Abstract
Heavy metal pollution has seriously disrupted eco-balance and transformed estuaries into sewage depots. Quanzhou bay is a typical heavy metal-contaminated estuary, in which Spartina alterniflora has widely invaded. Plant-associated microbial communities are crucial for biogeochemical cycles, studies of which would be helpful to demonstrate the invasion mechanisms of plants. Meanwhile, they are indispensable to phytoremediation by enhancing the heavy metal tolerance of plants, facilitating heavy metal absorption rate and promoting growth of plants. In the present study, S. alterniflora-associated rhizo- and endobacterial communities from 3 experimental sites were investigated by 454-pyrosequencing. Heavy metal screening generated 16 culturable isolates, further biochemical assays suggested these clones possess various abilities such as phosphate solubilization, indole-3-acetic acid (IAA) production and 1-aminocyclopropane-1-carboxylate (ACC) deaminase production to accelerate heavy metal uptake and growth of the host. This study revealed the bacterial community structures and characterized the predominant resident bacterial strains of S. alterniflora-associated rhizo- and endobacteria under heavy metal stress, and isolated several bacterial species with potential ecological function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
According to previous studies, plant invasion was believed to be not only a threat to native biodiversity, but also could affect ecosystem functioning and process through a variety of mechanisms, such as reducing plant and animal biodiversity, altering wetland hydrology and changing carbon or nitrogen cycling1. At present, two hypotheses of plant invasion mechanisms are commonly accepted: biological interactions, including resource availability and enemy release2. The invasion of exotic plant might change the original microbial community structure in the sediments. For example, Spartina alterniflora, native to the east coast of North America, is proved to affect the community structures of methanogens and sulfate-reducing bacteria in Phragmites australis vegetated sediments3. Meanwhile, plants (e.g., Lepidium sativum, Oryza sativa cultivars and maize) might also benefit from their associated rhizo- and endobacteria by enhancing their growth, productivity, stress resistance and even phytoremediation4,5,6.
Since the industrial revolution, heavy metal pollution has become a prevalent environmental threat7. Urban estuaries have been regarded as suitable places for the disposal of industrial and domestic wastes due to their proximity to the open ocean7,8. After long period of accumulation, natural biotic and physio-chemical balance of previously pristine system is disrupted and transformed into sewage depots. Unlike organic pollutants, heavy metal pollutants could not be removed by natural processes and start to move up the food chain once they accumulated in sediments. Moreover, the accumulation of potentially toxic heavy metals might also have a detrimental influence on physical metabolism, activity, biomass, diversity and composition of plant and microbial communities9. In order to survive under heavy metal stress, plants develop a series of coping strategies such as reducing water content, producing antioxidases and altering osmotic adjustment substances, to relieve the damage of heavy metal stress10. Wetland plants such as P. australis, Typha capensis and Spartina maritima can accumulate heavy metal from environment, and are considered as potential phytoremediators and indicators of heavy metal contamination11. Plant-associated microbes like endophytic and rhizosphere bacteria can enhance plant growth and increase the absorption of heavy metal at the same time. The plant-associated bacteria migrate from the bulk soil to the rhizosphere of living plant and aggressively colonize the rhizosphere and roots of plants12. Only a few of plants have been well studied regarding their rhizosphere and endophytic biology. Among the rhizo- and endobacteria involved in interactions between plants and metal-contaminated soil, the plant growth promoting bacteria (PGPB) are important. The metabolites released by PGPB (e.g., siderophores, biosurfactants, organic acids, plant growth regulator, etc.) can alter the uptake of heavy metals indirectly and directly13.
Spartina alterniflora was introduced to China in 1979 and had rapidly invaded the salt marshes on the east coast of China14. Its displacement of the native species had caused a number of ecological impacts14,15. Several studies investigated the effects of S. alterniflora on ecosystem15,16,17 and its rhizo- and endobacterial diversity18, etc. However, little was known about the rhizo- and endobacterial community of S. alterniflora under heavy metal stress.
The Quanzhou bay is a typical estuary where the water quality and ecological health of the system have been markedly polluted by inputs from the upstream regions. Industrial and domestic wastewater alongside the Luoyang River and Jinjiang River was discharged into Quanzhou bay, forming the “black and white” appearance of water body19. In Quanzhou bay estuary, the average salinity of soil is partially different in different sections, the average pH in soil has a small variation range (from 6.92 to 7.66) and the total organic carbon level vary from 0.81 to 1.88%20,21. Surveys on sediment and water quality indicated that heavy metals in the wastewater were the principal pollutant in Quanzhou bay20. Despite severe heavy metal pollution, S. alterniflora had widely invaded Quanzhou bay, offering an opportunity to understand the influence of rhizo- and endobacteria on the niche advantages of S. alterniflora.
Our study investigated the S. alterniflora-associated rhizo- and endobacterial community structures and diversity from 3 sampling sites, one (site 3) of which is allocated at an oil terminal. To analysis S. alterniflora-associated bacterial community composition and diversity, both culture-independent and traditional culture-dependent methods were applied. Biochemical assays were performed to screen for the heavy metal-resistant bacteria. These results would provide the basis for further study on the structure and function of rhizosphere and endophytic bacterial communities associated with S. alterniflora under heavy metal pollution, and established rhizo- and endobacterial libraries as references for future microbial application.
Results
Differences of microbial diversity between rhizosphere soil and roots of S. alterniflora
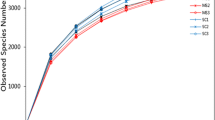
The heavy metal content of sediments and rhizosphere soil was assessed. The results showed that there are different degrees of heavy metal pollution in the 3 sampling sites (see Supplementary Table S1 online). To investigate the rhizo- and endobacterial diversity associated with S. alterniflora under different degrees of heavy metal contamination, we applied 454-pyrosequencing targeting V4 region of 16S rRNA. The 454-pyrosequencing generated over 155,346 reads. In total, 143,009 reads were obtained after quality control (average 29,403 reads per rhizosphere sample and 16,309 reads per endophytic sample). Trimmed sequences generated 14,537 operational taxonomic units (OTUs). On average, 4629 and 1191 OTUs per rhizosphere and endophytic sample were detected, respectively. Based on the rarefaction data (see Supplementary Fig. S1 online) and diversity indices of 454 tag sequences (Table 1), the sequencing depth could not completely cover full diversity of rhizosphere soil, indicating that the microbial community of rhizosphere soil was more diverse than that of the plant roots. It was worth noting that S3 hosted the most diverse and least abundant rhizobacterial community, while R3 displayed a reversed pattern. Weighted PCoA plot (Fig. 1) showed that R1/R2 and S1/S2 form clusters respectively, while S3/R3 was distant. The results suggested that both rhizo- and endobacterial communities were specific in geographical location.
Principal component analysis of all 454 libraries with weighted UniFrac. X-axis, 1st principal component and Y-axis, 2nd principal component. Numbers in brackets represents contributions of principal components to differences among samples. A dot represents each sample. Figure was drawn using R (v3.1.1, https://www.datavis.ca/R/).
Composition of the rhizo- and endobacterial communities
The composition of rhizo- and endobacterial communities was highly distinct (see Supplementary Fig. S2 online), and the phyla and genera (partial) with significant differences in relative abundance in rhizosphere soil and roots were shown in Fig. 2. The relative abundance of Actinobacteria and Firmicutes in root was significantly higher than that in rhizosphere soil. Chloroflexi constituted the majority of sequences in rhizosphere soil but showed less abundance in endophytic root. Acidobacteria was the second abundant phylum in both samples. The third abundant phylum allocated to Proteobacteria (12.69 ± 0.58% in soil, 9.66 ± 6.13% in root). Surprisingly, Actinobacteria seemed to concentrate in root (39.27 ± 8.74%) but not in rhizosphere soil (4.18 ± 2.80%). At genus level (see Supplementary Fig. S3 online), an uncultured group from Anaerolineaceae was dominant in rhizosphere soil (44.68 ± 15.11%), followed by Nitrospina (1.86 ± 0.79%), Acidothermus (1.78 ± 1.74%). For R1 and R2, Acidothermus appeared to be the dominant group (19.62 ± 12.01%) followed by Acidobacterium (13.53 ± 7.93%). Interestingly, R3 had a distinguished pattern, which showed the same dominant genus as rhizosphere soil (20.33%) followed by uncultured Propionibacteriaceae (18.50%) and Desulfovibrio (8.38%).
Phyla (a) and genera (b) with significant differences in relative abundance in rhizosphere soil and roots. R roots of Spartina alterniflora, S rhizosphere soil of S. alterniflora. Figure was drawn using R (v.3.1.1, https://www.datavis.ca/R/).
Differences of rhizo- and endobacterial community structure
For a better understanding of rhizo- and endobacterial communities, we screened out 8 phyla and 24 genera that had relative abundance > 1% in at least one site (Figs. 3, 4). Overall, rhizobacterial communities (S1, S2 and S3) were dominated by Chloroflexi. Acidobacteria, Actinobacteria and Fusobacteria were found enriched in S3 compared to S1 and S2. Actinobacteria was predominant in all rhizosphere samples. Propionigenium, and Acidobacteria_Gp1 were significantly enriched in S3. Notably, while Propionigenium occupied a large proportion of S3, it was barely found in S1 and S2. With the same screening strategy, 8 phyla and 14 genera were obtained in endobacterial database (Figs. 3, 4). Actinobacteria predominated R1 and R2, while Proteobacteria and Acidobacteria together made up majority of R3. Relative proportion of Proteobacteria, Firmicutes and Cyanobacteria/Chloroplast were higher in R3. Further analysis showed the R1 and R2 share similar bacterial community structure with Acidobacteria_Gp1 as the dominant genus. R3 was mainly consisted of members from Acidobacteria_Gp1, Desulfovibrio, Janibacter, Acetobacterium and Streptophyta.
16S rRNA sequencing
By using the same environmental DNA extracts and clones obtained from traditional cultivation method, small-scale verification conducted in lab by full-length 16S rRNA sequencing revealed a slightly different pattern. Taxonomic characterization suggested that most of the isolates were Firmicutes (in soil and endophytic leaves) and Proteobacteria (in endophytic roots). Results turned out to be the subsets of 454-pyrosequencing dataset, indicating 454-pyrosequencing an efficient way to get better insight of microbial community structure since large proportion of bacteria could be underrepresented due to disadvantages of traditional methods. But several genera like Xanthobacter, Methanoplanus, Exiguobacterium, Rheinheimera, Sinorhizobium, Yokenella, Planococcus, etc. that were believed to possess multiple potential ecological functions were identified by full-length sequencing but showed no representation in 454-pyrosequencing (see Supplementary Tables S2 and S3 online). Sequences with low abundance might be underrepresented by high-throughput methods. Therefore, combination of traditional and next-generation sequencing strategies would provide us a comprehensive understanding of microbiology community under natural status.
Identification and biochemical characterization of heavy metal resistant clones
Refer to previous studies on heavy metal content of wetland plants and preliminary experiments, proper concentrations were added for screening of heavy metal resistant endophytes (50 ppm Cu2+, 100 ppm Pb2+, 50 ppm Zn2+, 5 ppm Cd2+, 5 ppm Cr6+ and 30 ppm Ni2+). Most of the screened endophytes showed multiple resistances to different heavy metal, 5 isolates of them were able to grow on non-nitrogen medium. Biochemical characterization resulted in thirteen IAA producers, five ACC deaminase producer, one siderophore producer and one isolate that could solubilize phosphate (Table. 2). Blastn analysis of these sequences revealed that 34 of them were very similar to Psychrobacter sp. PRwf-1 (NR_074709), this isolate could tolerate 3 out of 5 examined heavy metal and also produce IAA, ACC deaminase. The second highest hits could be affiliated to Lysinibacillus fusiformis strain DSM 2898.
Discussion
Quanzhou bay is reported to have varying degrees of heavy metal contaminations. Despite the adverse environmental impacts, S. alterniflora still thrives and occupies dominant niche. A worldwide investigation indicated that wetlands soil consist of Proteobacteria, Bacteroidetes, Acidobacteria, Firmicutes and Actinobacteria as the five major phyla22. Meanwhile, a previous study also shows that Proteobacteria, Bacteroidetes, Chloroflexi and Firmicutes are major phyla of rhizoplane bacteria in S. alterniflora monoculture18. Based on our study, Chloroflexi (47.20%) was the most abundant phylum in rhizosphere soil. Its relative abundance was higher than the Chloroflexi recovered from the other heavily contaminated sediment samples23. Chloroflexi is generally found in intertidal sediment and moderately acidic wetland24,25, and is believed to be associated with nitrite-oxidizing26, biological nutrient removal (BNR) processes27, sediment carbon cycling28, reductive dehalogenation of polychlorinated biphenyls and organohalide-respiring29,30, etc.
Approximately 9% of OTUs were allocated to six classes in Proteobacteria. Among them, the Delta-, gamma- and betaproteobacteria together accounted for 85.3% of proteobacterial OTUs. It is documented that Deltaproteobacteria is a major group of sulfur-reducing bacteria and the major class of phylum Proteobacteria in the rhizosphere of mangrove3,31. Endophytic roots of monoculture S. alterniflora are predominated by Proteobacteria, Cyanobacteria, Bacteroidetes, Firmicutes and Spirochaetes18. Our data show that although Actinobacteria is sensitive to Cd, Zn contamination32, it formed the largest phylum (39.27%) in endobacterial community. Actinobacteria have been studied as soil bacteria occur abundantly in most plants. They are thought to be indispensable in organic material decomposition, and were revealed as endophytes in endophytic roots of S. alterniflora in the present study. Acidothermus was the most frequently observed genus, followed by Acidobacterium (13.5%), Demequina (5.3%) and sulfate-reducing bacteria Desulfovibrio. Members in these genus are believed to play important roles in the metabolism of nitrogen, phosphorus, sulfur and some other organic compounds in wetland system33. Above all, both rhizo- and endobacterial community compositions were distinct from previous studies on that of S. alterniflora under normal growing environment34,35. These interesting differences observed in our study might be the results of the heavy metal stress on S. alterniflora in situ.
Site 3 was located at an oil terminal, the heavy metal content assessment revealed that rhizosphere soil collected from site 3 showed the highest concentration of Cr (see Supplementary Table S1 online). As shown in PCoA plot, both rhizo- and endobacterial data at site 3 were distinguished with those from other sites. Comprehensive comparison of bacterial potential ecological functions and their abundance in each site revealed a relative higher abundance of ecological functional bacteria at site 3 (see Supplementary Table S4 online). Previous studies demonstrated that high richness and abundance of sulfate-reducing bacteria (SRB) occurred in rhizosphere soil of S. alterniflora during the late growing season, suggesting that the abundant SRB might have close relationships with decomposition of soil organic matters produced by S. alterniflora36. Notably, microorganisms involved in sulfur cycle had significantly higher abundance in rhizosphere soil (1.48%) and root (11%) collected from site 3 compared to that collected from site 1 and 2. These sulfur cycle participants (mainly associated with sulfate-reduction) might also possess ability to oxidize acetate or other organic compounds37, and are considered as numerically important members on macrophyte root surfaces38. Those root-associated bacteria of S. alterniflora were great contributors to sulfur accumulation in S. alterniflora-invaded stands and could cause the higher sulfur concentration in situ than that of native plants or unvegetated zones39.
Other bacteria groups possess potential ecological functions such as phosphate solubilization, biodegradation, aromatic compound degradation, crude-oil degradation etc., shared the same pattern. Commonly, Anaerolineae is often recognized as a large component of microbial communities in sludge wastewater treatment plants40, and has been known to be associated with anaerobic degradation of oil-related compounds. Inconsistent with the previous reports, Anaerolineae was found constituted of over 18% of R3 but could barely found in R1 and R2. Interestingly, its abundance in rhizosphere soil appeared to be the lowest at site 3, indicating that S. alterniflora might accumulate bacteria that possess ecological functional to help its survival under various environment stresses.
S. alterniflora could enrich a certain amount of heavy metals. Screening of heavy metal resistant bacteria resulted in 16 different endophytes that showed resistance against at least one of the tested heavy metal ions. Some of the identified endophytes are previously studied as functional bacteria for phytoremediation. For example, a clone (38.82% of leave endophytes) showed resistance against three different heavy metal ions (Cu2+, Pb2+, Cr6+) was allocated to genus Psychrobacter, members of which are suggested to be applied in phytoextraction41,42. A Lysinibacillus fusiformis strain had the second abundant hits (14.29%), which is proved to have potentials for plant growth promotion due to their abilities to resist/reduce chromate at high level and resist/accumulate boron43,44,45.
Nitrogen (N2)-fixing bacteria are able to form symbiotic association with various plants46. This functional type of bacteria (e.g. Azospirillum, Azotobacter, Oceanomonas) have been isolated from rhizosphere and proved to have significant impact on the nitrogen cycle of the wetland ecosystem. Endophytic N2-fixing bacteria likely constitute only a small percentage of total endophytic bacteria, and the increase of the endophytic N2-fixing bacteria population has been considered as possible way for plants to increase nitrogen fixation47,48. Endophytic N2-fixing bacteria were found in previous researches: a diversity of Azoarcus spp. has been recovered from Kallar grass49; and Klebsiella sp. strain Kp342 have been proved to fix N2 in wheat50. Likewise, N2-fixing endophytes seem to relieve nitrogen deficiencies of sweet potato in nitrogen-poor soils51. Moreover, some members of Paenibacillus are found to be N2-fixing bacteria52. In this case, we inoculated all endophytic isolations on non-nitrogen medium and obtained microbes from Paenibacillus, Lysinibacillus, and Chryseobacterium. Paenibacillus were identified in both leaves and roots, indicating that they might promote plant growth through fixing atmospheric nitrogen and dissolving phosphate.
Bacterial endophytes could promote plant growth by a number of different mechanisms, such as production of phytohormones53, phosphate solubilization activity54,55, nitrogen fixation56, siderophore biosynthesis57,58, and providing essential nutrients to host plants59. Like PGPR, endophytes can also promote plant growth by expressing ACC deaminase. In addition, the resistance of plants treated with ACC deaminase-containing PGPR to flood and heavy metal stress is significantly enhanced60,61. IAA, a plant hormone that does not apparently function as a hormone in bacterial cells, may have evolved in bacteria due to its importance in bacterium–plant relationship. In biochemical characterization of the isolated heavy metal-resistant clones, 13 IAA-producing isolates were identified. Among them, 5 isolates showed positive reaction in ACC-deaminase assay. The 16S rRNA sequencing results suggested that 5 ACC deaminase-containing endophytes were from Psychrobacter, Lysinibacillus and Bacillus. In addition, these isolates showed high levels of IAA synthesis. These results indicated that S. alterniflora was colonized by various kinds of endophytes. Some of the endophytes could tolerant a certain concentration of heavy metal and produce bacterial products to satisfy self-survival, improve plant tolerance to heavy metals and promote plant growth. To better understand how the rhizo- and endobacteria contribute to the heavy metal resistance of S. alterniflora, high-throughput sequencing assay focusing on functional genes is warranted for further study.
In conclusion, sediment of Quanzhou bay was contaminated with various degrees of heavy metals. This study contributes to our understanding of the composition and potential ecological functions of the rhizo- and endobacteria associated with S. alterniflora. The overall pattern of both rhizo- and endobacterial community structures were different from that reported in previous studies. The site 3 was located at an oil terminal with high level of Cr contamination. The rhizobacterial diversity decreased at site 3, root endophytes evolved to higher diversity with a large proportion of ecological functional bacteria for heavy metal accumulations, host plant growth promotion and crude-oil degradation. Some comments have suggested that S. alterniflora could alter the community structure of related functional microorganisms, even affect the carbon, nitrogen, and sulfur cycles in habitat62,63. Based on the analysis of datasets in the present study, root-associated bacteria of S. alterniflora might have the potential to affect nutrient metabolism in wetland ecosystem, especially nitrogen, phosphate, sulfur and carbon cycles. Culture-dependent method together with biochemical assay revealed that endophytes could tolerant certain concentration of heavy metals. Meanwhile, they could act through nitrogen-fixing, phosphate-solubilizing, IAA-producing and ACC-deaminase producing to participate in energy cycles and promote plant growth. Further investigation on the data indicated a considerable proportion of microorganisms with the potential to be applied in phytoremediation and natural medicine development. The functions of these microbial communities need to be further studied so as to elucidate the mechanism of S. alterniflora invasion and survival under heavy metal stress.
Materials and methods
Sample collection
Plants, rhizosphere soil and sediment samples were sampled from three sites of wetland in Quanzhou bay, Fujian, PR China in Dec 4, 2012. Sampling site 1 is located at a sluice of a residential quarter (N24°52.493′, E118°36.764′), site 2 was located at the north-west coast of Jinjiang bridge (N24°52.603′, E118°37.635′) and site 3 is at Houzhu oil terminal (N24°52.655′, E118°40.936′). All subsequent handling of samples was treated with sterilized gloves or tools. Plants with rhizosphere soil were immediately sealed in polyethylene bags and transported to the laboratory in transportable cooler (4 °C). Fresh samples were sorted as rhizosphere soil (S), roots (R) and leaves (L).
Sample pretreatments
Rhizosphere soil (S) was carefully removed from the root of the S. alterniflora, then stored at 4 °C. Plant samples were surface sterilized as Idris described64. Sterility was checked by blotting plant surface tightly onto tryptic soy agar (TSA) plates and incubating plates at 28 °C for 2 days. Surface-sterilized plant samples were cut into small pieces and classified into leaves (L) and roots (R), then stored at 4 °C.
Screening of heavy metal resistant microorganisms
After pretreatment, fresh rhizosphere soil (2.5 g) and sterilized plant samples (R and L) were soaked in tryptic soy broth and shaken for 2 h at 250 rpm at 28 °C, respectively. The solution was then left without shaking for 1 h to allow the settlement of particles. Various tenfold dilutions were plated on TSA (with 100 mg L−1 cycloheximide), nitrogen-free culture medium and TSA contain different concentrations of CrCl6 (5–50 ppm), CdCl2 (2–10 ppm), Pb(NO3)2 (20–100 ppm), ZnCl2 (100–500 ppm), CuSO4 (20–100 ppm), NiSO4 (10–50 ppm), respectively. Plates were incubated at 28 °C until visible clones were observed.
DNA extraction
TIANamp Bacteria DNA kit (TIANGEN) was applied for DNA extraction from clones. For environmental DNA extraction, rhizosphere soil (S), root (R) and leaves (L) were grinded using liquid nitrogen respectively, Soil DNA Kit (Sigma) and Plant DNA Kit (Sigma) were applied to acquire high-quality DNA from rhizosphere soil and plant samples. Equal amount of DNA isolated from 6 samples were pooled into one for further experiments (e.g. the leaf DNA contained the equal amount of DNA that isolated from 6 individual plants). Isolated DNA was resuspended in 50–200 μL sterile water and kept at − 80 °C until use.
Full-length and segment 16S rRNA sequencing
The 16S rRNA region of environmental DNA and DNA extraction from clones was amplified (primers 20F-1503R for rhizobacteria, while 773F-1513R for endophytes to avoid affection of chloroplast)65. PCR products were checked on 1.5% Sepharose gel. Amplified fragments were recovered by QIAquick Gel Extraction Kit (QIAGEN) and inserted into pMD18-T vector, then transformed into E. coli (DH5α) component cells. Colonies (~ 50) were randomly picked for sanger sequencing. The results were subjected to NCBI blast analysis to see whether the results obtained from sanger sequencing is a subset of the 454-sequencing results.
454 Pyrosequencing of V4 16S rRNA tags
Environmental DNA extraction of root and rhizosphere soil were obtained as described above, subsequently quantified with Qubit 2.0 DNA Kit (Invitrogen) and checked for integrity and concentration. High-quality DNA was then proceeded to PCR with barcode-infused universal primers (see Supplementary Table S5 online). PCR products were purified and quantified by Qubit 2.0 DNA Kit. All parallel samples were balanced mixed and sequenced at Sangon, using GS FLX titanium system.
Data analysis
Differentiating sequences by their own barcodes, low complexity sequences were ruled out by Prinseq (version 0.20.4, https://prinseq.sourceforge.net/). To obtain high quality reads, we applied Lucy (1.20p, https://lucy.sourceforge.net/) to trim the inferior quality (Q < 20) domain in both ends of the sequences and reserved sequences with length no less than 50 bp using sliding window method. Potential chimeras were identified and removed using ChimeraSlayer66. Ribosomal Database Project Classifier (RDP v2.2, https://rdp.cme.msu.edu/classifier/classifier.jsp) was applied to classify sequences. Sequences were clustered according to the distance between them, and were subsequently distributed into different operational taxonomic units (OTUs) at 97% similarity using QIIME Software (v1.80, https://qiime.org/). Alpha and beta diversity estimates were calculated by Mothur Software (mothur v1.31.2: https://www.mothur.org/). Bacterial diversity was estimated with Nobs (observed richness), Shannon Weiner diversity index (H’), Simpson index (D), evenness (E) ChaoI, Margalef index (dMa), Menhinick index (R2), Pielou index (Jgi), coverage and PIE after trimming each sample to an equal number of tags67,68,69. Algorithm Unifrac was performed to calculate sample intervals, clustering and principal component analysis (PCA). Figure was drawn using R (v3.1.1, https://www.datavis.ca/R/). Based on the results from RDP classifier (v2.2), relative abundance of each rank at different levels were calculated and compared to find the microflora that showed significant variation between samples (Fisher’s exact test) or groups (T statistic permutation test).
Phosphate solubilization assay
After sequencing, endophyte clones obtained from TSA (containing heavy metal) were preserved and preceded to following tests. Endophyte clones were inoculated to inorganic phosphorus medium, plates were incubated for 5 days at 28 °C. Colonies with soluble phosphorus circles were considered as phosphate-solubilizing strains.
IAA analysis
l-Tryptophan (2.5 mg mL−1) was filter sterilized before use. Medium containing 4 mL of nitrogen medium and 1 mL of l-tryptophan (2.5 mg mL−1) was inoculated with endophyte clone, and shaken for 4 days at 250 rpm at 28 °C. Removed 1 mL of each suspension and mixed thoroughly with 2 mL Sackowski’s reagent70, tubes were shielded from light at room temperature for 30 min. Positive reactions with IAA production showed pink color. Standard curve was established with various tenfold dilutions of IAA standard solution measured at 530 nm for absorbance. Endophyte clones were cultured in nitrogen medium with l-tryptophan (0.5 mg mL−1) for 48 h before measured for absorbance at 600 nm. Supernatants were mixed with isovolumetric Sackowski’s reagent, developed in dark for 30 min, and subjected to measurement for absorbance at 530 nm. Data were recorded for further analysis.
ACC-deaminase production analysis
Endophyte clones were inoculated on DF salts minimal medium respectively71, and incubated at 30 °C. Clones that could grow and pass for 5 times were considered able to produce ACC-deaminase. Positive clones were inoculated in 15 mL TSB, shaken for 24 h at 28 °C. Cells were harvested by centrifugation for 10 min at 8000×g at 4 °C, rinsed with DF salts medium for three times. Cells were resuspended with 7.5 mL ADF medium (DF salts medium with ACC final concentration of 3.0 mM), shaken for 24 h at 200 rpm at 30 °C to induce the production of ACC-deaminase. Cells were harvested by centrifugation for 10 min at 8000×g at 4 °C, rinsed with 0.1 M Tris–HCl (pH 7.6) twice. Samples were resuspended in 600 μL 0.1 M Tris–HCl (pH 8.5), added 30 μL methylbenzene and vortexed for 30 s to make crude enzyme. Protein concentration was determined by Bradford protein assay kit (Thermo Fisher) following the manufacturer’s instruction. Samples were subjected to ACC-deaminase activity test. Briefly, mixed 200 μL crude enzyme and 20 μL ACC (0.5 M), incubated at 30 °C for 15 min, added 1 mL 0.56 M HCL to terminate the reaction (reaction without crude enzyme and reaction without ACC were set up as control groups). Supernatant (1 mL) was removed to a new tube and mixed with 800 μL HCl (0.56 M) and 300 μL 0.2% 2,4-dinitrophenyl hydrazine (dissolved in 2 M HCl). After incubation at 30 °C for 30 min, samples were mixed thoroughly with 2 mL 2 M NaOH, and measured for the absorbance at 540 nm. Α-ketobutyrate standard curve was set up as followed: dissolved 0.102 g α-ketobutyrate in 10 mL 0.1 M Tris–HCl (pH 8.5) to obtain a stocking solution of 100 mM α-ketobutyrate. The stocking solution was diluted into 10 mM before use. Removed 0, 10, 20, 40, 60, 80, 100, 120 μL 10 mM dilution to tubes respectively and added 0.1 M Tris–HCl (pH 8.5) to final volume of 1 mL. The final concentration range of α-ketobutyrate was 0.024–0.293 μM. Samples were processed as described above to establish a standard curve. ACC-deaminase activity was defined by the μmol of α-ketobutyrate produced by 1 mg enzyme protein in the reaction per hour, enzyme-activity unit was α-ketobutyrate μmol/(mg·h).
Siderophore production analysis
Endophyte clones were inoculated on CAS agar plates72. Plates were incubated for 8–12 days at 30 °C. Colonies with yellow halos were considered as able to produce siderophore on CAS agar plate. To quantify siderophore production, solutions and procedures were carried out as previous described72. The quantitative index defined as As/Ar: 0–0.2, +++++; 0.2–0.4, ++++; 0.4–0.6, +++; 0.6–0.8, ++; 0.8–1.0, + Siderophore units were defined as below:
Heavy metal content analysis
Sediments and rhizosphere soil samples were determined using microwave digestion-ICP-MS following the prior description73.
References
Williams, S. L. & Grosholz, E. D. The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuar. Coasts 31, 3–20 (2008).
Blumenthal, D., Mitchell, C. E., Pysek, P. & Jarosik, V. Synergy between pathogen release and resource availability in plant invasion. Proc. Natl. Acad. Sci. U.S.A. 106, 7899–7904 (2009).
Jemaneh, Z. et al. Effects of Spartinaalterniflora invasion on the communities of methanogens and sulfate-reducing bacteria in estuarine marsh sediments. Front. Microbiol. 4, 243 (2013).
Miché, L., Battistoni, F., Gemmer, S., Belghazi, M. & Reinhold-Hurek, B. Upregulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol. Plant Microbe Interact. 19, 502–511 (2006).
Compant, S., Clément, C. & Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42, 669–678 (2010).
Sobariu, D. L. et al. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. Nat. Biotechnol. 39, 125–134 (2016).
Förstner, U. & Wittmann, G. T. W. Metal Pollution in the Aquatic Environment (Springer, Berlin, 1983).
Watling, R. J. & Watling, H. R. Metal surveys in South African estuaries. I. Swartkops River. Water S A. 8, 26–35 (1982).
Singh, J. & Kalamdhad, A. S. Chemical speciation of heavy metals in compost and compost amended soil—a review. Int. J. Environ. Eng. Res. 2, 27–37 (2013).
Sun, Q., Ye, Z. H., Wang, X. R. & Wong, M. H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant Physiol. 164, 1489–1498 (2007).
Phillips, D. P., Human, L. R. D. & Adams, J. B. Wetland plants as indicators of heavy metal contamination. Mar. Pollut. Bull. 92, 227–232 (2015).
Kloepper, J. W., Leong, J., Teintze, M. & Schroth, M. N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286, 885–886 (1980).
Ma, Y., Prasad, M. N. V., Rajkumar, M. & Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 29, 248–258 (2011).
Wan, S., Pei, Q., Liu, J. & Zhou, H. X. The positive and negative effects of exotic Spartina alterniflora in China. Ecol. Eng. 35, 444–452 (2009).
Zhang, Y., Huang, G., Wang, W., Chen, L. & Lin, G. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 93, 588–597 (2012).
Zhang, Q. et al. Abundance and composition of denitrifiers in response to Spartina alterniflora invasion in estuarine sediment. Can. J. Microbiol. 59, 825–836 (2013).
Zhao, C., Liu, X., Bai, J., Fengchun, L. & Li, J. Impact of Spartina alterniflora on benthic macro-invertebrates communities on mangrove wetland in Xicungang Estuary, Guangxi. Biodivers. Sci. 22, 630–639 (2014).
Youwei, H., Dan, L., Anyi, H., Han, W. & Jinsheng, C. Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartina alterniflora and native mangrove using Illumina amplicon sequencing. Can. J. Microbiol. 61, 723–733 (2015).
Yu, R. L. & Hu, G. R. Speciation and ecological risk of heavy metals in sediments from Quanzhou bay. J. Huaqiao Univ. 29, 419–423 (2008).
Hu, G., Yu, R., Zhao, J. & Chen, L. Distribution and enrichment of acid-leachable heavy metals in the intertidal sediments from Quanzhou Bay, southeast coast of China. Environ. Monit. Assess. 173, 107–116 (2011).
Wu, Y. & Liu, R. The Plants’ Adaptability to Environment of Quanzhou Bay Estuary Wetland (Science Press, Beijing, 2011).
Lv, X. et al. A meta-analysis of the bacterial and archaeal diversity observed in wetland soils. Sci. World J. 2014, 437684 (2014).
Zhu, J. et al. Phylogenetic analysis of bacterial community composition in sediment contaminated with multiple heavy metals from the Xiangjiang River in China. Mar. Pollut. Bull. 70, 134–139 (2013).
Wang, Y. et al. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 78, 8264–8271 (2012).
Wilhelm, R. C., Niederberger, T. D., Greer, C. & Whyte, L. G. Microbial diversity of active layer and permafrost in an acidic wetland from the Canadian High Arctic. Can. J. Microbiol. 57, 303–315 (2011).
Sorokin, D. Y. et al. Nitrification expanded: discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 6, 2245–2256 (2012).
Björnsson, L., Hugenholtz, P., Tyson, G. W. & Blackall, L. L. Filamentous Chloroflexi (green non-sulfur bacteria) are abundant in wastewater treatment processes with biological nutrient removal. Microbiology 148, 2309–2318 (2002).
Hug, L. A., Castelle, C. J., Wrighton, K. C., Thomas, B. C. & Banfield, J. F. Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 1, 1–17 (2013).
Krzmarzick, M. J. et al. Natural niche for organohalide-respiring Chloroflexi. Appl. Environ. Microbiol. 78, 393–401 (2012).
Watts, J. E., Fagervold, S. K., May, H. D. & Sowers, K. R. A PCR-based specific assay reveals a population of bacteria within the Chloroflexi associated with the reductive dehalogenation of polychlorinated biphenyls. Microbiology 151, 2039–2046 (2005).
Jiang, X. T. et al. Illumina sequencing of 16S rRNA tag revealed spatial variations of bacterial communities in a mangrove wetland. Microb. Ecol. 66, 96–104 (2013).
Yin, H. et al. An integrated insight into the response of sedimentary microbial communities to heavy metal contamination. Sci. Rep. 5, 14266 (2015).
Li, Y. H., Zhu, J. N., Zhai, Z. H. & Zhang, Q. Endophytic bacterial diversity in roots of Phragmites australis in constructed Beijing Cuihu Wetland (China). FEMS Microbiol. Lett. 309, 84–93 (2010).
Wang, M., Chen, J. K. & Bo, L. I. Characterization of bacterial community structure and diversity in rhizosphere soils of three plants in rapidly changing salt marshes using 16S rDNA. Pedosphere 17, 545–556 (2007).
Zhang, Q. et al. Endophytic bacterial communities associated with roots and leaves of plants growing in Chilean extreme environments. Sci. Rep. 9, 4950 (2019).
Nie, M., Wang, M. & Bo, L. Effects of salt marsh invasion by Spartina alterniflora on sulfate-reducing bacteria in the Yangtze River estuary, China. Ecol. Eng. 35, 1804–1808 (2009).
Muyzer, G. & Stams, A. J. M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 6, 441–454 (2008).
Vladár, P., Rusznyák, A., Márialigeti, K. & Andrea, K. B. Diversity of sulfate-reducing bacteria inhabiting the rhizosphere of Phragmites australis in Lake Velencei (Hungary) revealed by a combined cultivation-based and molecular approach. Microb. Ecol. 56, 64–75 (2008).
Zhou, H. W. et al. BIPES, a cost-effective high-throughput method for assessing microbial diversity. ISME J. 5, 741–749 (2011).
Halkjær, N. P., Caroline, K., Seviour, R. J. & Lund, N. J. Identity and ecophysiology of filamentous bacteria in activated sludge. FEMS Microbiol. Rev. 33, 6 (2009).
Ma, Y., Rajkumar, M. & Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 166, 1154–1161 (2009).
Ma, Y., Rajkumar, M. & Freitas, H. Isolation and characterization of Ni mobilizing PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere 75, 719–725 (2009).
He, M. et al. Characterization and genomic analysis of a highly chromate resistant and reducing bacterial strain Lysinibacillusfusiformis ZC1. J. Hazard. Mater. 185, 682–688 (2011).
Raja, C. E. & Omine, K. Characterization of boron resistant and accumulating bacteria Lysinibacillusfusiformis M1, Bacilluscereus M2, Bacilluscereus M3, Bacilluspumilus M4 isolated from former mining site, Hokkaido, Japan. J. Environ. Sci. Health A Toxic/Hazard. Subst. Environ. Eng. 47, 1341–1349 (2012).
Vendan, R. T., Yu, Y. J., Sun, H. L. & Rhee, Y. H. Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. J. Microbiol. 48, 559–565 (2010).
Gantar, M., Rowell, P., Kerby, N. W. & Sutherland, I. W. Role of extracellular polysaccharide in the colonization of wheat (Triticumvulgare L.) roots by N2-fixing cyanobacteria. Biol. Fertil. Soils 19, 41–48 (1995).
Barraquio, W. L., Revilla, L. & Ladha, J. K. Isolation of endophytic diazotrophic bacteria from wetland rice. Plant Soil. 194, 15–24 (1997).
Ladha, J. K., Barraquio, W. L. & Watanabe, I. Isolation and identification of nitrogen-fixing Enterobacter cloacae and Klebsiella planticola associated with rice plants. Can. J. Microbiol. 29, 1301–1308 (1983).
Reinhold-Hurek, B. et al. Azoarcus gen. nov., nitrogen-fixing proteobacteria associated with roots of kallar grass (Leptochloafusca (L.) Kunth), and description of two species, Azoarcusindigens sp. nov. and Azoarcuscommunis sp. nov.. Int. J. Syst. Bacteriol. 43, 574–584 (1993).
Iniguez, A., Dong, Y. & Triplett, E. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol. Plant Microbe Interact. 17, 1078–1085 (2004).
Reiter, B., Bürgmann, H., Burg, K. & Sessitsch, A. Endophytic nifH gene diversity in African sweet potato. Can. J. Microbiol. 49, 549–555 (2003).
Ryan, R. P., Kieran, G., Ashley, F., Ryan, D. J. & Dowling, D. N. Bacterial endophytes: recent developments and applications. FEMS Microbiol. Lett. 278, 1–9 (2008).
Lee, S. et al. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome c biogenesis genes. J. Bacteriol. 186, 5384–5391 (2004).
Subhash, C. V., Jagdish, K. L. & Anil, K. T. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 91, 127–141 (2001).
Wakelin, S., Warren, R., Harvey, P. & Ryder, M. Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol. Fertil. Soils 40, 36–43 (2004).
Compant, S. et al. Endophytic colonization of Vitisvinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 71, 1685–1693 (2005).
Wang, Y., Brown, H. N., Crowley, D. E. & Szaniszlo, P. J. Evidence for direct utilization of a siderophore, ferrioxamine B, in axenically grown cucumber. Plant Cell Environ. 16, 579–585 (1993).
Cindy, L., Jaco, V., Fiona, P., Edward, R. B. & Moore, S. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 21, 583–606 (2002).
Puente, M. E., Li, C. Y. & Bashan, Y. Endophytic bacteria in cacti seeds can improve the development of cactus seedlings. Environ. Exp. Bot. 66, 402–408 (2009).
Grichko, V. P., Filby, B. & Glick, B. R. Increased ability of transgenic plants expressing the bacterial enzyme ACC deaminase to accumulate Cd Co, Cu, Ni, Pb, and Zn. J. Biotechnol. 81, 45–53 (2000).
Grichko, V. P. & Glick, B. R. Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol. Biochem. 39, 11–17 (2001).
Liao, C. et al. Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 10, 1351–1361 (2007).
Thomas, F., Giblin, A. E., Cardon, Z. G. & Sievert, S. M. Rhizosphere heterogeneity shapes abundance and activity of sulfur-oxidizing bacteria in vegetated salt marsh sediments. Front. Microbiol. 5, 309 (2014).
Idris, R., Trifonova, R., Puschenreiter, M., Wenzel, W. W. & Sessitsch, A. Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl. Environ. Microbiol. 70, 2667–2677 (2004).
Wang, F., Men, X., Zhang, G., Liang, K. & Wu, L. Assessment of 16S rRNA gene primers for studying bacterial community structure and function of aging flue-cured tobaccos. AMB Express. 8, 182 (2018).
Haas, B. J. et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504 (2011).
Margalef, R. Information theory in ecology. Gen. Syst. 3, 36–71 (1958).
Menhinick, E. F. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 45, 859–861 (1964).
Pielou, E. C. An Introduction to Mathematical Ecology (Wiley, New York, 1969).
Gordon, S. A. & Weber, R. P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 26, 192–195 (1951).
Penrose, D. M. & Glick, B. R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 118, 10–15 (2010).
Payne, S. M. Detection, isolation, and characterization of siderophores. Method Enzymol. 235, 329–344 (1994).
Liang, S. X., Wang, X., Wu, H. & Sun, H. W. Determination of 9 heavy metal elements in sediment by ICP-MS using microwave digestion for sample preparation. Spectrosc. Spectr. Anal. 32, 809–812 (2012).
Acknowledgements
The authors would like to thank Professor Ke-Jian Wang (Xiamen University) for his guidance, his constant supervision and for providing us with the necessary equipment and information regarding the project. This work was funded by the National Natural Science Foundation of China (Grant No. 31070296), the Natural Science Foundation of Fujian Province, China (Grant No. 2012D120), the Fujian University industry research cooperation project (2019N5011) and the key special project of universities in Fujian province serving the construction of Western Taiwan Straits economic zone (No. A102).
Author information
Authors and Affiliations
Contributions
J.Y. conceived and conceptualized the study. Y.Y. and J.D. performed all the experiments, data analyses and wrote the original manuscript. J.Y. and Y.C. assisted the writing and revised the manuscript. J.Y. contributed all of the reagents/materials/analysis tools. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Ding, J., Chi, Y. et al. Characterization of bacterial communities associated with the exotic and heavy metal tolerant wetland plant Spartina alterniflora. Sci Rep 10, 17985 (2020). https://doi.org/10.1038/s41598-020-75041-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75041-5
- Springer Nature Limited
This article is cited by
-
Variations on the diazotrophic community in the rhizosphere soil of three dominant plant species in a lead–zinc mine area
Plant and Soil (2023)
-
Source apportionment of heavy metals and their effects on the species diversity of plant communities in the Caizi Lake wetland, China
Environmental Science and Pollution Research (2023)
-
Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations
Environmental Science and Pollution Research (2022)