Abstract
The solar dryer can reduce production costs, energy consumption, waste (use fruits outside the quality standard for fresh consumption) and is an alternative for small and medium producers. The solar dryer can reduce costs and is an alternative for small and medium producers worldwide. The consumption of fresh and processed tomatoes is high in the world, but post-harvest losses is also and drying is an alternative to reduce these losses. The temperature maintenance and drying time corresponds 30% of the costs. The objective was evaluated the tomato physicochemical characteristics after drying in handmade solar dryer. ‘Carmen’ tomato fruits were bleached in water, 2.5% NaCl solution, 2.5% NaCl + 0.5% CaCl2 solution and unbleached. Tomato slices were placed in a handmade solar dryer from 7:00 to 17:00. The solar dryer prototype was wood made, comprising a collector and a drying chamber. The average cost of the camera was US$ 13.08 (1 Brazilian Real = 0.26 United States Dollar). Water loss, drying kinetics, mathematical models and physicochemical characteristics of fresh and dried tomatoes were evaluated. The average length of solar drying for the four treatments was 30 h and the Midilli and Kucuk mathematical model was the most adjusted. The acidity, reducing sugars and soluble solids were concentrated by drying, while ascorbic acid was reduced. The pH did not change. Tomatoes 'Carmen' can be dried in a handmade solar dryer for 30 h while maintaining product quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Tomato is the second most horticultural crop in the world, with a production of 40 million tons per year1,2. The tomato fruits can be consumed in natura and/or processed in the form of dried tomato, powder, juice, puree, concentrated pulp, extract, ketchup and sauces3.
Tomato is a climacteric fruit and its post-harvest shelf life is short due to the rapid loss of firmness (softening) during maturation4. The main cause of rotting of fruit is the extent of softening that direct effect on palatability, shelf life, resistance to postharvest infections, transportation, storage and consumer acceptance5,6.
Drying of agricultural products is one of the most commonly used unitary operations to extend the shelf-life of perishable fruits by reducing water content to safe levels, as well as reducing storage and transport costs7. However, convection hot air-drying using fossil fuels such as coal, diesel oil and natural gas generates considerable energy costs and promotes negative environmental impacts8. Solar drying is a viable alternative to meet the energy demand, protecting the environment and reducing the energy cost of drying using fossil fuels9. In this method, the thermal energy absorbed by the solar collector is supplied to the air inside to dry products to a desired water content7.
Drying should still give the final product its own sensory characteristics and preserve its nutritional value to the maximum extent. Pre-treatments are applied to accelerate drying, reducing fruit exposure time and consequent nutritional loss. Bleaching facilitates internal mass transfer during drying by increasing tissue permeability10. Salts used in bleaching are also capable of increasing the diffusion of water from the interior of the food to its surface, accelerating drying11. Osmotic dehydration caused by salts also preserves the nutritional constituents and color compounds and aroma12.
The objective was to evaluate the efficiency of handmade solar dryer for drying tomatoes 'Carmen'.
Results
The mathematical model of Midilli and Kucuk was the one that most adjusted to the solar drying of tomatoes (Table 1).
The drying rate of sliced tomatoes did not differ between treatments, was higher in the first 10 h and progressively reduced until the end of the process. The mean drying time was 30 h and did not differ between treatments. The dryer temperature in the solar dryer varied from 40 (07:00–09:00 and 17:00) to 70 °C (09:00–16:00) (Figs. 1 and 2).
The water content (WC) in slices of pre-treated tomatoes with bleaching in 2.5% NaCl solution and 2.5% NaCl solution and 0.5% CaCl2 was lower after drying. Titratable acidity (TA), soluble solids (SS) and reducing sugars (RS) increased with drying and did not differ between treatments. The ascorbic acid content (AsA) decreased with drying and did not differ between treatments. The pH was not changed upon drying (Table 2).
Discussion
All applied models represented precisely the process, with R2 above 99%. Nevertheless, the model of Midilli and Kucuk fit better among the others.
The drying rate in the sliced tomatoes was higher at the beginning of the process due to the higher absorption of radiation by the high water content. Mature tomato fruits, variety regardless, contain, on average, 93–95% water13. Water is a good heat transfer fluid because it has high thermal capacity and low viscosity, accelerating drying14. The gradual reduction in the drying rate after 10 h is explained by the reduction in the water content during the process. The average time of 30 h of drying at 40–70 °C was satisfactory, since there were no costs in the energy consumption and the dry product quality was adequate. The optimal dehydration conditions for tomato slices are 35–44 h at 52–67 °C15. The maintenance of temperature and drying time correspond to 30% of the total cost of processing16, since fruits and vegetables generally contain more than 80% water and the drying process at desirable levels (5–10%) consumes a lot of energy16. Considering the low set-up cost, zero energy cost, reduced spatial impact17, favorable public acceptance18,19 and obtaining a product with acceptable qualities, the handmade solar dryer is an environmentally and socioeconomically beneficial system that characterizes the activity as an innovative business model for small and medium producers17.
The lower WC in the slices of pretreated tomatoes with bleaching in 2.5% NaCl solution and 2.5% NaCl and 0.5% CaCl2 after drying can be explained by the osmotic dehydration caused by the salt presence. In this phenomenon, water was removed from the inner tissues of the tomato slices (lower concentration of solute) to the surface (higher concentration) through semipermeable membranes to maintain equilibrium on both sides of the membrane20. On the surface, the water absorbed greater radiation accelerating the drying14 and resulting in products with lower WC. The increase of TA, SS and RS is basically explained by the concentration of organic acids and soluble solids due to the water loss. The AsA content reduced with drying, regardless of the treatment, due to the reduction in water content in tomato slices. The AsA loss during the tomato drying process depends on the temperature, drying time, product humidity21, luminosity and pH22 and follows a first order kinetics. This means that AsA degradation during tomato drying is a result of increased tomato concentration increasing the degradation rate23. The AsA initial rate degradation was faster as the moisture content decreased24. The presence of salts in the bleaching may also have influenced the AsA degradation. AsA was diffused from internal tissues to the tomato slices surface where chemical degradation occurred25. AsA photooxidation occurs in water at pH 4.5–11.622 similar to those found in this experiment.
Conclusions
The model of Midilli and Kucuk was the one that best represented the drying process, with R2 superior to 99%. ‘Carmen’ tomatoes can be dried with handcrafted solar dryer for 30 h while maintaining product quality.
Material and methods
Obtaining and processing raw material
Fruits tomatoes 'Carmen' were purchased in the municipal market Pombal, Paraíba, Brazil, selected in advanced stage of maturation (very mature) and taken to the laboratory. In the laboratory, the fruits were washed in double-filtered running water, sanitized in chlorinated solution at 100 ppm for 15 min, rinsed, sliced manually in four equal parts and the seeds were removed. One hundred grams of the sliced fruits were bleached in water (T2), 2.5% NaCl solution (T3), 2.5% NaCl solution and 0.5% CaCl2 (T4) or without bleaching (T1). Bleaching occurred at 100 °C for 2.5 min with immediate immersion in ice water (0 ± 4 °C).
Solar drying
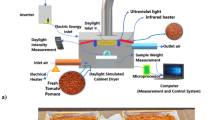
Drying was conducted in a homemade solar dryer direct exposure to light collector and 0.32 m2 drying chamber. The equipment was made in plywood in black. The collector and drying chamber were covered with 2 mm glass plates. The heat sink body of the solar collector was black zinc (Fig. 3).
The tomato slices, bleached or not, were randomly placed in the solar desiccator from 7:00 a.m. to 5:00 p.m. for 3 days, totaling 30 h of sun exposure. The slices were weighed every hour and drying was completed after obtaining constant weight over three consecutive weighings. The dried slices were packed in high density polypropylene, vacuum sealed, covered with aluminum foil and stored in a refrigerator. This process was performed in triplicate and on different days. Water loss, drying kinetics and mathematical models were evaluated. The moment of the removal of the solar dryer treatments was calculated:
where, Pi is the initial mass of the tomato (g), Mf is the final mass of tomato after dry (g), MOi is the initial moisture of fresh tomato and MOf is the desired final moisture of dried tomato (25–30%).
Analysis of the drying kinetics
The semi-theoretical mathematical models of Page, Henderson and Pabis and Midilli and Kucuk26 were applied to evaluate which was the most representative for drying (Table 3).
The models were applied by non-linear regressions using Statistica software version 5.0. The highest coefficient of determination (R2) and the lowest mean square deviation (MSD) (Eq. 2) were used as parameters for model evaluation27. Equation (2):
RTAexp is the experimental water content ratio, WCRpre is the ratio of water content predicted by equation, N is the observations number made during the experiment.
Physicochemical analysis
Fresh and dried tomatoes were evaluated:
-
Total titratable acidity five grams of pulp were homogenized in 45 mL of distilled water and the solution was titrated with sodium hydroxide (0.1 N) to pink color28;
-
pH determined by potentiometer reading (Tecnal, TEC-2);
-
Reducing sugar the reducing sugars content was determined by the dinitro alicyclic acid method29. An extract was prepared by diluting 1.0 g of pulp in 100 ml of distilled water. A solution containing 0.5 ml of the extract, 1.0 ml of distilled water and 1.0 ml of the dinitrosalicyclic acid solution was prepared in tubes. The tubes were agitated rapidly in a stirrer (Novainstruments, NI 1107, Piracicaba, São Paulo, Brazil) and placed in a thermostatic bath (Hemoquímica, HM 0128, Sabará, Minas Gerais, Brazil) at 100 °C for 5 min. The reducing sugars content was determined by spectrophotometry (Spectrum, 560 UV, Maharashtra, India) at 540 nm using glucose as reference for obtaining the standard curve.
-
Water content sliced tomato samples were spread separately on trays and dried by heat dehydration in an electric oven (SOLAB) at 70 °C to constant mass28;
-
Soluble solids drops of juice from fruit pulp pressed was placed on the digital refractometer prism and soluble solids was determinate by oBrix (Digital Refractometer, New Jersey, USA);
-
Ascorbic acid five grams of pulp were homogenized in 47 ml of oxalic acid (0.5%) and titrated with Tillmans solution until pink28.
Statistical analysis
The results of the physicochemical evaluation were submitted to Analysis of Variance (ANOVA) and the means were compared by the Tukey test at 5% significance by the software Assistat, version 7.730.
References
FAO—Food and Agriculture Organization of the United Nations. World Crops Production. http://www.wptc.to/releases-wptc.php (2016).
WPTC—World Processing Tomato Council. World production estimate. http://www.wptc.to/releases-wptc.php. (2016).
Silva, Y. P. A. et al. Characterization of tomato processing by-product for use as a potential functional food ingredient: Nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 70, 150–160 (2019).
Pereira, M. A. B. et al. Postharvest conservation of structural long shelf life tomato fruits and with the mutant rin produced, in edaphoclimatic conditions of the southern state of Tocantins. Ciênc. Agrotec. 39, 225–231 (2015).
Brummell, D. A. & Harpster, M. H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. PCW. 47, 311–340 (2001).
Meli, V. S. et al. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc. Natl. Acad. Sci. USA 107, 2413–2418 (2010).
Samimi-Akhijahani, H. & Arabhosseini, A. Accelerating drying process of tomato slices in a PV-assisted solar dryer using a sun tracking system. Renew. Energy 123, 428–438 (2018).
Tripathy, P. P. Investigação da secagem solar da batata: efeito da geometria da amostra na cinética de secagem e na mitigação das emissões de CO2. J. Ciênc. e Tecnol. Alim. 52, 1383–1393 (2015).
Badaoui, O., Hanini, S., Djebli, A., Haddad, B. & Benhamou, A. Experimental and modelling study of tomato pomace waste drying in a new solar greenhouse: Evaluation of new drying models. Renew. Energy 133, 144–155 (2019).
Mohsen, H. A., El-Rahmam, A. A. & Hassan, H. E. Drying of tomato fruits using solar energy. Int. J. Agric. Eng. 21, 204–215 (2019).
César, L. V. E., Lilia, C. M. A., Octavio, G. V., Isaac, P. F. & Rogelio, B. O. Thermal performance of a passive, mixed-type solar dryer for tomato slices (Solanum lycopersicum). Renew. Energy 147, 845–855 (2020).
Kingsly, A. R. P., Singh, R., Goyal, R. K. & Singh, D. B. Thin-layer drying behavior of organically produced tomato. Am. J. Food Tech. 2, 71–78 (2007).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959).
Silva, F. A. S. A. & Azevedo, C. A. V. Versão do programa computacional Assistat para o sistema operacional Windows. Rev. Bras. Prod. Agroindustriais 4, 71–78 (2002).
Klunklin, W. & Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 6, e56 (2017).
Azeez, L., Adebisi, S. A., Oyedeji, A. O., Adetoro, R. O. & Tijani, K. O. Bioactive compounds’ contents, drying kinetics and mathematical modelling of tomato slices influenced by drying temperatures and time. J. Saudi Soc. 10, 120–126 (2019).
Correia, A. F., Loro, K. A. C., Zanatta, S., Spoto, M. H. F. & Vieira, T. M. F. S. Effect of temperature, time, and material thickness on the dehydration process of tomato. Int. J. Food Sci. 1, e970724 (2015).
Eswara, A. R. & Ramakrishnarao, M. Solar energy in food processing—A critical appraisal. J. Food Sci. Technol. 50, 209–227 (2013).
Castillo, C. P., Silva, F. B. & Lavalle, C. An assessment of the regional potential for solar power generation in EU-28. Energy Policy 88, 86–99 (2016).
Tampakis, G., Tsantopoulos, G. & Arabatzis, I. R. Citizens’ views on various forms of energy and their contribution to the environment. Renew. Sust. Energ. Rev. 20, 473–482 (2013).
Tsantopoulos, G. & Arabatzis, T. G. Stilianos Public attitudes towards photovoltaic developments: Case study from Greece. Energy Policy 71, 94–106 (2014).
Tiwari, R. B. Application of osmo-air dehydration for processing of tropical frepical fruits in rural areas. Indian Food Ind. 24, 62–69 (2005).
Goula, A. M. & Adamopoulos, K. G. Retention of ascorbic acid during drying of tomato halves and tomato pulp. Drying Technol. 24, 57–64 (2006).
McAlpine, R. D., Cocivera, M. & Chen, H. Photooxidation and reduction of ascorbic acid atudied by E.S.R. Can. J. Chem. 51, 1682–1686 (1973).
Santos, P. H. S. & Silva, M. A. Retention of vitamin C in drying processes of fruits and vegetables—A review. Drying Technol. 26, 1421–1437 (2008).
Santos-Sánchez, N. F., Valadez-Blanco, R., Gómez-Gómez, M. S., Pérez-Herrera, A. & Salas-Coronado, R. Effect of rotating tray drying on antioxidant components, color and rehydration ratio of tomato saladette slices. LWT Food Sci. Technol. 46, 298–304 (2012).
Yadav, A. K. & Singh, S. V. Y. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 51, 1654–1673 (2014).
Gunhan, T., Demir, V., Hancioglu, E. & Hepbasli, A. Mathematical modeling of drying of bay leaves. Energy Convers. Manag. 46, 1667–1679 (2005).
Sacilik, K. & Unal, G. Dehydration characteristics of kastomonu garlic slices. Biosyst. Eng. 92, 207–215 (2005).
Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos 1020 (Instituto Adolfo Lutz, 2008).
Acknowledgements
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Programa de Pós-graduação em Fitotecnia da Universidade Federal de Viçosa for financial support.
Author information
Authors and Affiliations
Contributions
A.S.S. and W.S.R. designed the research; A.G.F.S., A.M.N., M.A.R.L. and W.S.R. performed the experiments; O.S.S., R.M.F.F. P.O.G. and P.A.S. performed statistical analysis, give reagents and laboratory support. W.S.R., F.B.C., S.M.S., F.L.F., P.A.S. and A.J.M.Q. wrote the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Souto Ribeiro, W., Sant’Ana Silva, A., Ferreira da Silva, Á.G. et al. Handmade solar dryer: an environmentally and economically viable alternative for small and medium producers. Sci Rep 11, 17177 (2021). https://doi.org/10.1038/s41598-021-94353-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-94353-8
- Springer Nature Limited