Abstract
Drug-related problems (DRP) cause preventable negative health outcomes, especially during hospital admissions. The aim of our study was to examine the prevalence and characteristics of DRP in regular clinical pharmacy, as well as to determine those factors associated with a higher risk of DRP in the hospital setting. We analyzed data from a standardized registry database of regular pharmacy practice (2015- 2016). DRP were classified according to the Pharmaceutical Care Network Europe v6.2 classification. Cross-sectional data were obtained from 1602 adults admitted to medical wards. Crude and adjusted binary logistic regressions were performed to identify associations between potential risk factors and DRP. Overall DRP prevalence was high across medical specialties (45,1%), in a population characterized by advanced age, polypharmacy and multimorbidity. Problems leading to DRP were mainly classified into two domains (effectiveness and adverse reactions), being drug and dose selection the most frequent causes. Interventions were accepted and DRP were totally or partially solved in 74.1% and 4.81% of cases, respectively. In the adjusted model polypharmacy, allergies, BMI > 25 kg/m2 and clearance < 30 mL/min were associated with a higher risk of DRP. The participation of clinical pharmacists into multidisciplinary teams promotes the detection and solution of DRP. Polypharmacy, obesity, renal impairment and allergy are associated with a higher risk of DRP during admission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Multimorbidity, the presence of several co-occurring conditions, is present in about 70% of the older adult population and becomes a major clinical and financial challenge for healthcare systems1,2. For example, most of the hospital medical admissions are the result of chronic diseases in the older adults3,4. There is a need of a comprehensive approach including the social sphere, nutrition and pharmacotherapy, to face with the increasing requirements of multimorbidity patients5.
Pharmacotherapy has been associated with negative health outcomes such as adverse effects, interactions, adherence problems, functional decline, cognitive problems, falls, urinary incontinence and metabolic or nutritional problems6,7,8,9,10,11,12,13. The risk of these problems increases with the number of drugs. Polypharmacy, defined as the use of more than four or five drugs, occurs in 40% of the adults over 65 years old14,15,16. Prevalence of polypharmacy reaches up to 90% of adults over 75 years at the moment of hospital admission17. Besides, during hospitalization, drug changes and new medicines for acute health problems will pose a higher risk of negative health outcomes. Up to 40% of hospitalized patients suffer from drug-related iatrogenesis6, emerging as the fourth to sixth mortality cause at this healthcare level18.
Several factors may significantly increase the risk of suffering a drug-related problem (DRP), defined as “an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes”19 as previously described, for example, in experiences of care transitions across the continuum of care20,21. In the hospital setting, DRP may occur at all stages, from admission to discharge20,21,22,23,24,25. Certain conditions, drugs in specific therapeutic groups and variability of pharmacology knowledge across healthcare professionals could also be related to DRP22,24,26,27,28. However, there is controversy on the impact of these variables and others such as gender, age, social factors or readmissions on the risk of developing DRPs, especially in clinical practice24,26,27,29,30,31.
Fortunately, a substantial proportion of DRP can be prevented32,33,34. Pharmacy practice implies the review of prescriptions and relevant clinical data of hospitalized patients to optimize the effectiveness and safety of treatments. The incorporation of hospital pharmacists into multidisciplinary teams has been shown to increase the detection of DRPs according to research35,36,37,38,39,40. Interventions described in research studies focusing on DRP are varied and cover a broad range of aspects, such as medication reconciliation, medication adherence, dose adjustment or therapeutic indication20,41,42,43,44,45,46,47,48,49. However, activities in real clinical practice are neither homogeneous nor standardized and data collection, such as the prevalence or the characterization of DRPs, is unusual.
In particular, the study of DRP in patients admitted to medical wards results of great interest as these patients may be at a higher risk of DRP due to several factors: acute conditions leading to the admission, advanced age with high burden of chronic comorbidities, younger patients with severe diseases, polypharmacy, risk of renal impairment, frequent changes in drug treatment and length of the stay29,37,50. Studies focusing on medical units have historically tended to focus on specific medical fields or ambulatory patients51,52,53,54,55,56,57. Also, many studies on medical wards are research projects that may not reflect real-life practice as there may have some of the following limitations: prospective studies with restrictive inclusion criteria, specific protocols and teaching programmes, small sample sizes, use of automatized DRP alerts without direct pharmacist intervention, poor methodology description of the pharmaceutical care process, lack of validated registration tools and reliable information in retrospective analyses, lack of DRP risk factors analysis or study of a limited list of potential factors, short study duration or pathology/drug-centered rather than patient-oriented approach26,27,38,40,44,45,47,48,49,58,59. Also, only a few studies have explored the degree of acceptance of recommendations by the medical team35,36,37,39,40.
The detection and characterization of DRPs, the study of their causes and the evaluation of the associated interventions are of special interest in daily clinical practice, especially in the hospital medical wards due to the high risk of iatrogenesis. An adequate evaluation should consider validated DRP classification systems, representative samples and study periods long enough to draw valid conclusions. Thus, the current study aims to examine the prevalence and characterize DRPs in regular clinical pharmacy as well as to determine those factors associated with a higher risk of DRPs in the hospital setting.
Methods
Study design
This hospital-based, observational, cross-sectional study was conducted in a 700 bed University Hospital (March 2015-February 2016). This reference hospital provides care to a population of 405,000 people in Barcelona, Spain.
Sample procedures
The sample included all adults over 18 years old admitted to the medical wards during the study period: Internal Medicine, Gastroenterology, Geriatrics, Neurology, Pneumology. Cardiology, Oncology and Haematology were excluded due to the presence of transplanted patients and special characteristics in relation to pharmacotherapy.
The study consisted of the assessment of activities and interventions made and registered in regular clinical pharmacy practice. Briefly, three pharmacists performed regular pharmaceutical care activities following their standard workflow (Fig. 1). Firstly, they assessed specific aspects regarding pharmacotherapy at the time of admission and subsequent changes in prescription, including a wide range of activities (i.e. medication reconciliation, allergy check, indication, posology) (Appendix 1). Secondly, certain patients were selected for daily follow-up based on pre-specified criteria (i.e. pharmacokinetics monitoring, risk of adverse effects, potential interaction, renal impairment) or any other clinical criteria (Appendix 1). Additionally, pharmacists received queries raised by physicians, nurses, caregivers or patients. All these activities resulted in specific pharmaceutical interventions.
All patients assessed throughout the aforementioned steps were registered in the pharmacy work database, as part of their standard practice, including selected variables. Pharmaceutical interventions were also registered for those patients with detected DRP.
Data collection and variable definitions
Data were obtained from a standardized registry database of regular pharmacy practice. Variables collected in the database were selected aiming to maximize pharmacotherapeutic utility of the data, taking into consideration clinical criteria and the reviews on factors related to DRP by Alomar and Kaufmann et al.24,30. Each of the variables described in the reviews was evaluated and those not applicable to the practice context were discarded (i.e. visual impairment, non-adherence; Appendix 2). Additional clinical variables of interest were included by consensus (Appendix 2).
Sociodemographic information included gender, age, country of birth, weight, height, body mass index (BMI). General clinical variables comprised creatinine clearance (Cockcroft-Gault), liver failure, number of chronic conditions, Charlson comorbidity index, allergies and medical specialty. Variables related to the use of health services were the number of last 12-month hospital admissions and coming from a nursing home. Pharmacotherapeutic variables were polypharmacy at admission (number of drugs), lack of information on regular treatment, monitoring drugs (amikacin, carbamazepine, cyclosporine, digoxin, everolimus, gentamicin, lithium, phenytoin, phenobarbital, sirolimus, tacrolimus, valproate, vancomycin), DRP detection, DRP description according to the Pharmaceutical Care Network Europe (PCNE) v6.2 DRP classification19, medication reconciliation problem, ATC group and an open field to be used in case further clarification was needed.
Statistical analyses
Due to the retrospective nature of the study, a priori sample size estimation was not possible. However, after data collection was finished, we calculated the theoretical needed sample size with the GRANMO calculator to confirm the adequacy of our ulterior analysis. According to the bibliography, DRP in hospitalizations range from 20 to 80%26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,62. A minimum sample size over 385 participants was needed considering an infinite population, 5% precision, 95% CI and a DRP prevalence of 50%. Hence, the sample obtained in our study was considered adequate for the analysis.
Frequencies, proportions, range, mean, SD, CIs and cross tabulations were applied for descriptive analysis. χ2 Test, Fisher's exact test and t-test were used to measure differences in prevalence of sociodemographic and clinical variables across DRP presence. T test, one-way ANOVA and simple linear regression were used to assess differences in polypharmacy across clinical and socio-demographic variables (age, gender, country, BMI, baseline chronic conditions, allergies, last-12 month admissions, creatinine clearance, liver failure, coming from nursing home, estimated 10-year survival, medical department and missing information defined as lack of data regarding chronic treatment in clinical records). Crude and adjusted binary logistic regressions were used to examine the relationship between theoretical factors related with DRP and their presence in our sample. The multivariate logistic regression model included those variables with an association in the bivariate analyses defined as p < 0.1. We fitted additional regression models for combinations of variables to test whether potential theoretical interactions were present with regard to the dependent variable: presence of DRP. Multicollinearity was also tested, defined as a condition index > 20 or VIF > 10. Both the presence of interactions (except number of chronic conditions with renal function) and multicollinearity were rejected. Results are reported as unadjusted and adjusted ORs with 95% CI. We conducted additional sensitivity analyses by age group (< 60 years, 60–69 years, ≥ 70 years). There were no missing data for most of the variables. Information on BMI and renal function were missing in 0.99% and 0.56% of the participants. These rates were considered low. We did not impute these variables as we could not guarantee whether these data were missing at random. Analyses were performed with IBM SPSS statistics V.19.
Results
Participant characteristics
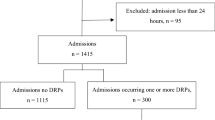
Overall, data from 1602 hospital admissions were reviewed and registered. Participants mean age was 72.8 years (SD:15.09). Most individuals were Spanish, 44.8% of them being women. Mean number of baseline chronic conditions was 5.99 (SD:3.06), BMI was greater than 25 kg/m2 in 17.5% of cases, and renal function was below 30 mL/min in 15.5% of the sample. As for additional healthcare data of interest, 8.8% of the admissions came from a nursing home, 44.4% have had a previous admission over the last 12 months and 2.9% were reported as having missing information on previous treatments. A summary of the full list of sociodemographic and clinical data is available at Table 1.
Regarding polypharmacy, mean number of drugs was 8.15 (SD:4.71). Certain variables showed a positive association with the degree of polypharmacy: last-12 months admissions (p = 0.001), coming from nursing home (p = 0.007), estimated 10-year survival below the median (p = 0.001), older age (p = 0.001), baseline chronic conditions (p = 0.001), and decreased renal function (0.001). Differences in polypharmacy were also found across departments (Respiratory = Geriatrics > Internal Medicine > Digestive = Neurology; p = 0.001); and across BMI (overweight > normoweight > infraweight; p = 0.001). For the specific distribution of polypharmacy across selected quantitative variables see Fig. 2.
Description of DRP
DRP were detected in 722 (45.1%) patients and their prevalence differed across BMI, allergy, polypharmacy, multimorbidity, last 12 months admissions and the estimated 10-year survival in (Table 1). DRP were related to medication reconciliation in 38.4% of the cases.
According to the PCNE V6.2 classification, most of the problems leading to DRP were included into two domains: effectiveness and adverse reactions. The principal problem was “toxic adverse drug-event”, followed by “untreated indication” and “effect of treatment not optimal” (Table 2). Regarding the cause of DRP, the most frequent domains were “drug selection” and “dose selection”. The leading causes were “indication for drug-treatment not noticed”, “pharmacokinetic problem requiring dose adjustment” and “drug dose too high”.
We divided interventions results into prescriber and drug levels. As for the first one, intervention was proposed and approved in two thirds of the cases, while not approved in only 5.8% of cases. Outcome was unknown in 13.9% interventions (for more categories, see Table 2). Pharmaceutical intervention results covered a wide range of aspects, such as: drug dose adjustment in patients with renal or hepatic impairment (e.g.: levofloxacin, simvastatin, paroxetine), treatment changes due to drug-drug interactions (e.g.: valproate with carbapenem antibiotics; high doses of simvastatin with diltiazem), need of dose adjustments (e.g.: low doses of antimicrobials for CNS infections infections in the central nervous system, excessive drug dose leading to potential harms), untreated indications (e.g.: hyperglycaemia, high-blood pressure, ACE Angiotensin-converting enzyme inhibitor induced hyperkalemia), contraindications (e.g.: oral bisphosphonates in patients with dysphagia), medication reconciliation (e.g.: dose/frequency, drugs to be stopped), IV intravenous administration issues (e.g.: excessive infusion rate of vancomycin or electrolytes; wrong serum for dilution, Y-Y incompatibilities), oral administration issues (e.g.: recommendation of available presentations for dysphagia, medicines to be taken on an empty stomach such as alendronic acid), contraindication or excessive duration treatment (e.g.: excessive antibiotic drug length, antihypertensive therapy in patients with low blood pressure), pharmacokinetics monitoring (e.g.: dosage increase/decrease or discontinuation of vancomycin, gentamicin, amikacin, etc.), wrong drug prescribed (e.g.: methimazole for metamizole), cost-efficacy interventions (e.g.: changes of low molecular weight heparins according to the hospital formulary). At a drug level, dosage was changed in 36.6% of cases, a new drug was started in 12.4% and the drug was stopped in 9.2% of the interventions, while 30.04% resulted in no changes. As for the final health outcomes, DRP were considered to be totally solved in 74.1% and partially solved in 4.8% of cases. The outcome was not known in 15.7% of interventions.
Considering pharmacist interventions on DRP as a continuum and clustering categories according to the domains of the PCNE V6.2, certain patterns were observed in the DRP pathway “cause → intervention → outcome” (Fig. 3).
Also, the five most frequent Anatomical Therapeutic Chemical (ATC) Classification System groups in the detected DRP (n = 1185) were: J “Antiinfectives for systemic use” (n = 337; e.g.: vancomycin, levofloxacin, amikacin), N “Nervous system” (n = 206; e.g.: valpropate, citalopram, sertraline), C “Cardiovascular system” (n = 195; e.g.: digoxin, simvastatin, enalapril), B “Blood and blood forming organs” (n = 114; e.g.: acetylsalicylic acid, enoxaparin, bemiparin) and A “Alimentary tract and metabolism” (n = 99; e.g.: omeprazole, potassium chloride, vitamin D). (Appendix 3).
Factors associated with DRP
Table 3 shows the crude and adjusted logistic regression odds ratios for the association between potential factors leading to DRP and the actual presence of these problems (n = 1602). In the crude analysis, number of diseases, previous last 12 month admissions, polypharmacy, allergies and BMI > 25 kg/m2 were associated with a great prevalence of DRP. A higher estimated 10-year survival was associated with a lower risk. In the adjusted model only polypharmacy, allergies, BMI > 25 kg/m2, renal function < 30 mL/min and the interaction “multimorbidity*renal function < 30 mL/min” were still associated with a higher risk of DRP (OR 1.040 [CI 1.010–1.071]; OR 1.281 [CI 1.004–1.634); OR 1.515 [CI 1.143–2.007]; OR 2.146 [1.200–3.837]; OR 0.908 [CI 0.838–0.985]) respectively).
Sensitivity analyses by age group showed similar results to those of the global analyses. For only a few variables, results maintained directionality and tendency but did not reach the statistical significance: Group 18–59 years: renal function < 30 mL/min [OR 3.365 (0.378–29.971); p = 0,2]; Group 60–69 years: BMI > 25 kg/m2 [OR 1.685 (0.902–3.148);p = 0,1]; polypharmacy [OR 1.202 (0.945–1.102); p = 0,6], Group 70 + years: allergy [OR 1.183 (0.884–1.585); p = 0,2]. Sample size of the youngest groups was smaller than the oldest group (< 60 years: 300 patients; 60–69 years: 246 patients; 70 + years: 1056 patients).
Discussion
To the best of our knowledge, this is one of the few studies to evaluate DRP prevalence and risk factors in real clinical practice during hospital admission in the context of a standardized pharmaceutical care programme, including a validated register of DRP interventions with a global representation of medical specialties. We found a high prevalence of DRP, most being caused by drug or dose selection, in a sample that highlights the worldwide demographical trends of ageing along with multimorbidity and polypharmacy. Pharmacist interventions were accepted in most cases, preventing potential negative health outcomes. As for potential risk factors, only polypharmacy, renal impairment, allergies and high BMI were associated with a higher prevalence of DRP. The main strengths of our study are the large sample size, the standardized procedures of clinical pharmacists throughout the admission in regular clinical pharmacy, the use of a validated DRP classification, the assessment of a comprehensive list of potential DRP risk factors and the inclusion of the most relevant medical specialties.
Our study found a great proportion of admissions with DRPs, a 45.1% of the cases. Most of the evidence available at a hospital level have focused on specific medical fields51,52,53, being limited to ambulatory patients in numerous occasions54,55,56. There are, however, some studies focusing on medical specialties, which found a DRP prevalence ranging from 15 to 81%26,27,60,61,62. Our results fit well into the related literature although it should be noted that literature on geriatrics show the higher results29,58,63. In contrast, results from studies which use automatized DRP alerts in computerized prescriber order entry systems have shown lower prevalence in previous studies26,40. Thus, these systems should be considered as tools to complement pharmacy practice rather than a substitution of clinical pharmacist functions due to the complexity of hospitalized patients.
Our study highlights the complexity of patients admitted in medical wards, with an average age over 72 years, in line with recent literature26,27,60,61,62. The World Health Organization has recognized demographic transitions as a major priority due to its burden at a health, social and economic levels64. Ageing involves a complex set of complications reflected in our results, such as multimorbidity, polypharmacy or functional impairment. Mean number of chronic conditions in our study was six and ranged up to 16 per patient, intimately related to a low expected 1-year survival of 25%. Most of the previous literature focusing on regular clinical pharmacy practice care in medical wards reported minimal data at this level as they had a more drug-centred approach26,27,60,61,62. Polypharmacy stands out as one of the most valuable variables of our study, ranging up to 26 drugs per patient, due to their potential harmful effects. In a context with a high burden of polypharmacy, there is need to move from the classical thresholds of 4+ or 5+ drugs14,15,16 to a more realistic linear approach, which we used in our analyses. A further complex approach, considering polypharmacy as a qualitative aspect is still being discussed. In the end, these characteristics and those regarding the own health system, such as communication across healthcare levels, will define the objectives for a specific patient and the type of interventions in drug treatment.
Regarding pharmacists’ interventions, acceptance accounted for almost 70% cases. This issue has not been properly assessed in many previous studies on DRPs in regular clinical practice26,27,60,61. Those studies documenting this data show similar interventions acceptance in regular clinical practice62 or even higher figures in prospective research studies compared with our results58. Also, final outcomes of the interventions showed total or partial resolution of the DRPs in our study, reinforcing the value of our results. Interventions registers are essential, as in other healthcare areas, to document professional activity and to assess the suitability of the approach being taken to eventually improve healthcare outcomes65. Despite its possible benefits, registries of clinical pharmacy have normally been present in specific areas, such as those linked to a computerized DRP alert system or nationwide voluntary reporting systems40,66.
Finally, one of the most relevant issues regarding DRPs is understanding their potential underlying factors to optimize interventions and preventive measures. As previously stated, there are many possible factors suggested by the literature. Certain theoretical frameworks or reviews have developed lists of risk factors but they may not totally apply to the care of admitted patients24,30. For example, issues such as self-medication, visual impairment, civil status or educational level would not be relevant from the acute drug management perspective. In contrast, we found that only polypharmacy, renal impairment, allergies and high BMI were associated with DRPs. A review of the literature on this topic at a hospital level used definitions not totally comparable to ours, i.e. medication errors, but polypharmacy and renal function also resulted to be risk factors29. We hypothesize that the idiosyncrasy and complexity of inpatients in our or similar environments may diminish the impact of alternative factors.
Our study has limitations. First, its cross-sectional nature identifies associations but does not allow causal relationships to be determined. Second, our results may differ from geographical areas with different healthcare characteristics, especially in terms of pharmaceutical care implementation. Nor can they be extrapolated to other specialties or primary care. Third, this study refers to DRPs, which by definition have a potential nature. Interventions where made in all detected DRPs, so that it is not possible to quantify the real negative health outcomes. As regular practice, it is not considered ethical to stop performing clinical activities for this reason. Fourth, administration errors, if not notified by nursing staff or detected by the pharmacist staff, were not registered. This limitation has been described in other studies previously. Fifth, underdetection may have happened to some extent, especially for infrequent or unfamiliar DRPs. This problem is partly covered by using a complete, standardized and validated classification system. Another factor that can influence underreporting is professional experience. Also, we did not include potential factors leading to DRP which were considered not applicable to practice context (e.g.: education level, visual impairment). These are relevant factors in primary care and could potentially impact on the results of specific hospitalizations. Moreover, our analyses have shown similar tendencies in the global analysis compared with the sensitivity age group analyses. However, some results did not achieve statistical significance in the younger groups, probably because sample sizes were smaller. Further research should focus on possible differences across age groups. Also, verbal interventions have been associated with higher acceptance rates compared with written interventions. In our study, most of them were verbal but this variable was not recorded and its impact could not be analyzed. Finally, the clinical relevance of interventions was not assessed as it is not collected routinely in regular clinical practice but future studies should address this issue.
DRPs have become a major challenge for health care systems due to their clinical and economic impact, especially in the context of the current demographical ageing trends that imply a high burden of multimorbidity and polypharmacy. The prevalence of DRP in hospitalized patients admitted to medical wards is high regardless of the specialty, which highlights the need of providing pharmaceutical care to prevent negative health outcomes during hospitalization. Problems leading to DRPs are mainly related to effectiveness and adverse reactions, while the most frequent causes are drug and dose selection. Thus, these domains should be prioritized in both pharmaceutical care programmes and general educational activities. The participation of clinical pharmacists into the multidisciplinary team promotes the detection and solution of DRP in the majority of cases, and should be considered as a rule in general clinical practice. Finally, only a limited number of factors may be associated with a higher risk of developing DRPs in the hospital setting, such as polypharmacy, allergies, BMI > 25 kg/m2 and renal function < 30 mL/min, which could be useful to prioritize actions. Better understanding of these issues may facilitate the implementation of general approaches in diverse settings and the study of these interactions in the future.
Ethics statement
The study was approved by the Clinical Research Ethics Committee of the Hospital de la Santa Creu i Sant Pau, Barcelona, Spain. This Ethics Committee waived the need to obtain informed consent due to the retrospective nature of the data, coming from a standardized regular practice database, and the anonymized analysis. All investigators worked according to the principles expressed in the Declaration of Helsinki.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
References
Garin, N. et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J. Gerontol. A Biol. Sci. Med. Sci. 71, 205–214 (2016).
Parekh, A. K. & Barton, M. B. The challenge of multiple comorbidity for the US health care system. JAMA 303, 1303–1304 (2010).
Cassell, A. et al. The epidemiology of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 68, e245–e251 (2018).
Kalyesubula, R. et al. Trends of admissions and case fatality rates among medical in-patients at a tertiary hospital in Uganda; a four-year retrospective study. PLoS ONE 14, e0216060 (2019).
Contel, J. C., Muntané, B. & Camp, L. L. atención al paciente crónico en situación de complejidad: el reto de construir un escenario de atención integrada. Atención Primaria. 44, 107–113 (2012).
Maher, R. L., Hanlon, J., Hajjar, E. R. & Hajjar, E. R. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Saf. 13, 57–65 (2014).
Noor, S., Ismail, M. & Khadim, F. Potential drug-drug interactions associated with adverse clinical outcomes and abnormal laboratory findings in patients with malaria. Malar J. https://doi.org/10.1186/s12936-020-03392-5 (2020).
Zelko, E., KlemencKetis, Z. & TusekBunc, K. Medication adherence in elderly with polypharmacy living at home: a systematic review of existing studies. Mater. Socio Med. 28, 129–132 (2016).
Lau, D. T., Mercaldo, N. D., Shega, J. W., Rademaker, A. & Weintraub, S. Functional decline associated with polypharmacy and potentially inappropriate medications in community-dwelling older adults with dementia. Am. J. Alzheimers Dis. Other Demen. 26, 606–615 (2011).
Campbell, N. et al. The cognitive impact of anticholinergics: a clinical review. Clin. Interv. Aging. 4, 225–233 (2009).
de Jong, M. R., Van Der Elst, M. & Hartholt, K. A. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther. Adv. Drug Saf. 4, 147–154 (2013).
Tsakiris, P., Oelke, M. & Michel, M. C. Drug-induced urinary incontinence. Drugs Aging 25, 541–549 (2008).
Ma, R. C. W., Kong, A. P. S., Chan, N., Tong, P. C. Y. & Chan, J. C. N. Drug-induced endocrine and metabolic disorders. Drug Saf. 30, 215–245 (2007).
Barrett, K., Lucas, E. & Alexander, G. C. How polypharmacy has become a medical burden worldwide. Clin. Pharm. https://doi.org/10.1211/CP.2016.20201251 (2016).
Martin-Pérez, M. et al. Prevalencia de polifarmacia en la población mayor de 65 años en España: análisis de las Encuestas Nacionales de Salud 2006 y 2011/12. Rev. EspGeriatr. Gerontol. 52, 2–8 (2017).
Morin, L., Johnell, K., Laroche, M.-L., Fastbom, J. & Wastesson, J. W. Theepidemiology of polypharmacy in olderadults: register-basedprospectivecohortstudy. Clin. Epidemiol. 10, 289–298 (2018).
Olmos, R., Garcia, O., Velasco, J. & de la Rubia, A. Prevalence of polypharmacy in older hospitalised patients. Eur. J. Hosp. Pharm. 19(242), 3–243 (2012).
Lazarou, J., Pomeranz, B. H. & Corey, P. N. Incidence of adverse drug reactions in hospitalized patients. JAMA 279, 1200–1205 (1998).
Pharmaceutical Care Network Europe Foundation. Classification for Drug related problems: The PCNE Classification V 6.2. PCNE. https://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf (2010).
Gleason, K. M. et al. Results of the medications at transitions and clinical handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J. Gen. Intern. Med. 25, 441–447 (2010).
Kwan, J. L., Lo, L., Sampson, M. & Shojania, K. G. Medication reconciliation during transitions of care as a patient safety strategy. Ann. Intern. Med. 158, 397–403 (2013).
Freyer, J. et al. Drug-related problems in geriatric rehabilitation patients after discharge—a prevalence analysis and clinical case scenario-based pilot study. Res. Soc. Adm. Pharm. 14, 628–637 (2018).
Maxwell, K., Harrison, J., Scahill, S. & Braund, R. Identifying drug-related problems during transition between secondary and primary care in New Zealand. Int. J. Pharm. Pract. 21, 333–336 (2013).
Kaufmann, C. P., Stampfli, D., Hersberger, K. E. & Lampert, M. L. Determination of risk factors for drug-related problems: a multidisciplinary triangulation process. BMJ Open 5, e006376–e006376 (2015).
Unroe, K. T. et al. Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am. J. Geriatr. Pharmacother. 8, 115–126 (2010).
Ferrández, O. et al. Validation of a score to identify inpatients at risk of a drug-related problem during a 4-year period. Saudi Pharm J. 26, 703–708 (2018).
Blix, H. S. et al. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur. J. Clin. Pharmacol. 60, 651–658 (2004).
Keijsers, C. J. P. W. et al. Pharmacists’ and general practitioners’ pharmacology knowledge and pharmacotherapy skills. J. Clin. Pharmacol. 55, 936–943 (2015).
Krähenbühl-Melcher, A. et al. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 30, 379–407 (2007).
Alomar, M. J. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J. 22, 83–94 (2014).
Wilmer, C. M. et al. Drug-related problems in a clinical setting: a literature review and cross-sectional study evaluating factors to identify patients at risk. Eur. J. Hosp. Pharm. 22, 229–235 (2015).
Leendertse, A. J., Egberts, A. C. & Stoker, J. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch. Intern. Med. 168, 1890–1896 (2008).
Leape, L. L. et al. The nature of adverse events in hospitalized patients. N. Engl. J. Med. 324, 377–384 (1991).
Howard, R. L. et al. Which drugs cause preventable admissions to hospital? A systematic review. Br. J. Clin. Pharmacol. 63, 136–147 (2007).
Blix, H. S., Viktil, K. K., Moger, T. A. & Reikvam, Å. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm. World Sci. 28, 152–158 (2006).
Bedouch, P. et al. Trends in pharmacists’ medication order review in French hospitals from 2006 to 2009: analysis of pharmacists’ interventions from the Act-IP© website observatory. J. Clin. Pharm. Ther. 40, 32–40 (2015).
Guignard, B. et al. Drug-related problems identification in general internal medicine: the impact and role of the clinical pharmacist and pharmacologist. Eur. J. Intern. Med. 26, 399–406 (2015).
Abunahlah, N., Elawaisi, A., Velibeyoglu, F. M. & Sancar, M. Drug related problems identified by clinical pharmacist at the Internal Medicine Ward in Turkey. Int. J. Clin. Pharm. 40, 360–367 (2018).
Delgado Silveira, E. et al. The impact of Pharmacy Intervention on the treatment of elderly multi-pathological patients. Farm Hosp. 39, 192–202 (2015).
Ferrández, O. et al. Análisis de los problemas relacionados con los medicamentos en un hospital de tercer nivel de Barcelona. GacSanit. 33, 361–368 (2019).
Pippins, J. R. et al. Classifying and PredictingErrors of InpatientMedicationReconciliation. J. Gen. Intern. Med. 23, 1414–1422 (2008).
Reeder, T. A. & Mutnick, A. Pharmacist- versus physician-obtained medication histories. Am. J. Health Pharm. 65, 857–860 (2008).
Buckley, M. S. et al. Impact of a clinical pharmacy admission medication reconciliation program on medication errors in “High-Risk” patients. Ann. Pharmacother. 47, 1599–1610 (2013).
Shanika, L. G. T. et al. Ward-based clinical pharmacists and hospital readmission: a non-randomized controlled trial in Sri Lanka. Bull. World Health Organ. 96, 155–164 (2018).
Stuhec, M., Bratović, N. & Mrhar, A. Impact of clinical pharmacist’s interventions on pharmacotherapy management in elderly patients on polypharmacy with mental health problems including quality of life: a prospective non-randomized study. Sci. Rep. https://doi.org/10.1038/s41598-019-53057-w (2019).
Tefera, G. M., Zeleke, A. Z., Jima, Y. M. & Kebede, T. M. Drug therapy problems and the role of clinical pharmacist in surgery ward: Prospective observational and interventional study. Drug Healthc. Patient Saf. 12, 71–83 (2020).
Erku, D. A. et al. The impact of pharmacist-led medication therapy management on medication adherence in patients with type 2 diabetes mellitus: a randomized controlled study. Pharm. Pract. (Granada) https://doi.org/10.18549/PharmPract.2017.03.1026 (2017).
Falcão, F. et al. Hospital pharmacist interventions in a central hospital. Eur. J. Hosp. Pharm. 22, 94–97 (2015).
Reis, W. C. T., Scopel, C. T., Correr, C. J. & Andrzejevski, V. M. S. Analysis of clinical pharmacist interventions in a tertiary teaching hospital in Brazil. Einstein (Sao Paulo) 11, 190–196 (2013).
Taxis, K., Dean, B. & Barber, N. Hospital drug distribution systems in the UK and Germany—a study of medication errors. Pharm. World Sci. 21, 25–31 (1999).
Ali, M. A. S., Khedr, E. M. H., Ahmed, F. A. H. & Mohamed, N. N. E. Clinical pharmacist interventions in managing drug-related problems in hospitalized patients with neurological diseases. Int. J. Clin. Pharm. 40, 1257–1264 (2018).
Pfister, B., Jonsson, J. & Gustafsson, M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacol. Toxicol. https://doi.org/10.1186/s40360-017-0157-2 (2017).
Sutherland, A., Phipps, D. L., Tomlin, S. & Ashcroft, D. M. Mapping the prevalence and nature of drug related problems among hospitalised children in the United Kingdom: a systematic review. BMC Pediatr. https://doi.org/10.1186/s12887-019-1875-y (2019).
Nasir, B. B. et al. Drug therapy problems and treatment satisfaction among ambulatory patients with epilepsy in a specialized hospital in Ethiopia. PLoS ONE 15, e0227359 (2020).
Belaiche, S., Romanet, T., Allenet, B., Calop, J. & Zaoui, P. Identification of drug-related problems in ambulatory chronic kidney disease patients: a 6-month prospective study. J. Nephrol. 25, 782–788 (2012).
Farha, R. A., Basheti, I., Al Ruz, H. A., Alsaleh, A. & AbuRuz, S. Assessment of drug-related problems and their impact on blood pressure control in patients with hypertension. Eur. J. Hosp. Pharm. 23, 126–130 (2016).
Zhu, Y. et al. Identification and resolution of drug-related problems in a tertiary hospital respiratory unit in China. Int. J. Clin. Pharm. 41, 1570–1577 (2019).
Wincent, M. M., Potrilingam, D., Anagha, V., Jacob, S. C. & Andhuvan, G. Assessment of drug related problems in patients with chronic diseases in the general medicine units of a tertiary care hospital. Int. J. Pharm. Pharm. Sci. 9, 194–200 (2017).
Hailu, B. Y., Berhe, D. F., Gudina, E. K., Gidey, K. & Getachew, M. Drug related problems in admitted geriatric patients: the impact of clinical pharmacist interventions. BMC Geriatr. 20, 13. https://doi.org/10.1186/s12877-020-1413-7 (2020).
Reinau, D., Furrer, C., Stämpfli, D., Bornand, D. & Meier, C. R. Evaluation of drug-related problems and subsequent clinical pharmacists’ interventions at a Swiss university hospital. J. Clin. Pharm. Ther. 44, 924–931 (2019).
Tasaka, Y. et al. Potential drug-related problems detected by routine pharmaceutical interventions: safety and economic contributions made by hospital pharmacists in Japan. J. Pharm. Health Care Sci. 4, 33. https://doi.org/10.1186/s40780-018-0125-z (2018).
Peterson, C. & Gustafsson, M. Characterisation of drug-related problems and associated factors at a clinical pharmacist Service-Naïve Hospital in Northern Sweden. Drugs Real World Outcomes 4, 97–107 (2017).
Van Der Linden, L. et al. Factors associated with the number of clinical pharmacy recommendations: findings from an observational study in geriatric inpatients. ActaClin. Belg. Int. J. Clin. Lab. Med. 23, 1–8 (2019).
World Health Organization. World report on Ageing And Health. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland. ISBN 978 92 4 069479 8. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1 (2015).
Hoque, D. M. E. et al. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS ONE https://doi.org/10.1371/journal.pone.0183667 (2017).
Laatikainen, O., Sneck, S. & Turpeinen, M. The risks and outcomes resulting from medication errors reported in the finnish tertiary care units: a cross-sectional retrospective register study. Front Pharmacol. https://doi.org/10.3389/fphar.2019.01571 (2020).
Author information
Authors and Affiliations
Contributions
N.G.: Participated in the study design, acquisition of data, database management, statistical analyses, interpretation of data and paper draft. N.S.: Participated in the study design, acquisition of data, database management and interpretation of data. B.L.: Participated in the acquisition of data, database management, statistical analyses, interpretation of data and paper draft. L.M.: Participated in the acquisition of data and interpretation of data. D.M.: Participated in the acquisition of data and interpretation of data. A.R.: Participated in the acquisition of data and interpretation of data. L.G.: Participated in the acquisition of data, database management and interpretation of data. N.F.: Participated in the study design, acquisition of data, database management and interpretation of data. All authors reviewed the manuscript and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garin, N., Sole, N., Lucas, B. et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep 11, 883 (2021). https://doi.org/10.1038/s41598-020-80560-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80560-2
- Springer Nature Limited
This article is cited by
-
Adverse drug reactions and drug-related problems with supportive care medications among the oncological population
Discover Oncology (2024)
-
Intervention of pharmacist included in multidisciplinary team to reduce adverse drug event: a qualitative systematic review
BMC Health Services Research (2023)
-
Drug-related problems in hospitalized patients with chronic kidney diseases and clinical pharmacist interventions
BMC Geriatrics (2023)
-
Development, implementation and evaluation of a seven-day clinical pharmacy service in a tertiary referral teaching hospital during surge-2 of the COVID-19 pandemic
International Journal of Clinical Pharmacy (2023)
-
Framework for measuring the efficiency and efficacy of sale of distressed mortgaged properties using imports of statistical tests deployed in clinical studies
SN Business & Economics (2023)