Abstract
Novel heterotrophic bacterial strains—Bzr02 and Str21, effective in nitrogen transformation, were isolated from sequential sedimentation-biofiltration systems (SSBSs). Bzr02, identified as Citrobacter freundii, removed up to 99.0% of N–NH4 and 70.2% of N–NO3, while Str21, identified as Pseudomonas mandelii, removed up to 98.9% of N–NH4 and 87.7% of N–NO3. The key functional genes napA/narG and hao were detected for Bzr02, confirming its ability to reduce nitrate to nitrite and remove hydroxylamine. Str21 was detected with the genes narG, nirS, norB and nosZ, confirming its potential for complete denitrification process. Nitrogen total balance experiments determined that Bzr02 and Str21 incorporated nitrogen into cell biomass (up to 94.7% and 74.7%, respectively), suggesting that nitrogen assimilation was also an important process occurring simultaneously with denitrification. Based on these results, both strains are suitable candidates for improving nutrient removal efficiencies in nature-based solutions such as SSBSs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The excessive inflow of nitrogen compounds has been a serious problem for water bodies in urban areas, including rivers and ponds. High concentrations of NH4+, NO3− and NO2− contribute to the occurrence of favourable conditions for the proliferation of phytoplankton, including cyanobacteria, which consequently affect aquatic and human health with the production of toxins, the decrease of light penetration and the depletion of oxygen in the pelagic zone1,2,3. To address the above-mentioned problem in urban polluted rivers, sequential sedimentation-biofiltration systems (SSBSs) have been implemented. These systems are designed according to the principles of ecohydrology to enhance the capacity of natural systems to remove environmental pollutants and are considered as nature-based solutions (NBS)4,5. These eco-friendly systems use a combination of natural processes for water treatment, i.e., sedimentation of solids, absorption of phosphorus, reduction of excessive nitrogen compounds by stimulating denitrification and nitrification processes and phytoremediation. SSBSs are constructed upstream of ponds or reservoirs to reduce anthropogenic eutrophication and, among others, the development of harmful algal blooms including toxic cyanobacteria. These systems have been observed to remove nitrogen compounds up to 59.8% of NH4+, 55% of NO2−, 91.3% of NO3− and 56.9% of total nitrogen (TN)6,7,8,9. The protection of urban ponds is needed because they regulate water flow and soil erosion during storms, increase the water retention, provide humidity, promote plant evapotranspiration and influence the cooling of urban areas. Moreover, urban ponds also offer aesthetic value, environmental education and recreational opportunities10,11,12.
The important elements for the effective functioning of SSBSs are the structure and metabolic activity of microorganism inhabiting sediments. Microbial communities, with special consideration on bacteria, have been recently studied in working SSBSs9. The significant positive correlations observed between the measured concentration of nutrients (NO3− and NH4+) and the abundance of bacterial genes involved in nitrification and denitrification processes indicated that bacterial communities have played an important role in nitrogen transformations. Therefore, in the present study we focused on the characteristics of isolated bacterial strains capable of nitrogen removal.
The nitrification involves two consecutive reactions (NH4+ → NO2− → NO3−), and it has been studied in different autotrophic strains: (i) the first reaction was described in ammonia oxidizing bacteria (AOB), in the genera Nitrosomonas, Nitrosospira (β-Proteobacteria) and Nitrosococcus (ϒ-Proteobacteria)13,14; while (ii) the second reaction in nitrite oxidizing bacteria (NOB), in the genera Nitrobacter (α-proteobacteria), Nitrococcus (ϒ-Proteobacteria) and Nitrospina15. Nitrification also occurs in direct oxidation of NH4+ → NO3− (complete ammonium oxidation, COMAMMOX) by autotrophic strains of Nitrospira spp. (Class Nitrospirae)16,17. Moreover, nitrification via the hydroxylamine (NH2OH) pathway, which is an intermediary product between the first nitrification reaction (ammonia oxidation to hydroxylamine), has also been described for Nitrosomonas18 and heterotrophic strains of Acinetobacter19, Janthinobacterium20, Alcaligenes21, Enterobacter22, and Pseudomonas23,24,25. Denitrification—a dissimilatory nitrate reduction (DNR) pathway—involves four cascade reactions for the transformation of NO3− → NO2− → NO → N2O → N2, which was initially described for heterotrophic facultative anaerobic bacterial strains26,27. More recently, research has been focused in the identification of aerobic denitrifying strains that can perform parallel nitrification due to their potential utilization in waste water treatment plants (WWTPs) for the complete removal of nitrogen compounds. Several strains have been isolated and reported to perform simultaneous nitrification–denitrification (SNdN), with the most common genera represented by Acinetobacter, Agrobacterium, Alcaligenes, Bacillus, Klebsiella, Enterobacter and Pseudomonas28.
The majority of the above described nitrogen transforming bacteria have been isolated from sewage in WWTPs, constructed wetlands (CWs) or biofilm formations in experimental bioreactors28. To our knowledge, the bacteria carrying out nitrogen transformation processes have not yet been isolated and characterized within the SSBSs. Additionally, there is a limited number of studies discussing the nitrogen balance, most of which were in controlled experiments for selected bacterial strains, in order to confirm their preferred metabolic pathways29,30,31,32,33.
Therefore, the present study aimed to isolate and characterize heterotrophic bacterial strains that naturally occur in SSBSs, which are responsible for nitrogen transformation in nitrification and denitrification processes. To reach the objective, culturable bacteria were isolated from sediments, nitrogen transformation pathways were determined, and nitrogen balance was described. Additionally, the preference of the strains to perform nitrogen assimilatory over dissimilatory transformation processes was also investigated. Our results were compared with the nitrogen removal efficiency of other published isolated bacterial strains and discussed in the context of biotechnological potential of selected strains to improve the nutrient removal efficiency in NBS technologies.
Results and discussion
Selection and identification of potential nitrogen transforming bacteria
Initial screening of bacteria capable of nitrogen utilization
Ten bacterial strains were selected for their ability to transform nitrogen compounds and were summarized in Table 1. All mentioned strains were able to utilize NO3− in Giltay denitrifying medium (GiDM). Seven strains (Str21, Bzr07, Sok01, Sok03, Sok06, Sok20 and Sok41) presented no accumulation of NO2−, suggesting that it was further reduced by bacteria (Table 1). In contrast, three strains (Bzr02, Str01 and Sok05), only transformed NO3− to NO2−, which was then accumulated in the medium with no further utilization (Table 1).
In turn, seven among 10 selected strains (Str21, Bzr02, Bzr07, Str01, Sok03, Sok05 and Sok41) were able to utilize NH4+ on various nitrifying media with different carbon sources (Table 1). The most efficient removal of NH4+ was found in nitrifying medium containing glucose—GNM (up to 48 h for the strains Str21, Bzr02, Bzr07, Sok05 and Sok41; Table 1).
Taxonomic and phylogenetic characteristics
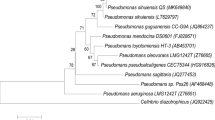
Taxonomical characteristics of selected bacterial isolates, based on the 16 s rRNA, were presented in Table 1, and their phylogenetic relationships were described in Fig. 1. The sequence homology revealed that the studied bacteria belong to significantly different taxonomical groups (Supplementary Table S1). Seven of them were clustered within the phylum Proteobacteria but different bacterial families: (i) the strains Str21, Bzr07 and Sok03, within the family Pseudomonadaceae, presented high similarity with Pseudomonas mandelii (99.55%), P. migulae (99.83%) and P. guineae (99.45%), respectively, (ii) the Sok01 was similar to Hydrogenophaga taeniospiralis (99.32%) and the Sok41 to Acidovorax radicis (99.29%), both strains within the family Comamonadaceae, (iii) the Bzr02 was similar to Citrobacter freundii (99.39%), which belongs to the family Enterobacteriaceae, and (iv) the strain Sok20 to Janthinobacterium lividum (99.34%), which belongs to the family Oxalobacteriaceae (Fig. 1). Furthermore, the strains Str01 and Sok06, within the phylum Firmicutes, presented high similarity to Bacillus simplex (98.36%) and B. aereus (99.63%), respectively, and the strain Sok05 to Kocuria rosea (99.08%) in the phylum Actinobacteria (Fig. 1).
Neighbour-joining phylogenetic tree construction for the nitrogen transforming bacteria isolated in SSBSs. The tree was constructed using the 16S rRNA sequences obtained from GenBank (accession number inside the brackets). The bar under the graph represents the nucleotide substitutions per position. The sequence of Microcystis aeruginosa was used as an outgroup to cluster the representative strains in the phylum Proteobacteria, and the sequence of Methanimicrococcus blatticola PA (Archaea) as an outgroup to cluster the different bacteria phyla.
Proposed metabolic pathways for nitrogen transformation
Possible bacterial metabolic pathways for nitrogen transformation were described based on the amplification of key functional genes involved in the nitrogen cycling process (Table 1 and supplementary Fig S2). The strains Str01 and Sok05 were considered to be nitrate reducers, since NO2− was accumulated in GiDM (Table 1). The above suggestion was supported with the detection of the narG gene (respiratory nitrate reductase), which is involved in the reduction of NO3− → NO2− in anaerobic conditions (Table 1 and supplementary Fig S2). The strains Sok01, Sok06, and Sok20 were considered to be facultative anaerobic denitrifiers, since they were able to continue the reduction of NO3− to gas in GiDM, but could not utilize NH4+ in any of the nitrifying media in aerobic conditions (Table 1 and supplementary Fig S2). In contrast, the strains Sok41, Sok03 and Bzr07 were considered to be facultative anaerobic denitrifiers that could also utilize NH4+ in aerobic conditions (Table 1). All six facultative anaerobic denitrifiers (Sok01, Sok41, Sok20, Sok06, Sok03 and Bzr07) presented the nosZ gene (Table 1), which is involved in the last step of denitrification, and therefore, suggested that they performed complete reduction of NO3− → N2.
Bzr02 (Citrobacter freundii) and Str21 (Pseudomonas mandelii), isolated from the Bzr-SSBS and Str-SSBS, respectively, presented the best results during the screening experiments on transformation of nitrogen compounds. Both strains were able to grow and remove NO3− and NH4+ in a lower time of incubation in different culture media, and were observed with the highest number of studied key functional genes involved in assimilation, nitrification or denitrification processes (Table 1). Moreover, the Bzr02 was the only strain capable to utilize NH4+ with the presence of hydroxylamine in GNM, suggesting that hydroxylamine could be an intermediary product in the nitrification process. Therefore, Bz02 and Str21 were selected for further quantitative experiments in nitrogen transformation assays.
Nitrogen transforming processes—strains Bzr02 and Str21
Ammonium transformation in nitrifying medium
Bzr02 and Str21 were cultivated in nitrifying medium (NM) under aerobic conditions, and their growth and utilization of N–NH4 were followed for 24 h (Fig. 2a,b). The average and maximum removal rates of N–NH4 for both strains were described in Table 2. Both strains were able to utilize N–NH4 as a sole nitrogen source. Bzr02 presented a 4 h lag phase with minimal growth at the beginning of the assay (Fig. 2a). The log phase was observed after 4 h of incubation (Fig. 2a), which correlated with the maximum removal rate of N–NH4 (16.17 ± 0.97 mg L−1 h−1, Table 2). A stationary phase occurred between 12 and 18 h, however, the strain was able to remove 82.6% of N–NH4 until 14 h of incubation (Fig. 2a). The maximum removal of N–NH4 was observed at 22 h of incubation (99.0 ± 0.2%; Table 2). The average removal rate of N–NH4 was 5.41 ± 0.13 mg L−1 h−1 (Table 2), which was significantly higher from other published strains: Alcaligenes denitrificans WY200811 (0.69 mg L−1 h−1)34, Klebsiella pneumonae EGD-HP19-C (2.29 mg L−1 h−1)35, K. pneumonae CF-S9 (4.3 mg L−1 h−1)36 and Enterobacter cloacae CF-S27 (2.22 mg L−1 h−1)22.
Str21 presented a 6 h lag phase, however, utilization of N–NH4 started after 2 h of incubation (Fig. 2b). The maximum removal rate of N–NH4 was observed after 8 h (10.2 ± 0.25 mg L−1 h−1; Table 2), which continued until almost complete depletion under 16 h of incubation (98.9 ± 0.6%; Table 2). The average removal rate of N–NH4 was 7.21 ± 0.12 mg L−1 h−1 (Table 2), which was significantly higher from other strains in the family Pseudomonadaceae: Pseudomonas sp. JQ-H3 (2.7 mg L−1 h−1)33, P. stutzeri YZN-001 (5.53 mg L−1 h−1)37, P. stutzeri AD1 (3.1 mg L−1 h−1)38, P. tolaasii Y-11 (2.04 mg L−1 h−1)39, and similar to P. putida Y-9 (7.4 mg L−1 h−1)24 and P. stutzeri T13 (7.09 mg L−1 h−1)30.
The concentrations of N–NO2 and N–NO3 were insignificant through the complete assays for Br02 and Str21, and therefore no nitrification products were observed to occur (Fig. 2a,b, respectively). Similar results were published for all the above-mentioned strains and other genera, i.e., Bacillus SB140 and Acinetobacter sp. SYF2641.
Nitrate transformation in denitrifying medium
Bzr02 and Str21 were cultivated in denitrifying medium (DM) under aerobic conditions, and their growth and utilization of N–NO3 were followed for 32 h (Fig. 2c,d). The average and maximum removal rates of N–NO3 for both strains were described in Table 2. Bzr02 was not able to grow and transform N–NO3 when it was added to the medium as the sole nitrogen source. Similar results were reported for Acinetobacter calcoaceticus HNR19, and it was proposed that the strain was sensitive to an initial high concentration of N–NO3 (40 mg L−1) in denitrifying medium. The above observation suggests that Bzr02 was also sensitive to the high initial concentration of N–NO3 (100 mg L−1) in DM.
On the contrary, Str21 was able to utilize N–NO3 as a sole nitrogen source in DM (Fig. 2d). After a 6 h lag phase, the strain began to grow until the log phase was observed from 12 h of incubation (Fig. 2d). The maximum removal rate of N–NO3 was 6.66 ± 0.27 mg L−1 h−1 (Table 2). Str21 removed N–NO3 to a maximum of 87.7 ± 0.16% during 28 h of incubation. The average removal rate of N–NO3 was 3.89 ± 0.27 mg L−1 h−1, which was significantly higher than other strains in the family Pseudomonadaceae: Pseudomonas sp. JQ-H3 (1.78 mg L−1 h−1)33, P. tolaasii Y-11 (2.04 mg L−1 h−1)39 and P. stutzeri AD1 (1.98 mg L−1 h−1)38, and other bacteria: Klebsiella pneumonae CF-S9 (2.2 mg L−1 h−1)36 and Bacillus cereus GS-5 (2.7 mg L−1 h−1)31. The formation of N–NO2 was detected in DM, which was a result from the oxidation of N–NO3. A maximum concentration of N–NO2 was observed at 16 h (18.66 ± 1.68 mg L−1 h−1) and decreased until it was completely utilized in 20 h of incubation (Fig. 2d). However, N–NO3 was not completely removed at the end of the assay (13.54 ± 0.60 mg L−1 in 32 h; Fig. 2d), suggesting that the denitrification process by Str21 was partially inhibited by the aerobic condition.
Ammonium and nitrate transformation in simultaneous nitrifying-denitrifying medium
Bzr02 and Str21 were cultivated in simultaneous nitrification–denitrification medium (SNDM) under aerobic conditions, and their growth and utilization of N–NH4 and N–NO3 were followed for 36 h (Fig. 2e,f). The average and maximum removal rates of N–NH4 and N–NO3 for both strains were described in Table 2. Bzr02 was able to remove 94.1 ± 1.3% of N–NH4 and 70.2 ± 3.6% of N–NO3 after 36 h of incubation (Table 2). A total of 16.80 ± 1.24 mg L−1 of N–NO3 was accumulated in SNDM after 24 h of incubation, with no further utilization by Bzr02 (Fig. 2e). The formation of N–NO2 was detected in SNDM, which was a result from the N–NO3 oxidation. The concentration of N–NO2 increased to a maximum of 20.02 ± 1.15 mg L−1 after 6 h, however, 9.48 ± 0.99 mg L−1 of N–NO2 remained accumulated in SNDM from 24 h of incubation (Fig. 2e). The average removal rate of N–NH4 (5.07 ± 0.09 mg L−1) was significantly higher than N–NO3 (1.44 ± 0.16 mg L−1), which suggests that Bzr02 preferred to utilize N–NH4 in SNDM (Table 2).
Similarly, Str21 was able to remove a higher amount of N–NH4 (95.6 ± 1.5%) than of N–NO3 (75.4 ± 2.6%) (Table 2), however, the utilization of N–NO3 was not significant until after 12 h of incubation (Fig. 2f). The formation of N–NO2 was detected in SNDM, which was a result from the reduction of N–NO3, however, some differences were observed when Str21 was compared to Bzr02: (i) the maximum concentration of N–NO2 was lower (12.19 ± 0.77 mg L−1) and it was observed after 12 h of incubation, and (ii) N–NO2 was almost completely utilized after 24 h of incubation (Fig. 2f). Moreover, a lower concentration of N–NO3 (12.07 ± 0.91 mg L−1) was accumulated after 24 h of incubation (Fig. 2f), when compared to Bzr02. The average removal rate of N–NH4 (3.35 ± 0.04 mg L−1) was higher than of N–NO3 (2.29 ± 0.22 mg L−1), which also suggested that Str21 preferred to utilize N–NH4 in SNDM (Table 2). Similar results for other strains, where the removal rate of N–NH4 was faster than of N–NO3, have been described for Klebsiella pneumoniae CF-S9 (3.3 and 2.6 mg L−1, respectively)36 and Pseudomonas tolaasii Y-11 (2.13 and 0.52 mg L−1, respectively)39. However, other strains have been found to remove N–NO3 faster than of N–NH4, i.e.: Bacillus cereus GS-5 (2.94 and 2.69 mg L−1, respectively)31 and Janthinobacterium svalbardensis F19 (1.19 and 0.62 mg L−1, respectively)20.
Hydroxylamine influence in the ammonium transformation by the strain Bzr02 in nitrifying medium
Bzr02 was cultivated in NM supplemented with NH2OH in different concentrations, and the growth and utilization of N–NH4 and NH2OH were followed for 30 h (Fig. 3). The experiment was performed to corroborate the nitrification process by Bzr02 since the oxidized products (N–NO2 and N–NO3) were not observed during incubation with N–NH4 as the sole nitrogen source. Bzr02 presented a log phase after 4 h of incubation in the control medium without hydroxylamine, which also corresponded with the maximum removal rate of N–NH4 (23.80 ± 0.84 mg L−1, Fig. 3a). When NH2OH was added to 10 mg L−1 in NM after 4 h of incubation, the log phase of Bzr02 was observed until after 6 h of incubation (Fig. 3b). The maximum removal of N–NH4 was 8.03 ± 0.60 mg L−1 h−1 during the addition of 10 mg L−1 NH2OH, which was significantly lower when compared to the control (Fig. 3a,b). When 20 and 50 mg L−1 of NH2OH were added to NM after 4 h of incubation, the log phase was observed after 8 and 12 h of incubation, respectively (Fig. 3c,d). Moreover, the maximum removal rates of N–NH4 were 2.05 ± 0.90 and 0.86 ± 0.67 mg L−1, respectively, which were significantly lower when compared to the control (Fig. 3a,c,d). These results suggested that NH2OH, in high concentrations, significantly inhibited the growth of Bzr02, and in consequence, the removal of N–NH4. However, the transformation of N–NH4 was resumed when significant amount of NH2OH was removed by Bzr02. Furthermore, N–NO2 was not detected as product from the oxidation of NH2OH (Fig. 3b,c,d). Similar results in other strains have been reported for: Enterobacter cloacae CF-S2722, Alcaligenes faecalis21, and Thiosphaera pantotropha (formerly Paracoccus denitrificans)42.
Confirmation of bacterial nitrogen transforming pathways
The nitrogen balance during the transformation processes for Bzr02 and Str21 was calculated and presented in Table 3. The detection of key functional genes involved in nitrogen cycling was also summarized in Fig. 4, and the results were used to corroborate their nitrogen transforming pathways. For the ammonium transformation assay using NM, Bzr02 and Str21 utilized almost complete nitrogen and incorporated it into their cell biomass (94.7 ± 1.4 and 94.3 ± 2.0 mg L−1, respectively) (Table 3). Only a small fraction of nitrogen was lost for Bzr02 and Str21 (0.75 and 1.25 mg L−1, respectively; Table 3), suggesting that it was assimilated when N–NH4 was given as the sole nitrogen source. The nitrification process seemed not to have occurred, especially because the products from the oxidation of N–NH4 (N–NO2 and N–NO3) were not significantly detected through the entire assays (Fig. 2a,b).
The nitrification process seemed to have occurred for Bzr02 when NH2OH was added to NM, which is another intermediary product during the first reaction of nitrification (NH4+ → [NH2OH] → NO2−). Bzr02 removed NH2OH from the NM while there was no significant bacterial growth or removal of N–NH4 (Fig. 3), suggesting that NH2OH was oxidized (nitrification) rather than assimilated. Additionally, the detection of the gene hao (hydroxylamine oxidoreductase, HAO) supports the nitrification process by Bzr02 (Fig. 4a); however, the concentration of N–NO2 -the product from NH2OH oxidation- was not significantly detected in all experiments (Fig. 3). These results are different from other strains that produced NO2− from the oxidation of NH2OH, i.e., Nitrosomonas europaea18 and Pseudomonas PB1623. Other studies suggest that the enzyme HAO also catalyzes a different reaction where NH2OH is transformed to nitric oxide (NO) in Alcaligenes faecalis No.443 or reduced to N2 in A. facecalis44,45 and Acinetobacter calcoaceticus HNR19. The above results suggests that Bzr02 could have reduced NH2OH to a nitrogen gas (Fig. 4b), rather than being oxidized to NO2− in the process of nitrification.
In the nitrate transformation assay in DM, only Str21 was able to grow and utilize N–NO3 as the only nitrogen source (Fig. 2d). The initial nitrogen content in DM (105.0 ± 1.2 mg L−1) was utilized by Str21 until 11.40 ± 0.62 mg L−1 remained in the medium at the end of the experiment (Table 3). The majority of nitrogen was detected in the cell biomass of Str21 (68.2 ± 1.2 mg L−1) and 25.4 mg L−1 was estimated to be lost (Table 3). The above results suggested that Str21 transformed 89.1% of total nitrogen, from which 65.0% was assimilated and the remaining 24.1% was probably lost as a nitrogen gaseous form in the process of denitrification. Str21 was found to contain the gene nasA (assimilatory nitrate reductase, NAS; Fig. 4b) that confirmed the process of assimilatory NO3− reduction to NO2−, and subsequently to NH4+. The gene nasA is involved in the synthesis of cell biomass46 (Fig. 4d). Moreover, Str21 was found to contain all studied genes involved in the process of denitrification (narG, nirS, norB and nosZ; Fig. 4b), suggesting that it is a facultative anaerobic denitrifier (Fig. 4d). The denitrification activity in anaerobic conditions for a similar strain—Pseudomonas mandelii strain PD30—has already been described with the gene expression of nirS and norB47,48. In the above research, it was argued that the gene expression was significantly inhibited in aerobic conditions, and therefore, it was concluded that P. mandelii PD30 performed denitrification in exclusive anaerobic conditions. In contrast, for other Pseudomonas strains, i.e., P. stutzeri YG-2429, P. sp. JQ-H333 and P. mendocina GL649, the removal of nitrogen content as gas was up to 46.0—74.4% in aerobic conditions, suggesting that there was a similar preference for nitrogen denitrification and assimilation, and sometimes, denitrification could be significantly higher. The detection of the gene napA, rather than the gene narG, was probably the most important factor influencing aerobic denitrification in the above three mentioned strains. In the case of Str21, only the gene narG was detected (Fig. 4b), however, the process of denitrification was not completely inhibited when it was incubated in DM, during aerobic conditions (Fig. 2d). We believe that the aerobic conditions in the media could have partially influenced the reduction of N–NO3 to subsequent forms of nitrogen for Str21, resulting in an evident preference to assimilate nitrogen rather than performing denitrification.
For the N–NH4 and N–NO3 transformation assays in SNDM, Bzr02 and Str21 were able to utilize N–NH4 and N–NO3 in aerobic conditions. For Bzr02, a total of 68.3 ± 1.2 mg L−1 of nitrogen was found in the cell biomass and 29.2 ± 2.1 mg L−1 remained in the medium (Table 3). The remaining nitrogen was mostly from N–NO3 and the accumulation of its reduction to N–NO2, that were not completely depleted by Bzr02 (Fig. 2e). The above results could be associated from the difficulty of Bzr02 to reduce NO3− to NO2− in aerobic conditions, as it was explained when it was incubated with higher N–NO3 concentrations in DM (Fig. 2c). Despite the above, only 1.4 mg L−1 of nitrogen was lost (Table 3), suggesting that the dominant metabolic pathway presented by Bzr02 was nitrogen assimilation (Fig. 4c). The gene nasA was not detected for Bzr02, indicating that N–NO3 was rather reduced by a dissimilatory nitrate reductase (NAR or NAP), and then, part of N–NO2 was incorporated into the cell biomass through the process of assimilatory nitrite reduction46,50 (Fig. 4c).
Str21 presented 74.3 ± 1.6 mg L−1 of nitrogen in the cell biomass and 14.6 ± 0.2 mg L−1 remained in the medium with no further utilization (Table 3). A significant concentration of nitrogen (12.6 mg L−1) was lost at the end of incubation for Str21 (Table 3) in comparisson to Bzr02, suggesting that the process of denitrification took place. Moreover, the N–NO2—produced from the reduction of N–NO3—was not accumulated in Strs21 as it was observed for Bzr02 (Fig. 2e,f), also supporting that N–NO2 was further reduced into nitrogen gaseous forms in the process of denitrification. Similarly as it was described during the experiment in DM, the detection of nasA suggested that N–NO3 was incorporated into the cell biomass through the process of assimilatory nitrate reduction, and the detection of all four nitrogen reductase genes (narG, nirS, norB and nosZ) supported that the lost nitrogen escaped as nitrogen gas during dissimilatory nitrate reduction (denitrification; Fig. 4d). The low denitrification activity by Str21 in SNDM was also the influece of the aerobic conditions, which could be appreciated for the long lag phase were N–NO3 was not significantly utilized at the first 12 h of incubation (Fig. 2d).
Conclusion
Bzr02 and Str21 (isolated from SSBSs sediments), identified as Citrobacter freundii and Pseudomonas mandelii, respectively, were found to have potential applications in nature-based solutions to enhance nitrogen compounds removal, such as SSBSs. Nitrate reduction to nitrite in the denitrification process was found for both strains. Str21 seemed to be a facultative anaerobic denitrifier, and therefore, could participate in nitrogen cycling in SSBSs sediments, where oxygen limiting conditions occur. In turn, Bzr02 and Str21 were observed to significantly assimilate N–NH4 and N–NO3 into their cell biomass in aerobic conditions, which could subsequently help to improve the efficiency of SSBSs in the nitrogen removal with its sequestration in the sediments. Therefore the application of both strains could be recommended for sedimentation zones, where the release of nitrogen would be controlled by: i) other decomposing microbial communities dwelling in the sediments, and ii) the periodical removal of sediments to maintain the proper operation of SSBSs.
Materials and methods
Samples collection and isolation of bacteria
Sediment samples were collected from the sedimentation zone (August 2018) in three SSBSs constructed for different urban rivers: (i) the River Sokołówka (Sok-SSBS) and (ii) the River Bzura (Bzr-SSBS) in the city of Łódź, and (iii) the River Struga Gnieźnieńska (Str-SSBS) in the city of Gniezno, Poland.9 Complete description of structure and function for Bzr-SSBS is detailed in Szulc et al.51 and Jurczak et al.8, and for Sok-SSBS and Str-SSBS in Font-Nájera et al.9 Sediment samples were suspended in sterile 0.75% NaCl w/v (10 g of sediment in 90 mL) and shacked for 30 min at 25 °C. Samples were allowed to settle for 15 min and supernatant was used to prepare serial dilutions (1 × 10–1–1 × 10–6) according to Mankiewicz-Boczek et al.52. 100 µL of each dilution was plated on to Soil Extract Agar (SEA), a solid medium according to Hamaki et al.53, and incubated for seven days at 25 °C. For each SSBS, 50 heterotrophic bacterial isolates (150 in total) were randomly streaked out and re-plated on to nutrient agar solid medium (NA, Karl Roth).
Screening of nitrogen transforming bacteria
A total of 150 well-separated bacterial colonies were picked from NA and checked for nitrogen transformation abilities in different culturable media (See also media description in supplementary material):
(i) in Giltay denitrifying medium (GiDM) with high content of NO3− (N: 277 mg L−1) according to Alexander54, at 25 °C. Bacterial ability to reduce NO3−, under oxygen limited condition (Becton Dickinson Gas Pak System), was qualitatively monitored every 12 h with the semi-quantitative test strips QUANTOFIX nitrate/nitrite (Macherey–Nagel) for 7 d. A total of 10 different bacterial strains were able to completely or partially reduce NO3− (denitrification process), and therefore, were selected for further experiments;
(ii) the 10 selected bacterial isolates were incubated in 15 mL glucose nitrifying medium (GNM) described in Pahdi et al.22, with a small modification—KH2PO4 was used instead of NaH2PO4 (0.10 g MgSO4 · 7H2O, 3.84 g K2HPO4, 1.5 g KH2PO4, 0.802 g NH4Cl [N: 212 mg L−1], 5.3 g glucose C6H12O6 [C: 2120 mg L−1]), and 2 mL of trace elements were added per 1000 mL of GNM, final pH was 7.2, shacked at 150 rpm and incubated at 25 °C. The trace element solution was prepared according to Pahdi et al.22. The effect of different carbon sources was also screened with changes to the nitrifying medium where glucose was replace by: (i) sodium succinate (11.9 g)—succinate nitrifying medium (SNM), (ii) sodium acetate (10.0 g) – acetate nitrifying medium (ANM), and (iii) sodium citrate (8.65 g)—citrate nitrifying medium (CNM). The carbon and nitrogen ratio was kept constant (C:N = 10) in all used media.
Bacteria were also tested for the transformation of NH4+ under the presence of hydroxylamine in GNM. Cultures were grown in GNM for 6 h and spiked with high concentration of hydroxylamine (100 mg L−1 final concentration) according to Padhi et al.22. For the screening purpose, their ability to transform NH4+ was qualitatively monitored with the semi-quantitative test strips QUANTOFIX ammonium (Macherey–Nagel), every 12 h during 7 d.
DNA isolation and detection of key functional genes involved in nitrogen transformation processes
DNA was isolated from overnight bacterial cultures (Luria Bertani broth, LB) according to the specification in Wizard Genomic DNA purification kit (Promega, Madison, Wisconsin). The 10 previously selected bacterial strains (Chapter 2.2.) were screened for the presence of key functional genes involved in nitrification (hao22), denitrification (napA38, narG55, nirS56, norB57 and nosZ58) and nitrogen assimilation (nasA59) processes using conventional PCR (Supplementary Table S4). PCR products for the strains Bzr02 and Str21 were purified with the QIAEX II Gel Extraction Kit (Promega, Madison, Wisconsin) and sequenced by Genomed laboratories in Warsaw, Poland (http://www.genomed.pl/). DNA sequences were edited using the software MEGA7 (http://www.megasoftware.net/) and similarity with other published bacterial strains was verified with the nucleotide BLAST tool. Sequences were deposited in the GenBank database with the accession numbers for Str21: nosZ (MW286255), cnorB (MW286256), nirS (MW286257), narG (MW286258), and nasA (MW286259), and for Bzr02: napA (MW286261), hao (MW286262), and narG (MW286263).
Taxonomic characteristics and phylogenetic analysis
The 16S rRNA bacterial molecular marker was amplified for the 10 selected strains, with 27F / 1492R primers according to Lane60. PCR products were processed (purification, sequencing and nucleotide BLAST analysis) similarly as specified for the functional genes in Chapter 2.3. A neighbour-joining phylogenetic tree was constructed for bacteria using the software MEGA7. Bacterial 16S rRNA sequences were deposited in the GenBank database with the accession numbers for Str21 (MW282158), Bzr02 (MW282159), Bzr07 (MW282160), Str01 (MW282161), Sok03 (MW282162), Sok05 (MW282163), Sok41 (MW282164), Sok01 (MW282165), Sok06 (MW282166), and Sok20 (MW282167).
Additional methods to corroborate the taxonomical identification of two strains (Bzr02 and Str21) were described in supplementary material. The strain Bzr02 was incubated on GEN III Biolog MicroPlates with different carbon substrates, according to the manufacturer specifications61, and the taxonomic characteristics of bacterium were determined using the GEN III Biolog database. For Str21, the gene rpoB (coding for the β sub-unit of the RNA bacterial polymerase) was used as a molecular marker, since it has been recommended for the optimal differentiation between Pseudomonas species62. The DNA sequence of the rpoB gene was published in GenBank database for Str21 (MW286260).
Ammonium transformation
Bzr02 and Str21 were cultured overnight in LB at 25 °C and 120 rpm. Cells were harvested by centrifugation (8000 rpm, 10 min, 4 °C), and washed three times with sterile water. Then, each strain was inoculated into the nitrifying medium NM (0.1 final OD600) with adjusted concentrations of NH4+ (N: 100 mg L−1) and glucose (C: 1000 mg L−1), incubation was performed at 25 °C and 150 rpm. Bacterial growth (optical density OD 600 nm) was checked at 2 h intervals using an Eppendorf Biophotometer in a 24 h experiment22. Supernatant was also collected during each interval (13,000 rpm, 10 min, 4 °C) for the measurement of N–NH4, N–NO3, N–NO2 and extracellular TN. The pellet was washed three times with sterile water and used to estimate intracellular TN32.
Nitrate transformation
Bzr02 and Str21 were inoculated into denitrifying medium (DM). The denitrifying media was similar to NM with the use of KNO3 (N: 100 mg L−1 final concentration) as the source of nitrogen. The check of bacterial growth and the collection of samples were performed similarly as explained for the ammonium transformation assays in a 32 h experiment22. The supernatant was used to measure N–NH4, N–NO3, N–NO2 and extracellular TN, and the bacterial pellet for intracellular TN.
Simultaneous ammonium and nitrate transformation
Bzr02 and Str21 were inoculated into the simultaneous nitrifying-denitrifying medium (SNDM). The media was similar to NM with the use of KNO3 and NH4CL (N: 50 mg L−1 each; TN: 100 mg L−1 final concentration) as sources of nitrogen. The check of bacterial growth and the collection of samples was performed similarly as explained for the ammonium transformation assay, in a 50 h experiment22. The supernatant was used to measure N–NH4, N–NO3, N–NO2 and extracellular TN, and the bacterial pellet for intracellular TN.
The impact of hydroxylamine for ammonium transformation
Bzr02 was the only strain capable of growth in the presence of hydroxylamine during the screening experiments (described in Chapter 2.2.). Therefore, in a parallel experiment, the transformation of ammonium by Bzr02 was also investigated with different concentrations of hydroxylamine (0, 10, 20 and 50 mg L−1 as final concentrations) added after 4 h of growth in NM. The bacterial growth and the collection of samples were performed similarly as explained for the ammonium transformation assay, at 0 and 2 h (before the addition of hydroxylamine), and 4, 8, 12, 24 and 30 h of incubation (after the addition of hydroxylamine)22. The supernatant was used to measure N–NH4, N–NO3, N–NO2 and NH2OH concentrations.
Analytical methods
Concentration of nitrogen sources were measured with the Multiskan Sky Microplate Spectrophotometer (Thermo Fisher Scientific) according to standard methods63: (i) N–NH4 by the Nessler’s colorimetric assay, (ii) N–NO3 by the ultraviolet spectrophotometric method, and (iii) N–NO2 by the Griess colorimetric assay. The Hydroxylamine was measured by indirect spectrophotometry64. The TN was calculated with the total Kjeldal reagent set65 as follows: (i) using the supernatant for the extracellular TN, and (ii) reconstitution of the cell pellet with sterile water for intracellular TN32. All measurements were performed in triplicate.
Analysis of data
Nitrogen balance was monitored with the formula:
where NL is the loss of nitrogen at the end of the experiment, the TNFe and TNFi are the final extracellular and intracellular TN, respectively, and the TNIe is the initial extracellular TN (adapted from Fidélis Silva et al.32).
Bacterial removal rates for N–NH4−, N–NO3− and NH2OH (mg L−1 h−1) were estimated as follows:
where Ci and Cf are the initial and final concentration of the nitrogen source, respectively, and the t is the final time of the experiment29.
References
Chahal, M., Shi, Z. & Flury, M. Nutrient leaching and copper speciation in compost-amended bioretention systems. Sci. Total Environ. 556, 302–309. https://doi.org/10.1016/j.scitotenv.2016.02.125 (2016).
Wang, S. et al. Nitrogen removal from urban stormwater runoff by stepped bioretention systems. Ecol. Eng. 106, 340–348. https://doi.org/10.1016/j.ecoleng.2017.05.055 (2017).
Codd, G. A., Meriluoto, J. & Metcalf, J. S. Introduction: Cyanobacteria, Cyanotoxins, Their Human Impact and Risk Management. In Handbook of Cyanobacterial Monitoring and CYanotoxin Analysis (eds Meriluoto, J. et al.) 3–8 (John Wiler & Sons Ltd, 2017).
Zalewski, M. (2012). Blue-green city for compensating global climate change. The Parliament Magazine, 350, 2-
Zalewski, M. et al. Low cost, nature-based solutions for managing aquatic resources: integrating the principles of Ecohydrology and the Circular Economy. Ecohydrol. Hydrobiol. 18, 309–310. https://doi.org/10.1016/j.ecohyd.2018.12.001 (2018).
Negussie, Y. et al. Efficiency analysis of two sequential biofiltration systems in Poland and Ethiopia - the pilot study. Ecohydrol. Hydrobiol.. https://doi.org/10.2478/v10104-012-0028-9 (2012).
Szklarek, S., Wagner, I., Jurczak, T. & Zalewski, M. Sequential Sedimentation-Biofiltration System for the purification of a small urban river (the Sokołówka, Łódź) supplied by stormwater. J. Environ. Manage. 205, 201–208. https://doi.org/10.1016/j.jenvman.2017.09.066 (2018).
Jurczak, T. et al. Comprehensive approach to restoring urban recreational reservoirs. Part 1 - Reduction of nutrient loading through low-cost and highly effective ecohydrological measures. Ecol. Eng. 131, 81–98. https://doi.org/10.1016/j.ecoleng.2019.03.006 (2019).
Font-Nájera, A., Serwecinska, L., Szklarek, S. & Mankiewicz-Boczek, J. Characterization and comparison of microbial communities in sequential sedimentation-biofiltration systems for removal of nutrients in urban rivers. Ecol. Eng. 149, 105796. https://doi.org/10.1016/j.ecoleng.2020.105796 (2020).
Manteghi, G., Lamit, H. & Ossen, D. R. Water bodies and urban microclimate: a revies. Mod. Appl. Sci. 9, 6. https://doi.org/10.5539/mas.v9n6p1 (2015).
Wagner, I. K., & Krauze, K. (2015). How to safely retain stormwater in the city: technical tools Introduction: on-site stormwater management. In T. Bergier, K. Jakub, & W. Iwona, Sustainable Development Applications Journal 5/2015 (Water in the City) (pp. 71–87). Sedzimir Foundation.
Oertli, B., & Parris, K. M. (2019). Review: Toward management of urban ponds for freshwater biodiversity. Ecosphere, 10(7). https://doi.org/https://doi.org/10.1002/ecs2.2810
Kowalchuk, G. A. & Stephen, J. R. Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 55, 485–529. https://doi.org/10.1146/annurev.micro.55.1.485 (2001).
Guo, J. et al. Pathways and organisms involved in ammonia oxidation and nitrous oxide emission. Crit. Rev. Environ. Sci. Technol. 43, 2213–2296. https://doi.org/10.1080/10643389.2012.672072 (2013).
Prosser, J. I. Nitrogen in soils, nitrification. Encycl. Soils Environ.. https://doi.org/10.1016/B0-12-348530-4/00512-9 (2005).
Holger, D. et al. Complete nitrification by Nitrospira bacteria. Nature. 528, 504–509. https://doi.org/10.1038/nature16461 (2015).
Hu, H. W. & He, J. Z. Comammox - a newly discovered nitrification process in the terrestrial nitrogen cycle. Frontiers in soils and sediments 17, 2709–2717. https://doi.org/10.1007/s11368-017-1851-9 (2017).
de Bruijn, P., van de Graaf, A. A., Jetten, M. S., Robertson, L. A. & Kuenen, J. G. Growth of Nitrosomonas europaea on hydroxylamine. FEMS Microbiol. Lett. 125(2–3), 179–184. https://doi.org/10.1016/0378-1097(94)00495-D (1995).
Zhao, B., He, Y. L., Hughes, J. & Zhang, X. F. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Biores. Technol. 101(14), 5194–5200. https://doi.org/10.1016/j.biortech.2010.02.04 (2010).
Chen, Y., Jin, P., Cui, Z. & Xu, t. ,. Identification and Characterization of Janthinobacterium svalbardensis F19, a Novel Low-C/N-Tolerant Denitrifying Bacterium. Appl. Sci. 9(9), 1937. https://doi.org/10.3390/app9091937 (2019).
Otte, S., Schalk, J., Kuenen, J. G. & Jetten, M. S. Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis. Appl. Microbiol. Biotechnol. 51, 255–261. https://doi.org/10.1007/s002530051390 (1999).
Padhi, S. K., Tripathy, S., Mohanty, S. & Maiti, N. K. Aerobic and heterotrophic nitrogen removal by Enterobacter cloacae CF-S27 with efficient utilization of hydroxylamine. Biores. Technol. 232, 285–296. https://doi.org/10.1016/j.biortech.2017.02.049 (2017).
Jetten, M. S., de Bruijn, P. & Kuenen, J. G. Hydroxylamine metabolism in Pseudomonas PB16: involvement of a novel hydroxylamine oxidoreductase. Antonie Van Leeuwenhoek 71, 69–74. https://doi.org/10.1023/a:1000145617904 (1997).
Huang, X. et al. Ammonium transformed into nitrous oxide via nitric oxide by Pseudomonas putida Y-9 under aerobic conditions without hydroxylamine as intermediate. Biores. Technol. 277, 87–93. https://doi.org/10.1016/j.biortech.2019.01.040 (2019).
Yang, L. et al. Simultaneous removal of nitrogen and phosphorous by heterotrophic nitrification-aerobic denitrification of a metal resistant bacterium Pseudomonas putida strain NP5. Biores. Technol.. https://doi.org/10.1016/j.biortech.2019.121360 (2019).
Tiedje, J. M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In Environmental Microbiology of Anaerobes (ed. Zehnder, A. J.) 179–244 (John Wiley and Sons, 1988).
Devol, A. H. Denitrification, anammox, and N(2) production in marine sediments. Ann. Rev. Mar. Sci. 7, 403–423. https://doi.org/10.1146/annurev-marine-010213-135040 (2015).
Holmes, D. E., Dang, Y. & Smith, J. A. Chapter Four - Nitrogen cycling during wastewater treatment. Adv. Appl. Microbiol. 106, 113–192. https://doi.org/10.1016/bs.aambs.2018.10.003 (2019).
Li, C. et al. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a phosphate accumulating bacterium Pseudomonas stutzeri YG-24. Biores. Technol. 182, 18–25. https://doi.org/10.1016/j.biortech.2015.01.100 (2015).
Sun, Y. et al. Ammonium assimilation: An important accessory during aerobic denitrification of Pseudomonas stutzeri T13. Biores. Technol. 234, 264–272. https://doi.org/10.1016/j.biortech.2017.03.053 (2017).
Rout, P. R., Bhunia, P. & Dash, R. R. Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorus removal. Biores. Technol. 244, 484–495. https://doi.org/10.1016/j.biortech.2017.07.186 (2017).
Fidélis Silva, L. C. et al. Heterotrophic nitrifying/aerobic denitrifying bacteria: Ammonium removal under different physical-chemical conditions and molecular characterization. J. Environ. Manage.. https://doi.org/10.1016/j.jenvman.2019.109294 (2019).
Wang, X., Wang, W., Zhang, Y., Sun, Z., Zhang, J., Chen, G., & Li, J. (2019). Simultaneous nitrification and denitrification by a novel isolated Pseudomonas sp. JQ-H3 using polycaprolactone as carbon surce. Bioresource Technology, 288, 121506. https://doi.org/https://doi.org/10.1016/j.biortech.2019.121506
Wen, Y. & Wei, C.-H. Heterotrophic nitrification and aerobic denitrification bacterium isolated from anaerobic/anoxic/oxic treatment system. Afr. J. Biotech. 10(36), 6985–6990. https://doi.org/10.5897/AJB10.1855 (2011).
Pal, R. R., Khardenavis, A. A. & Purohit, H. J. Identification and monitoring of nitrification and denitrification genes in Klebsiella pneumoniae EGD-HP19-C for its ability to perform heterotrophic nitrification and aerobic denitrification. Funct. Integr. Genomics 15, 63–76. https://doi.org/10.1007/s10142-014-0406-z (2015).
Padhi, S. K. et al. Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int. Biodeterior. Biodegradation 78, 67–73. https://doi.org/10.1016/j.ibiod.2013.01.001 (2013).
Zhang, J., Wu, P., Hao, B. & Yu, Z. Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Biores. Technol. 102, 9866–9869. https://doi.org/10.1016/j.biortech.2011.07.118 (2011).
Qing, H. et al. Novel heterotrophic nitrogen removal and assimilation characteristic of the newly isolated bacterium Pseudomonas stutzeri AD-1. J. Biosci. Bioeng. 3, 339–345. https://doi.org/10.1016/j.jbiosc.2018.03.010 (2018).
He, T., Li, Z., Sun, Q., Xu, Y., & Ye, Q. (2016). Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresource Technology(200), 493–499. doi: https://doi.org/https://doi.org/10.1016/j.biortech.2015.10.064
Sheela, B., Beebi, S. K., & Rao, O. Y. (2015). Simultaneous Nitrification and Denitrification of Ammonical Wastewaters Using Bacillus Species SB1 Isolated from Domestic Sewage. International Journal of Life Sciences Biotechnology and Pharma Research, 4(1), 17–21. https://doi.org/https://doi.org/10.12720/ijlbpr.4.1.17-21
Su, J.-F. et al. Heterotrophic nitrification and aerobic denitrification at low nutrient conditions by a newly isolated bacterium, Acinetobacter sp. SYF26. Microbiology 161, 829–837. https://doi.org/10.1099/mic.0.000047 (2015).
Robertson, L. A. & Kuenen, J. G. Combined heterotrophic nitrification and aerobic denitrification in Thiosphaera pantotropha and other bacteria. Antonie Van Leeuwenhoek. https://doi.org/10.1007/BF00403948 (1990).
Caranto, J. D. & Lancaster, K. M. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. PNAS. https://doi.org/10.1073/pnas.1704504114 (2017).
Joo, H. S., Hirai, M. & Shoda, M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis NO. J. Biosci. Bioeng. 100, 181–191. https://doi.org/10.1263/jbb.100.184 (2005).
Zhao, B., An, Q., He, Y. L. & Guo, J. S. N2O and N2 production during heterotrophic nitrification by Alcaligenes faecalis strain NR. Biores. Technol.. https://doi.org/10.1016/j.biortech.2012.03.113 (2012).
Sparacino-Watkins, C., Stolz, J. F. & Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 43, 676–706. https://doi.org/10.1039/C3CS60249D (2014).
Saleh-Lakha, S. et al. Nitric oxide reductase gene expression and nitrous oxide production in nitrate-grown Pseudomonas mandelii. Appl. Environ. Microbiol. 74(22), 6876–6879. https://doi.org/10.1128/AEM.01533-08 (2008).
Saleh-Lakha, S. et al. Effect of pH and temperature on denitrification gene expression and activity in Pseudomonas mandelii. Appl. Environ. Microbiol. 75(12), 3903–3911. https://doi.org/10.1128/AEM.00080-09 (2009).
Zhang, W., Yan, C., Shen, J., Wei, R., Gao, Y., Miao, A., Xiao, L., Yang, L. (2019). Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. international Journal of Environmental Research and Public Health, 16(364). https://doi.org/https://doi.org/10.3390/ijerph16030364
Moreno-Vivián, C., Cabello, P., Marínez-Luque, M., Blasco, R. & Castillo, F. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181(21), 6573–6584 (1999).
Szulc, B., Jurczak, T., Szulc, K. & Kaczkowski, Z. The influence of the ecohydrological rehabilitation in the cascade of Arturówek reservoirs in Łódź (Central Poland) on the cyanobacterial and algae blooming. Oceanol. Hydrobiol. Stud. 44(2), 236–244. https://doi.org/10.1515/ohs-2015-0022 (2015).
Mankiewicz-Boczek, J. et al. The removal of nitrogen compounds from farming wastewater - The effect of different carbon substrates and different microbial activators. Ecol. Eng. 105, 341–354. https://doi.org/10.1016/j.ecoleng.2017.05.014 (2017).
Hamaki, T. et al. Isolation of novel bacteria and actinomycetes using soil-extract agar medium. J. Biosci. Bioeng. 99(5), 485–494. https://doi.org/10.1263/jbb.99.485 (2005).
Alexander, M. (1965). Denitrifying bacteria. In A. G. Norman, & C. A. Black (Ed.), Methods of soil analysis. Chemical and microbiological properties (pp. 1484–1486). Madison, Wisconsin , USA: American Society of Agronomy. https://doi.org/https://doi.org/10.2134/agronmonogr9.2.c52
Philippot, L., Piutti, S., Martin-Laurent, F., Hallet, S. & Germon, K. C. Molecular analysis of the nitrate-reducing community from unplanted and maize-planted soils. Appl. Environ. Microbiol. 68(12), 6121–6128. https://doi.org/10.1128/AEM.68.12.6121-6128.2002 (2002).
Braker, G. & Tiedje, J. M. Nitric oxide reductase (norB) genes from pure cultures and environmental samples. Appl. Environ. Microbiol. 69(6), 3476–3483. https://doi.org/10.1128/AEM.69.6.3476-3483.2003 (2003).
Braker, G., Fesefeldt, A. & Witzel, K. P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 64(10), 3769–3775 (1998).
Rich, J. J., Heichen, R. S., Bottomley, P. J., Cromack, K. & Myrold, D. F. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl. Environ. Microbiol. 69(10), 5974–5982. https://doi.org/10.1128/AEM.69.10.5974-5982.2003 (2003).
Allen, A. E. et al. Diversity and detection of nitrate assimilation genes in marine bacteria. Appl. Environ. Microbiol. 67(11), 5343–5348. https://doi.org/10.1128/AEM.67.11.5343-5348.2001 (2001).
Lane, D. J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics (eds Stackebrandt, E. & Goodfellow, M.) 115–175 (John Wiley and Sons, 1991).
BIOLOG. (2016, 10 01). BIOLOG GEN III MicroPlates Protocol. Retrieved from Biolog Cell Phenotyping, Microbial Identification to Human Cell Analysis: https://www.biolog.com/wp-content/uploads/2020/04/00P_185_GEN_III_MicroPlate_IFU.pdf
Tayeb, L., Ageron, E., Grimont, F. & Grimont, P. A. Molecular phylogeny of the genus Pseudomonas based on rpoB sequences and application for the identification of isolates. Res. Microbiol. 156(5–6), 763–773. https://doi.org/10.1016/j.resmic.2005.02.009 (2005).
APHA. Standard methods for the examination of water and wastewater 20th edn. (American Public Health Association, 1998).
Frear, D. S. & Burrell, R. C. Spectrophotometric method for determining hydroxylamine reductase activity in higher plants. Anal. Chem. 27(10), 1664–1665 (1955).
Hach. Water analysis handbook 3rd edn. (HACH Company, 1997).
Acknowledgements
The research was partially supported by the NCRD, TANGO2/339929/NCBR/2017 “AZOSTOP”, Development and implementation of innovative biotechnological products for agriculture and wastewater management in order to reduce water pollution. The authors sincerely thank Lauren Zielinski from the IHE Delft Institute for Water Education for her help in the edition of the text in the English language.
Author information
Authors and Affiliations
Contributions
A.F.N.: Formal analysis, Investigation – literature review, Writing – Original Draft, visualization. L.S.: Methodology, Supervision – microbiological and biochemical analysis, Writing – Review & editing. J.M.-B.: Conceptualization, Supervision – genetic analysis, Writing – Review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Font Nájera, A., Serwecińska, L. & Mankiewicz-Boczek, J. Culturable nitrogen-transforming bacteria from sequential sedimentation biofiltration systems and their potential for nutrient removal in urban polluted rivers. Sci Rep 11, 7448 (2021). https://doi.org/10.1038/s41598-021-86212-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86212-3
- Springer Nature Limited
This article is cited by
-
Water Level Fluctuations Modulate the Microbiomes Involved in Biogeochemical Cycling in Floodplains
Microbial Ecology (2024)
-
Triticum aestivum: antioxidant gene profiling and morpho-physiological studies under salt stress
Molecular Biology Reports (2023)