levels of active community-dwelling older adults and investigate the possible correlation of HGS with the physical fitness parameters of older adults.
Participants and methods
Study design and participants
In this cross-sectional study, we reviewed the data of community-dwelling older adults who participated in a community health promotion program run by the Center of Community Medicine at National Yang Ming Chiao Tung University Hospital, Taiwan, between July 10, 2017, and December 25, 2019. During recruitment, experienced medical professionals (P-JP and M-JL) evaluated the health status of the participants. All eligible community-dwelling older adults were subjected to initial screening. The inclusion criteria were as follows: residence in Taiwan’s Yilan County, age ≥ 65 years, desire to undergo evaluation for physical fitness, no major medical concerns, and an ability to perform study-related tasks. Older adults with severe neurological or cognitive impairment, a history of recent traumatic events, acute illnesses such as sepsis, unstable vital signs, or complete dependence on caregivers for the activities of daily living were excluded from this study. All procedures performed in this study were in accordance with the ethical principles of the World Medical Association Declaration of Helsinki and National Yang Ming Chiao Tung University Hospital. This study was reviewed and approved by the Human Subject Research Ethics Committee of National Yang Ming Chiao Tung University Hospital (approval number, 2020A012). Written informed consent for participation was obtained from all participants.
Data collection
Eligible older adults were invited to our health promotion center at National Yang Ming Chiao Tung University Hospital. The study procedure was divided into the following five steps:
-
1.
Interviewing participants to obtain demographic data
-
2.
Administering a frailty questionnaire: a modified questionnaire from short version of the International Physical Activity Questionnaire (IPAQ)37
-
3.
Conducting physical fitness tests to evaluate the participants’ body composition (body mass index [BMI]), muscular strength and endurance (30-s arm curl and 30-s chair stand tests)38, flexibility (back scratch and chair sit-and-reach tests; negative score if the fingertips do not overlap or pass the 0 mark)38, aerobic endurance (2-min step test)39, and balance (single-leg standing40 and 8-foot up-and-go38 tests); the tests were conducted in accordance with the guidelines of the Sports Department of the Ministry of Education in Taiwan.
-
4.
Screening for sarcopenia with measurements of HGS, calf circumference, and 6-m walking speed41
-
5.
Measuring bone density
In the waiting area of the aforementioned health promotion center, a face-to-face interview was conducted with the participants to obtain data regarding their demographics and a frailty questionnaire was administered. Subsequently, all participants were subjected to physical fitness assessment and sarcopenia and osteoporosis screening at a sports ground; the sensor-assistive system (SHM; Acutek) and bone density measurement were located there.
Outcome measures
Demographics
Well-trained researchers collected data regarding participant demographics (e.g., age, sex, and exercise habits) during the interview.
Physical activity levels
The aforementioned frailty questionnaire was used to collect data regarding the participants’ HGS, energy level, waking speed, physical activity level, and unintentional weight loss37. To evaluate their physical activity levels, the participants were interviewed; data regarding their calorie consumption was collected using the modified short version of the IPAQ (Taiwan version)37.
Physical fitness levels and sarcopenia and osteoporosis
Trained hospital volunteers assisted a qualified physical therapist (Y-YL) and a physical fitness coach in data collection. Intra- and interrater variabilities were not assessed in this study. Nevertheless, the qualified physical therapist (Y-YL) completed official training and qualified assessments on standard operating procedures. Training sessions were conducted before the health promotion program to ensure that the researchers can duly conduct all study tasks. Furthermore, the aforementioned sensor-assistive system for assessing geriatric physical fitness levels was used in our study to improve test efficiency and precision; this system has also been used previously42. We measured the participants’ height and weight (InBody 570; InBody), calf circumference, and bone density (Pegasus; Medilink) and conducted the following tests: 30-s arm curl (SHM16; Acutek), back scratch, 30-s chair stand (SHM12; Acutek), chair sit-and-reach (SHM11; Acutek), single-leg standing (SHM 15; Acutek), 8-foot up-and-go (SHM13; Acutek), 2-min step (SHM14; Acutek), HGS (TTM Digital Hand Grip Dynamometer; TTM110D), and 6-m walking speed tests.
HGS
The participants’ HGS was measured twice, and the higher of the two obtained values was recorded. On the basis of the Asian Working Group for Sarcopenia 2019 consensus, low muscle strength was defined as an HGS of < 28 kg for men and < 18 kg for women41.
BMI
In the present study, the participants were categorized as underweight (BMI < 18.5 kg/m2), normal weight (BMI, 18.5–24 kg/m2), overweight (BMI, 24–27 kg/m2), and obese (BMI > 27 kg/m2) on the basis of guidelines of the Taiwanese Ministry of Health and Welfare43.
Osteoporosis
The T-score is a standard score used for bone density measurements with respect to the mean bone density of a population of 30-year-old healthy individuals. Individuals with a T-score of + 1 to − 1 are regarded as healthy. A T-score between − 1 and − 2.5 indicates low bone density, whereas a T-score of less than − 2.5 suggests osteoporosis. A highly negative value of the T-score indicates severe osteoporosis.
Statistical analysis
Descriptive statistics, including mean, standard deviation, and percentile (5th, 10th, 25th, 50th, 75th, 90th, and 95th), were used to describe the demographics and physical fitness levels of the participants. The data were stratified by age (groups: 65–69, 70–74, 75–79, 80–84, 85–89, and ≥ 90 years) and sex (men and women). For the community-dwelling older adults, a stepwise multiple linear regression model (entry probability = 0.05; removal probability = 0.10) was constructed for the predictive analysis of HGS (kg; response variable) on the basis of age; sex (men, 1; women, 0); BMI; calf circumference; bone density; physical activity levels; and 30-s arm curl, 8-foot up-and-go, back scratch, single-leg standing, chair sit-and-reach, 30-s chair stand, 2-min step, and timed 6-m walking speed tests. Separate regression models were used for men and women. Moreover, the correlations between age and various physical fitness parameters were investigated using simple linear regression analyses. A p value of < 0.05 indicated statistical significance. Adjusted R-squared (R2) values indicate variations (%) in parameters affecting a response variable. Variance inflation factors indicate the amount of multicollinearity in a set of regression variables. Furthermore, 95% confidence interval and prediction interval indicate uncertainty levels in statistical estimates. Statistical analyses were performed using SPSS (version 22.0; IBM Corp., Armonk, NY, USA).
Ethics approval and consent to participate
It was a cross-sectional study, and the medical datasets were analyzed. Permission for accessing patients’ medical datasets was granted by the Director of the Center of Community Medicine, National Yang Ming Chiao Tung University Hospital, Yilan, Taiwan. All procedures performed in this study were in accordance with the ethical principles of the World Medical Association Declaration of Helsinki and National Yang Ming Chiao Tung University Hospital.
Study approval statement
This study was reviewed and approved by the Human Subject Research Ethics Committee of National Yang Ming Chiao Tung University Hospital (approval number, 2020A012).
Consent to participate statement
Written informed consent for participation was obtained from all participants.
Results
A total of 2,878 older adults were assessed for their eligibility to participate in the present study. This study excluded a total 691 individuals aged < 65 years, 28 whose ages were unknown, 8 who lived outside Yilan County, and 21 who met any one of the study exclusion criteria. Thus, a total of 748 people were excluded from this study; finally, a total of 2,130 people were included (Fig. 1). All physical fitness tests involved a total of 2,130 individuals aged ≥ 65 years (men, 547; mean age, 74.83 ± 6.54 years). The older adults were divided into six age groups: 65–69, 70–74, 75–79, 80–84, 85–89, and ≥ 90 years. The 65–69-year age group had the highest number of participants. The frailty questionnaire, which is used to assess recalled the average weekly activity levels in the previous month, revealed an average activity level of 13.74 ± 39.94 metabolic equivalent hours per week (men, 14.44 ± 44.92 MET-hours/week; women, 13.04 ± 38.07 MET-hours/week). Table 1 summarizes the participant demographics.
Physical fitness levels of community-dwelling older adults in Taiwan
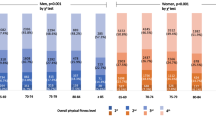
Table 2 presents the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentiles of the participants’ (stratified by age and sex) physical fitness levels, height and weight, sarcopenia results (e.g., HGS, timed 6-m walking speed test, and calf circumference), and bone density. These percentile values can be used to determine the fitness levels of active community-dwelling older adults in Taiwan with regard to their age and sex. Younger age was associated with improved performance.
Among men, older adults aged 65–69 years had the highest BMI (25.61 ± 3.64 kg/m2), whereas those aged 80–84 years had the lowest BMI (24.24 ± 2.99 kg/m2). Regarding the single-leg standing test, the best results were noted in older adults aged 65–69 years (28.45 ± 1.70 s) and the worst results were noted in those aged ≥ 90 years (3.57 ± 0.48 s). Older adults aged 65–69 and 70–74 years exhibited the best results in the back scratch (− 14.28 ± 1.27 cm) and chair sit-and-reach (1.23 ± 0.95 cm) tests, respectively; however, older adults aged ≥ 90 years had the poorest performance in both tests (− 24.54 ± 2.41 and − 5.36 ± 2.71 cm, respectively). In 30-s arm curl and 30-s chair stand tests, older adults aged 65–69 years exhibited the best performance (20.6 ± 0.41 and 18.7 ± 0.55 repeats, respectively), whereas those aged ≥ 90 years exhibited the worst performance (12.2 ± 0.64 and 9.3 ± 0.58 repeats, respectively). Moreover, older adults aged 85–89 years took the longest time to complete 8-foot up-and-go test (12.05 ± 0.80 s), whereas those aged 65–69 years took the least time (6.79 ± 0.21 s). In the 2-min step test, older adults aged 65–69 years exhibited the highest performance (115.9 ± 1.75 steps), whereas those aged ≥ 90 years exhibited the lowest performance (89.0 ± 7.46 steps). Furthermore, in HGS test, older adults aged 65–69 years had the highest strength (35.11 ± 0.80 kg), whereas those aged ≥ 90 years had the lowest strength (24.27 ± 1.02 kg). The highest calf circumference (35.8 ± 0.40 cm) was observed among older adults aged 65–69 years, whereas the lowest calf circumference (31.7 ± 0.95 cm) was noted among those aged ≥ 90 years. In the timed 6-m walking speed test, older adults aged 65–69 years had the best performance (4.82 ± 0.15 s), whereas those aged ≥ 90 years had the worst performance (7.76 ± 0.47 s). In addition, physical performance was poorer (gait speed < 1.0 m/s) in older adults aged 80–84, 85–89, and ≥ 90 years than in the other age groups. Among men, bone density decreased with age (measured as the T-score; decreased from − 0.80 ± 0.15 to − 1.59 ± 0.63). Table 2 summarizes the test results of older men.
Among women, BMI was the highest in older adults aged 65–69 years (25.89 ± 4.07 kg/m2) and the lowest those aged ≥ 90 years (23.32 ± 3.27 kg/m2). In the single-leg standing test, older adults aged 65–69 years exhibited the best performance (25.55 ± 0.63 s), whereas those aged ≥ 90 years exhibited the worst performance (5.64 ± 2.16 s). In back scratch and chair sit-and-reach tests, best performance levels were noted in older adults aged 65–69 years (− 3.98 ± 0.47 and 8.26 ± 0.43 cm, respectively), whereas the worst performance was noted in those aged ≥ 90 years. Moreover, in 30-s arm curl and 30-s chair stand tests, older adults aged 65–69 years had the best performance (20.5 ± 0.18 and 18.6 ± 0.22 repeats, respectively), whereas those aged ≥ 90 years had the worst performance (13.5 ± 1.09 and 8.5 ± 0.75 repeats, respectively). Older adults aged 85–89 years took the longest time to complete 8-foot up-and-go test (13.29 ± 0.65 s), whereas those aged 65–69 years took the least time (7.02 ± 0.09 s). In the 2-min step test, older adults aged 65–69 years exhibited the best performance (116.4 ± 0.83 steps), whereas those aged ≥ 90 years exhibited the worst performance (89.6 ± 8.99 steps). In HGS, older adults aged 65–69 years had the highest strength (23.95 ± 0.21 kg), whereas those aged 85–89 years had the lowest strength (17.63 ± 0.36 kg). Calf circumference exhibited a tendency to decrease with age; older adults aged ≥ 90 had the lowest calf circumference (29.8 ± 1.17 cm). In the timed 6-m walking speed test, older adults aged 65–69 years had the best results (4.81 ± 0.05 s) whereas those aged 85–89 years had the worst results (8.51 ± 0.49 s). Slow gait speed was associated with poor physical performance in older adults aged 75–79, 80–84, 85–89, and ≥ 90 years. Bone density (T-score) decreased with age (from − 0.72 ± 0.08 to − 1.85 ± 0.35). Table 2 summarizes the test results of older women.
HGS analyzed using a stepwise multiple linear regression model in older adults
We performed stepwise regression analysis to evaluate the changes (R2) in the result of each physical fitness test. High adjusted R2 values suggest that the regression model can predict the dependent variable and also explain the variance in the response variable around its mean. Thus, we constructed an HGS model; high R2 values obtained using this model indicated that the results of the stepwise regression analysis fit well with our data. For community-dwelling older adults, 59.3% of the variance in HGS equations could be explained by age; sex; BMI; and the results of 30-s arm curl, 8-foot up-and-go, and back scratch tests. The HGS model for older adults was as follows: HGS = 24.688 + (11.954 × sex) + (0.294 × 30-s arm curl test result) – (0.548 × 8-foot up-and-go test result) – (0.12 × age) + (0.222 × BMI) + (0.036 × back scratch test result). The 95% prediction interval for this estimate was approximately ± 9.28. As indicated in Table 3, no evidence of multicollinearity was noted among the predictive factors, such as age, sex, BMI, and the results of 30-s arm curl, 8-foot up-and-go, and back scratch tests. The stepwise regression analysis revealed that HGS had a negative correlation with age and 8-foot up-and-go test result (p < 0.05) but a positive correlation with sex (p < 0.05), BMI (p < 0.05), and back scratch test result (p < 0.05). Furthermore, the variance inflation factors were all < 5, which indicated that the predictors were not correlated.
HGS analyzed separately for men and women through stepwise multiple linear analysis
Sex was strongly correlated with HGS, and the R2 value of the total population corroborated this result. Table 4 presents the results of stepwise regression analysis with the data of participants stratified by sex. The HGS equation for older men was as follows: HGS = 19.973 + (0.754 × calf circumference) + (0.318 × 30-s chair stand test result) − (0.827 × 8-foot up-and-go test result) − (0.151 × age). The factors in this equation were calf circumference, age, and the results of 30-s chair stand and 8-foot up-and-go tests (adjusted R2: male, 0.36). By contrast, the HGS equation for older women was as follows: HGS = 27.489 + (0.305 × 30-s arm curl test result) − (0.495 × 8-foot up-and-go test result) – (0.098 × age) + (0.138 × BMI) + (0.032 × back scratch test result). The factors in this equation were age; BMI; and 30-s arm curl, 8-foot up-and-go, and back scratch results (adjusted R2: women, 0.271).
Results of the simple linear regression with age as the predictor variable and physical fitness parameters as the dependent variables
All physical fitness parameters declined with age in both older men and women. A strong negative correlation was noted between age and HGS. With increasing age, the HGS of older men and women decreased by 0.437 and 0.289 kg every year, respectively. The decrease in HGS with age was more rapid in men than in women. Furthermore, with increasing aging, bone density and the results of 30-s chair stand, 2-min step, and back scratch tests were less favorable and timed 6-m walking was more favorable in women than in men. Table 5 presents the correlation between age and physical fitness parameters.
Discussion
We evaluated the physical fitness levels of a rural community-dwelling older adult population in Taiwan and investigated the predictive factors for sarcopenia and osteoporosis. Although several studies have been conducted to assess the fitness levels of older adults across the globe27,28,30,31,32,44, only one study focused on the older adults in Taiwan33. However, in the aforementioned study conducted in Taiwan, the participants were not active community-dwelling older adults. Furthermore, in most studies, only a few aspects of physical fitness have been evaluated22,23,24,25,26,28,29,30,31,32,33,44; thus, the findings might not have reflected the full potential of older adults to safely engage in mundane daily physical activities without requiring assistance or becoming tired. The present study was conducted to devise strategies for the comprehensive long-term care of older adults. To the best of our knowledge, this study is the first to explore all aspects of physical fitness in active community-dwelling older adults and use a battery of widely recognized and acceptable physical fitness tests27 in addition to demographic and frailty surveys. The reference values for physical fitness were age-specific (5-year span) and sex-specific, which increases their clinical applicability. Our findings related to the percentile distribution of each physical fitness test result may be useful for physicians to design tailored programs for their patients on the basis of the patients’ physical fitness levels. These findings aid the development of various health promotion programs for active community-dwelling older adults; these individuals can also compare their physical performance levels with the standard for their age group. According to a study conducted > 10 years ago33, reference values for physical fitness must be updated, particularly for older adults who participate in community activities. Furthermore, physical performance reportedly worsens with age, which suggests that more precise models are needed to evaluate the performance levels of adults aged > 50 years44. The high variability and low explained variance of previous predictive models for older adults suggest that these estimates of the physical tests are not completely reliable. In the present study, HGS test was used as a simple and an effective assessment tool because the test can be completed rapidly and it helps obtain valuable information regarding the health status of older adults. Thus, we developed the aforementioned HGS equations on the basis of the stepwise regression analysis results; our finding may improve the understanding of physicians regarding the correlation between HGS and complete physical performance. Furthermore, on the basis of our study, various therapeutic strategies and educational programs may be developed to prevent or delay disability and promote health in community-dwelling older adults, thus reducing the economic burden associated with the treatment and hospitalization of these individuals.
As mentioned, the HGS test is a widely used, convenient tool for physical fitness assessment. Low HGS has been associated with physical limitations in individuals aged ≥ 60 years35 and also mortality36. HGS is a clinically important indicator of the health status of older adults. Therefore, it can be used as a substitute for other complex and time-consuming tests to evaluate the overall status of community-dwelling older adults. As mentioned, our model revealed that HGS is positively correlated with sex; BMI; and the results of 30-s arm curl, 8-foot up-and-go, and back scratch tests. These findings are consistent with those of a study reporting an association between HGS and BMI among Greek women45. By contrast, we found a negative correlation between age and HGS. Similar findings have been reported for other ethnicities25,26,44,45,46,47. HGS is a parameter of physical fitness and may indicate the physiological health status of an individual. The correlation of HGS with other commonly used physical fitness parameters, as shown in the present study, suggests that the associated physical fitness parameters can decline with decreasing HGS. Thus, our model represents an age- and sex-specific reference system for evaluating the efficacy of health promotion programs for community-dwelling older adults. Although the physical activity levels may influence the HGS of older adults, the stepwise process did not include them in the final regression model statistically. Physical activity differs from exercise. In the present study, the participants engaged in various activities in the community, such as calisthenics, strolling, and Qigong; however, their physical activity was neither intense nor sufficient for improving their overall strength. Therefore, well-designed programs for effectively improving the physical fitness of community-dwelling older adults are needed. Furthermore, in the domain of physical rehabilitation, the specific adaptation to imposed demands (SAID) principle indicates that the human body adapts specifically to imposed demands. If someone simply performs pull-up exercise using a pull-up bar, the body adapts to this unique physical effort but not necessarily to other climbing patterns or settings. To substantially improve HGS, exercise regimens must be designed according to the SAID principle.
In the present study, the strongest correlation was noted between HGS and sex. Although HGS was correlated with the results of 30-s chair stand test in men, it was correlated with the results of 30-s arm curl test in women. Notably, a study including American college students reported that female students exhibited 37%–68% of the muscular strength generally noted in men; compared with male students, female students exhibited high muscle strength in the upper body but low muscle strength in the lower body. The lower limbs of women are often stronger than their upper limbs and shoulders48. In the present study, we used the 30-s chair stand and 30-s arm curl tests to evaluate the strength/endurance of lower and upper extremities, respectively. Contrasting findings were noted in our study, and these findings might be due to the adaptive behaviors of the participants; specifically, in Yilan County, men are generally involved in farming and labor work that requires more crouching and sit-to-stand movements, whereas women are generally engaged in household chores and crafting (handicrafts). As shown in Table 5, the result of 30-s chair stand test decreases by 0.318 and 0.350 with 1-year increase in age among both men and women, respectively. Because the lower limb strength of women reduces faster than that of men, HGS may be more directly correlated with the results of 30-s arm curl test in women than in men.
The findings of the present study indicate that levels of performance in all physical fitness tests decline with aging in a consistent and steady manner in both men and women. We compared the results obtained using the HGS model with those of a study conducted in Taiwan. Adults with a BMI of < 18.5 kg/m2 are considered to be underweight by the Taiwanese Ministry of Health and Welfare; this low value may suggest malnutrition, eating disorders, or other health concerns. By contrast, adults with a BMI of ≥ 24 kg/m2 are considered to be overweight. In the present study, the BMI values of men and women were 24.24–25.61 and 23.32–25.89 kg/m2, respectively. Almost all older men and women were overweight, except women aged ≥ 90 years whose BMI was within the normal range (23.32 ± 3.27). A study conducted in Taiwan33 reported a BMI of 23.3–25.2 and 23.0–25.0 kg/m2 in men and women, respectively. In their study, men aged 60–64 years and women aged 65–69 years were overweight. We demonstrated that older adults who participate in community activities tend to have a high BMI. A study published in The Lancet in 2009, including a total of 900,000 adults, reported that individuals with underweight and overweight exhibit higher risks of mortality than do those with normal weight (determined using BMI)49. Despite good health and a low percentage of body fat, some people may be regarded as overweight on the basis of their BMI. Our findings indicate that many community-dwelling older adults have body composition–related problems, even if they participate in community activities. Thus, further studies on the distribution of muscle and fat mass are warranted.
In the present study, the 8-foot up-and-go test was used to assess agility and dynamic balance and determine the differences between recurrent faller and nonfaller groups used in a previous study50. Fallers are defined as older adults who take ≥ 8.5 s to complete the test (overall prediction rate, 82%). This test has a sensitivity of 78% and a specificity of 86%50. In the present study, men and women aged > 75 years took > 8.5 s; this highlights importance of fall prevention programs for this age group. Although the percentiles of all physical fitness scores of older adults stratified by age and sex were not reported in a study conducted in Taiwan34, their participants (both men and women) exhibited lower performance levels in 8-foot up-and-go and 30-s arm curl tests than did our participants. This difference may be explained by the inherent differences between the two older adult populations. In the aforementioned study, the participants were recruited through preannouncement at local communities by health-care facilities, clubs, and sports centers34. In the present study, the participants were older adults who engaged in community activities, such as calisthenics and walking, which might have improved their performance. Regarding the results of the flexibility test, older adults are less flexible than their younger counterparts. Women tend to be more flexible than their same-aged male counterparts. This is reasonable because of the metabolic changes occurring in the muscles; in addition, the loss of mitochondrial DNA may exert negative effects on the overall fitness levels of older adults51.
Our study has some limitations. First, this study included community-dwelling older adults who were functionally independent and engaged in community activities; the participants were the residents of a rural area in Taiwan. Thus, our findings may not be generalized beyond the study population because our data may not represent normative data. Normative data can be obtained by conducting studies involving a large and randomly selected representative sample from a wide population. Age, sex, and ethnicity should all be considered when defining the reference population. Moreover, older adults who do not participate in community activities must also be assessed. Online surveys may be conducted to collect normative data—something we wish to explore in our future studies given the suitability of our test model. Second, women outnumbered men in the present study. Similarly, in a study on the fitness levels of older people residing in the United States, the number of female participants was higher than that of male participants (men, 2,135; women, 5,048). By contrast, in a similar study conducted in Taiwan, the number of men was higher than that of women. This difference between the participation of men and women may be attributed to cultural and geographical factors; however, further studies are needed. Owing to the sex-specific differences, the sample size of men in our study (n = 547) might not have been adequate. Third, the cross-sectional design of the present study precluded causal inference. To investigate the possible causal relationship between HGS and physical fitness, a longitudinal design or qualitative methodology must be used in future studies. Finally, several researchers conducted the physical fitness tests and managed (i.e., arrangement, input, and evaluation) the obtained data. Thus, human error might have occurred. Nevertheless, to reduce data collection–related variability and enhance test efficiency and accuracy, we used a sensor-assisted physical fitness test system to evaluate the physical fitness of the participants.
Conclusions
The age- and sex-specific reference values for physical fitness may facilitate the interpretation of physical fitness test results. These values can be used by health-care professionals to warn their patients regarding any risk of functional decline. In addition, the reference values can be used to design effective exercise programs for community-dwelling older adults. The stepwise regression analysis indicated that young men with high BMI values and high levels of performance in the 8-foot up-and-go and back scratch tests exhibit high HGS. Furthermore, the sex-specific analysis suggested that HGS is associated with upper and lower limb strength in women and men, respectively. Thus, our model and reference values may be useful for designing tailored programs for promoting health in older adults.
Data availability
The data that support the findings of this study are not publicly available because their containing information that could compromise the privacy of research participants but are available from the corresponding author upon reasonable request.
References
Wong, C. H. et al. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin. Exp. Res. 22, 54–62 (2010).
Kim, H. et al. Sarcopenia: Prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr. Gerontol. Int. 16, 110–122 (2016).
Yu, X. et al. Effects of different living conditions on the risk of osteoporosis in Chinese community-dwelling elderly: a 3-year cohort study. J. Int. Med. Res. 48, 300060520943450–300060520943450. https://doi.org/10.1177/0300060520943450 (2020).
Liu, L. K. et al. Association between frailty, osteoporosis, falls and hip fractures among community-dwelling people aged 50 years and older in Taiwan: Results from I-LAN longitudinal aging study. PLoS ONE 10, e0136968. https://doi.org/10.1371/journal.pone.0136968 (2015).
Ryan, C. G. et al. Individuals with chronic low back pain have a lower level, and an altered pattern, of physical activity compared with matched controls: An observational study. Aust. J. Physiother. 55, 53–58. https://doi.org/10.1016/s0004-9514(09)70061-3 (2009).
Hodselmans, A. P., Dijkstra, P. U., Geertzen, J. H. & van der Schans, C. P. Nonspecific chronic low back pain patients are deconditioned and have an increased body fat percentage. Int. J. Rehabil. Res. 33, 268–270. https://doi.org/10.1097/MRR.0b013e328335213f (2010).
Wu, S. C., Leu, S. Y. & Li, C. Y. Incidence of and predictors for chronic disability in activities of daily living among older people in Taiwan. J. Am. Geriatr. Soc. 47, 1082–1086. https://doi.org/10.1111/j.1532-5415.1999.tb05231.x (1999).
Medicine, A. C. o. S. ACSM's health-related physical fitness assessment manual. (Lippincott Williams & Wilkins, 2013).
Caspersen, C. J., Powell, K. E. & Christenson, G. M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 100, 126 (1985).
Rikli, R. E. & Jones, C. J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 7, 129–161 (1999).
Jette, D. U., Halbert, J., Iverson, C., Miceli, E. & Shah, P. Use of standardized outcome measures in physical therapist practice: perceptions and applications. Phys. Ther. 89, 125–135 (2009).
Bennell, K., Dobson, F. & Hinman, R. Measures of physical performance assessments: Self-Paced walk test (SPWT), stair climb test (SCT), Six-Minute walk test (6MWT), chair stand test (CST), timed up & go (TUG), sock test, lift and carry test (LCT), and car task. Arthritis Care Res. 63, S350–S370 (2011).
Jones, C. J., Rikli, R. E. & Beam, W. C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 70, 113–119 (1999).
Benton, M. J. & Alexander, J. L. Validation of functional fitness tests as surrogates for strength measurement in frail, older adults with chronic obstructive pulmonary disease. Am. J. Phys. Med. Rehabil. 88, 579–583 (2009).
Bohannon, R. W. Dynamometer measurements of grip and knee extension strength: are they indicative of overall limb and trunk muscle strength?. Percept. Mot. Skills 108, 339–342 (2009).
Roberts, H. C. et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 40, 423–429 (2011).
Perret, C. et al. Validity, reliability, and responsiveness of the fingertip-to-floor test. Arch. Phys. Med. Rehabil. 82, 1566–1570 (2001).
Burr, J. F., Bredin, S. S., Faktor, M. D. & Warburton, D. E. The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. Phys. Sportsmed. 39, 133–139 (2011).
Hovington, C. L., Nadeau, S. & Leroux, A. Comparison of walking parameters and cardiorespiratory changes during the 6-minute walk test in healthy sexagenarians and septuagenarians. Gerontology 55, 694–701 (2009).
Cataneo, D. C. & Cataneo, A. J. M. Accuracy of the stair climbing test using maximal oxygen uptake as the gold standard. J. Bras. Pneumol. 33, 128–133 (2007).
Koegelenberg, C. F., Diacon, A. H., Irani, S. & Bolliger, C. T. Stair climbing in the functional assessment of lung resection candidates. Respiration 75, 374–379 (2008).
Schlüssel, M. M., dos Anjos, L. A., de Vasconcellos, M. T. L. & Kac, G. Reference values of handgrip dynamometry of healthy adults: a population-based study. Clin. Nutr. 27, 601–607 (2008).
Hanten, W. P. et al. Maximum grip strength in normal subjects from 20 to 64 years of age. J. Hand Ther. 12, 193–200 (1999).
Massy-Westropp, N. M., Gill, T. K., Taylor, A. W., Bohannon, R. W. & Hill, C. L. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC. Res. Notes 4, 1–5 (2011).
Günther, C. M., Bürger, A., Rickert, M., Crispin, A. & Schulz, C. U. Grip strength in healthy caucasian adults: reference values. J. Hand Surg. 33, 558–565 (2008).
Pan, P.-J. et al. Normative data and associated factors of hand grip strength among elderly individuals: The Yilan Study Taiwan. Sci. Rep. 10, 1–8 (2020).
Rikli, R. E. & Jones, C. J. Functional fitness normative scores for community-residing older adults, ages 60–94. J. Aging Phys. Act. 7, 162–181 (1999).
Krause, M. P. et al. A comparison of functional fitness of older Brazilian and American women. J. Aging Phys. Act. 17, 387–397 (2009).
Gusi, N. et al. Normative fitness performance scores of community-dwelling older adults in Spain. J. Aging Phys. Act. 20, 106–126 (2012).
Bhattacharya, P. K., Deka, K., Roy, A. & Saikia, H. normative values of physical fitness test in the elderly: A community based study in an urban population in northeast India. J. Clin. Diagnost. Res. 11 (2017).
Kim, J. K., Son, W. I., Sim, Y. J., Lee, J. S. & Oli Saud, K. The study of health-related fitness normative scores for nepalese older adults. Int. J. Environ. Res. Public Health 17, 2723 (2020).
Ignasiak, Z. et al. Functional fitness normative values for elderly polish population. BMC Geriatr. 20, 1–9 (2020).
Chen, H.-T., Lin, C.-H. & Yu, L.-H. Normative physical fitness scores for community-dwelling older adults. J. Nurs. Res. 17, 30–41 (2009).
Lee, P.-F. et al. Cross-sectional associations of physical fitness performance level and sleep duration among older adults: Results from the national physical fitness survey in Taiwan. Int. J. Environ. Res. Public Health 17, 388 (2020).
Germain, C. M., Vasquez, E., Batsis, J. A. & McQuoid, D. R. Sex, race and age differences in muscle strength and limitations in community dwelling older adults: Data from the Health and Retirement Survey (HRS). Arch. Gerontol. Geriatr. 65, 98–103. https://doi.org/10.1016/j.archger.2016.03.007 (2016).
Lera, L. et al. Reference values of hand-grip dynamometry and the relationship between low strength and mortality in older Chileans. Clin. Interv. Aging 13, 317–324. https://doi.org/10.2147/CIA.S152946 (2018).
Chen, L.-J., Chen, C.-Y., Lue, B.-H., Tseng, M.-Y. & Wu, S.-C. Prevalence and associated factors of frailty among elderly people in Taiwan. Int. J. Gerontol. 8, 114–119 (2014).
Rikli, R. E. & Jones, C. J. Assessing physical performance in independent older adults: Issues and guidelines. J. Aging Phys. Act. 5, 244–261 (1997).
Bohannon, R. W. & Crouch, R. H. Two-minute step test of exercise capacity: systematic review of procedures, performance, and clinimetric properties. J. Geriat. Phys. Therapy 42, 105–112 (2019).
Vellas, B. et al. One-leg standing balance and functional status in a population of 512 community-living elderly persons. Aging Clin. Exp. Res. 9, 95–98 (1997).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300–307, https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Lin, W.-S. et al. Correlation analysis of physical fitness and its impact on falls in 2130 community- dwelling older adults: a retrospective cross-sectional study. BMC Geriatr. 22, 447. https://doi.org/10.1186/s12877-022-03138-9 (2022).
Health Promotion Administration, Ministry of Health and Welfare. Taiwan’s Obesity Prevention and Management Strategy. 1st edn, 1, 55 (Health Promotion Administration, Ministry of Health and Welfare, 2018).
Tveter, A. T., Dagfinrud, H., Moseng, T. & Holm, I. Health-related physical fitness measures: Reference values and reference equations for use in clinical practice. Arch. Phys. Med. Rehabil. 95, 1366–1373 (2014).
Mitsionis, G. et al. Normative data on hand grip strength in a Greek adult population. Int. Orthop. 33, 713–717. https://doi.org/10.1007/s00264-008-0551-x (2009).
Koley, S. & Singh, A. P. An association of dominant hand grip strength with some anthropometric variables in Indian collegiate population. Anthropologischer Anzeiger, 21–28 (2009).
Wu, S.-W., Wu, S.-F., Liang, H.-W., Wu, Z.-T. & Huang, S. Measuring factors affecting grip strength in a Taiwan Chinese population and a comparison with consolidated norms. Appl. Ergon. 40, 811–815 (2009).
Chen, G., Liu, L. & Yu, J. A comparative study on strength between American college male and female students in Caucasian and Asian populations. Sport Sci. Rev. 21, 153 (2012).
Collaboration, P. S. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. The Lancet 373, 1083–1096 (2009).
Rose, D. J., Jones, C. J. & Lucchese, N. Predicting the probability of falls in community-residing older adults using the 8-foot up-and-go: A new measure of functional mobility. J. Aging Phys. Act. 10, 466–475 (2002).
Cortopassi, G. A., Shibata, D., Soong, N. W. & Arnheim, N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc. Natl. Acad. Sci. USA 89, 7370–7374. https://doi.org/10.1073/pnas.89.16.7370 (1992).
Acknowledgements
The authors thank all the community participants and the staffs of Center of Community Medicine, National Yang Ming Chiao Tung University Hospital in this health promotion program. Furthermore, the authors would like to thank Pau-Jar Charity Foundation and Social affairs department, Yilan County. This manuscript was edited by Wallace Academic Editing.
Author information
Authors and Affiliations
Contributions
P.-J.P.: study design, study supervision, writing—review and editing, result interpretation and project administration; N.-W.H., M.-J.L. and Y.-Y.L.: subject recruitment, data acquisition and project administration; C.-C.T.: statistical analysis; W.-S.L.: writing—original draft preparation and result interpretation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, PJ., Hsu, NW., Lee, MJ. et al. Physical fitness and its correlation with handgrip strength in active community-dwelling older adults. Sci Rep 12, 17227 (2022). https://doi.org/10.1038/s41598-022-21736-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21736-w
- Springer Nature Limited
This article is cited by
-
Gingival bleeding as a predictor of handgrip strength—an observational study and a pilot randomized clinical trial
Clinical Oral Investigations (2024)
-
Effects of Taiwan’s COVID-19 alert levels on the physical activity behaviors and psychological distress of community-dwelling older adults
BMC Geriatrics (2023)