Abstract
Mangroves are critical in global carbon budget while vulnerable to exotic plant invasion. Spartina alterniflora, one of typical salt marsh plant grows forcefully along the coast of China, has invaded the native mangrove habitats in Zhangjiang Estuary. However, the effects of S. alterniflora invasion on soil carbon gases (CH4 and CO2) emission from mangroves are not fully understood. Accordingly, we conducted a field experiment to investigate the soil CH4 and CO2 emission during growing seasons in 2016 and 2017 at four adjacent wetlands, namely bare mudflat (Mud), Kandelia obovata (KO), Avicennia marina (AM) and S. alterniflora (SA). Potential methane production (PMP), potential methane oxidation (PMO), functional microbial abundance and soil biogeochemical properties were measured simultaneously. Our results indicate that S. alterniflora invasion could dramatically increase soil CH4 emissions mainly due to the enhancement in PMP which facilitated by soil EC, MBC, TOC and mcrA gene abundance. Additionally, S. alterniflora invasion decreases soil CO2 emission. Both heterotrophic microbial respiration (16S rRNA) and methane oxidation (pmoA and ANME-pmoA) are responsible for CO2 emission reduction. Furthermore, S. alterniflora invasion greatly increases GWP by stimulating CH4 emissions. Thus, comparing with mangroves, invasive S. alterniflora significantly (p < 0.001) increases CH4 emission while reduces CO2 emission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The concentrations of atmospheric carbon gases (mainly CO2 and CH4) have drastically increased since the industrial era, playing a pivotal role in global climate change1. Since the industrial era, the atmospheric CO2 concentration had increased from 280 ppm to 403.3 ppm in 2016 with an annual growth rate of 2.21 ppm2. In addition, by 2016, atmospheric CH4 concentration had reached 1853 ppb, which is 257% of the pre-industrial level2. Although the fossil fuel burning, cement industry and other land use changes largely enrich the atmospheric CO21, small changes of CO2 emission from natural soil in long-period may also strongly alter atmospheric CO2 concentration3. For CH4, emission from natural ecosystems accounts for 30–40% of total CH4 emission1,4. Therefore, many studies have grown up around the theme of soil greenhouse gases emission from natural ecosystems5,6,7,8.
Coastal wetland ecosystems play a critical role in global carbon budget and climate change9. Among them, mangrove ecosystems are reported as highly productivity and most carbon rich10,11. As estimated, approximately 75% of total carbon of tropical mangrove ecosystems are stored in mangrove soils11. Therefore, many studies focused on the soil carbon gases emission from mangroves8,12,13,14. However, mangrove ecosystems are vulnerable to invasion from exotic plant species15. Spartina alterniflora, a cordgrass native to North America, has grown forcefully along the northernmost to the southernmost coast of China since it was introduced to China in 197916. As a consequence, S. alterniflora invasion has become one of the most serious ecological problems, challenging the local biodiversity and ecosystem functions17. S. alterniflora are replacing the mangrove habitats in the recent two decades, which will be more and more serious in southeastern China in the future16. S. alterniflora is also one of the most productive ecosystems, as mangrove is, functioning as a large carbon sink in wetland ecosystems11,18.
Although many studies have been conducted to investigate the soil greenhouse gas emission from S. alterniflora salt marsh ecosystems6,19,20,21,22,23, there are very little agreements on the effects of S. alterniflora invasion on soil carbon gases emission. For instance, comparing with the native salt marsh species, S. alterniflora invasion was reported to stimulate CH4 emission due to its higher plant biomass19,20,24. In contrast, some studies showed no significant differences in greenhouse gas emissions between S. alterniflora and Phragmites australis stands in New England25. Moreover, S. alterniflora invasion was reported to increase soil CO2 emission while reducing CH4 emission in Yancheng National Nature Reserve (YNNR) in southeastern China, comparing with native P. australis marshes21. Up to now, inadequate attention has been paid to investigate the effects of S. alterniflora invasion on soil carbon gases emission from mangrove wetlands8,26. Besides, the responses of mangrove soil carbon gases emission to S. alterniflora invasion in relevant researches reached no agreement. For example, in Jiulong River Mangrove Reserve (JRMR), the soil CH4 emission from S. alterniflora invaded mangrove stand was much higher than that from native mangrove stands26. In terms of CO2, however, the highest emission was observed in mangrove Sonneratia apetala stands26. Even at the same locations in JRMR, much uncertainty still exists in the trend and magnitude of soil carbon gases emission after S. alterniflora invasion8. For instance, CH4 emission from S. alterniflora and mangrove Kandelia obovata sites are comparable, which are higher than that from bare mudflat in JRMR8. This inconsistency implies that the effects of S. alterniflora invasion on soil carbon cycle are complicated. As the rapid expansion of S. alterniflora in mangrove wetlands in China, there is an urgent need to reveal the soil carbon gases emission from mangroves as well as its driven factors according to S. alterniflora invasion.
Therefore, the specific objective of this study was to investigate the influences of S. alterniflora invasion on mangrove soil carbon gases emission and to explore its underlying mechanisms. It is estimated that S. alterniflora has expanded rapidly and the areal extent increased from 57.94 ha to 116.11 ha from 2003 to 2015 in Zhangjiang Mangrove Estuary27. The mangrove and S. alterniflora habitats in this area are currently experiencing similar tidal dynamics and soil texture, which provide our field experiment with natural advantages. Several environmental factors exhibited difference between growing season and non-growing season, including average total solar radiation (growing season: 198.24 W/m2; non-growing season: 144.75 W/m2), mean air temperature (growing season: 25.78 °C; non-growing season: 15.28 °C) and total precipitation (growing season: 513.5 mm; non-growing season: 358.9 mm). However, there is no difference in the tidal range and tidal cycle between these two seasons. Due to the higher temperature in the growing season, microbial activities and organic matter decomposition are enhanced8,21, which in turn affect soil carbon gases emission. Additionally, most field studies have demonstrated that a large proportion of soil carbon gases was emitted during warming seasons6,8,26. Similarly, our study in this particular area showed that CH4 and CO2 emission exhibited significant (p < 0.005) spatial difference only during the growing season (May to Oct) over the year (unpublished data). Hence, a field study was conducted during growing seasons (May, Jun and Jul) in 2016 and 2017 at Zhangjiang Mangrove Estuary to investigate soil carbon gases (CH4 and CO2) emission at four adjacent sites, namely bare mudflat (Mud), Kandelia obovata (KO), Avicennia marina (AM) and S. alterniflora (SA). Meanwhile, soil functional microbial abundance (16S rRNA, pmoA, ANME-pmoA and M. oxyfera-pmoA), potential methane production (PMP), potential methane oxidation (PMO), extracellular enzyme activities (invertase and β-glucosidase activity) and other biogeochemical properties (TOC, MBC and so on) were measured simultaneously.
Results
Spatial variations of carbon gases emission
Figure 1 provides the results obtained from the field observations of soil carbon gases emission. On average, KO site (30.24 mg m−2 h−1) exhibited the highest CO2 emission, followed by AM site (23.96 mg m−2 h−1) and Mud site (9.97 mg m−2 h−1). Compared with KO, AM and Mud site, CO2 emission from SA site decreased by about 81.00%, 76.02% and 42.38%, respectively (Fig. 1a). For CH4, it showed no significant difference (p > 0.05) among native mangrove habitats (Mud, AM and KO). However, CH4 emission from SA site reached 2261.23 µg m−2 h−1, which was approximately 275, 5 and 57 times higher than those at Mud, AM and KO site, respectively (Fig. 1b).
Differences in soil biogeochemical properties
The spatial variations of soil biogeochemical properties are shown in Table 1. In contrast to mangrove forest, S. alterniflora invasion significantly (p < 0.05) increased pore-water salinity, water content, soil microbial biomass carbon (MBC) and invertase activity. In addition, the lowest TC, total organic carbon (TOC), CN ratio and organic matter content were observed at Mud site, compared with AM, KO and SA site. While among those vegetation habitats (AM, KO and SA), no significant (p > 0.05) differences were shown. The lowest electrical conductivity (EC) was also observed at Mud site. Besides, S. alterniflora invasion decreased soil pH while mangrove species (AM and KO) showed no remarkable influence. The highest SO42− concentration was observed at KO and Mud site, which was significantly (p < 0.05) higher than that at AM and SA site. Additionally, there were no significant (p > 0.05) differences in soil NH4+, NO3−, NO2− and β-glucosidase activity among all the mentioned sites.
Shifts of functional microbial abundance based on qRT-PCR analysis
Soil functional microbial abundance varied from different sites, which shown in Fig. 2. Soil total microbes, which could be expressed as 16S rRNA gene abundance, was highest at KO site and lowest at Mud site (Fig. 2a). However, the highest mcrA gene abundance was observed at SA site, followed by AM, KO and Mud site (Fig. 2b). About pmoA and ANME-pmoA gene abundance, the trend that be found were highest at KO site, slightly decreased at SA site and the lowest at Mud site (Fig. 2c,d). No significant (p = 0.68) differences were found in M. oxyfera-pmoA gene abundance among these sites (Fig. 2e).
Soil functional microbial abundance 16S rRNA (a); mcrA (b); pmoA (c); ANME-pmoA (d) and M. oxyfera-pmoA (e) in different sites. Mud: bare mudflat; KO: K. obovata; AM: A. marina; SA: S. alterniflora. DW: dry weight. Different lowercase letters in each column indicate a significant difference (p < 0.05). Data were presented as mean ± SE.
Changes of soil PMP and PMO
There were significant (p < 0.005) differences in PMP and PMO among the sites, as shown in Fig. 3. The highest PMP was detected at SA site, reaching 176.94 ng g−1 d−1, which was about 3, 6 and 11 times higher than that at AM (58.01 ng g−1 d−1), KO (27.36 ng g−1 d−1) and Mud site (16.28 ng g−1 d−1), respectively. However, no significant (p > 0.10) differences in PMP were observed among AM, KO and Mud site (Fig. 3a). In contrast, PMO at SA site (24.47 ng g−1 d−1), which has no significant (p > 0.05) differences with Mud and AM site, was lower than that at KO (85.76 ng g−1 d−1) site (Fig. 3b).
Spatial variations of potential methane production (a) and potential methane oxidation (b). Data were presented as positive values with mean ± SE. Mud: bare mudflat; KO: K. obovata; AM: A. marina; SA: S. alterniflora. Different lowercase letters on each column indicate a significant difference (p < 0.05).
Estimation of annual emission of carbon gases and global warming potential (GWP)
In order to assess the effects of S. alterniflora invasion on GWP, we further estimated the annual emission of CO2 and CH4 based on the investigations during the growing season in the present study (Table 2, Fig. 4). We found that the level of soil CO2 and CH4 emission during the ebb tide were relatively close to the daily average emission level (Supplementary Fig. S1 on dashline), which could change over the course of the season. Therefore, the GWP was expressed as g eq-CO2 m−2 year−1 and calculated as following: GWP = annual CO2 emissions + 28 * annual CH4 emissions21. Table 2 showed that the annual CO2 emissions at AM, KO and Mud site were 209.80, 264.90 and 87.34 g m−2 year−1, respectively, which were higher than that at SA site (50.28 g m−2 year−1). On the contrary, in comparison with mangrove habitats, S. alterniflora invasion increased anuual CH4 emission remarkably, estimated as 19808.37 mg m−2 year−1. As calculated and shown in Fig. 4, the GWP was highest at SA site (604.91 g eq-CO2 m−2 year−1), which was approximately 1.9, 2.2 and 6.8 times higher than that from AM, KO and Mud, respectively. CH4 emission only accounts for 2.3–33.5% of total GWP at native mangrove habitats, while this proportion reached 91.7% at SA site.
Discussion
The current study found that S. alterniflora invasion drastically increased CH4 emission by 5–275 folds, which was significantly (p < 0.0001) higher than that from native mangrove habitats (Fig. 1b). Our results were well in agreement with the study conducted in JRMR which revealed that S. alterniflora site has the highest soil CH4 emission, comparing with mangrove K. obovata and S. apetala site26. However, Wang and co-authors found no significant differences in soil CH4 emission between S. alterniflora and K. obovata site in JRMR8. In salt marshes ecosystem of southeastern China, S. alterniflora invasion was reported to increase soil CH4 emission, comparing with P. australis in YNNR6 and Cyperus malaccensis in Shanyutan wetland20 as well as Scirpus mariqueter in Yangtze River estuary19. Previous studies concluded that S. alterniflora stimulated soil CH4 emission mainly due to its higher plant biomass than that of native salt marsh plant20,24. Nevertheless, soil CH4 emission from S. alterniflora site was slightly lower than P. australis site in YNNR21 or comparable with P. australis site in New England25. Many abiotic factors, such as salinity which influencing the activities of methanogens28, inorganic nitrogen (NH4+ and NO3−) which impacting CH4 oxidation29,30, can influence soil CH4 emission. Other than that, soil functional microbes play an important role in regulating soil CH4 dynamic31. In JRMR, S. alterniflora invasion was reported to increase soil bacterial richness and change microbial community structure32. Therefore, we inferred that the distinct effects of S. alterniflora invasion on soil CH4 emission may be mainly due to the highly discrepancy in soil functional microbial abundance (Fig. 2).
Based on redundancy analysis (RDA), CH4 emission was strongly positively correlated with PMP (Fig. 5). This relationship between CH4 emission and PMP was also found in the rice paddy field at Shizukuishi, Japan33. Nevertheless, the PMP showed no spatial differences and was not correlated with soil CH4 flux in Great Xing’an Mountains34. This inconsistency may be due to the subtle environmental differences as well as the complex processes of CH4 emission. Our results showed that S. alterniflora invasion enhanced PMP by 3–11 folds (Fig. 3a). In salt marsh ecosystem, S. alterniflora invasion was reported to increase PMP20,35. Firstly, the PMP was positively correlated with soil TOC (Fig. 5). Previous studies also observed a significant correlation between PMP and soil organic carbon in YNNR35. In our study, TOC were significantly (p < 0.05) higher at vegetation site (AM, KO and SA) than that at Mud site (Table 1). Liu et al. (2007) reported that TOC in S. alterniflora salt marshes was significantly higher than that in mudflats in Jiangsu36. Other relative studies have demonstrated that S. alterniflora could enhance TOC storage comparing with native salt marshes37,38 and mangrove K. obovata26. However, TOC showed no significant (p > 0.80) spatial differences among AM, KO and SA site (Table 1). This may be explained by the facts that the carbon sequestration rates were comparable between mangroves and S. alterniflora salt marshes39. Secondly, the PMP was enhanced as the MBC increased after S. alterniflora invasion (Fig. 5). It was reported that S. alterniflora invasion significantly increased MBC by increasing soil available substrates40. MBC is an important factor in the regulation of soil carbon cycle41. Thirdly, EC, as an important indicator of total soluble salt, was positively correlated with PMP (Fig. 5). Previous studies in S. alterniflora salt marshes in eastern China demonstrated that EC was significantly positively correlated with MBC and TOC40. In our study, we found higher EC in S. alterniflora sites than that in mangrove sites (Table 1), indicating that S. alterniflora invasion could supply methanogens with more available substrates, such as ‘non-competitive’ substrate trimethylamine6. Previous study found that CH4 production was primarily associated with trimethylamine in S. alterniflora site35. Fourthly, PMP was also influenced by methanogens (mcrA) abundance. The increase in mcrA abundance will lead to an enhancement of PMP (Fig. 5). In our study, S. alterniflora invasion significantly (p < 0.005) increased the mcrA abundance (Fig. 2b), which matched the conclusions in earlier studies in coastal salt marsh in YNNR35 and in Dongtan42. It was reported that methanogen community was regulated by trimethylamine which produced by S. alterniflora35. Besides, soil TOC may facilitate the growth of methanogens population43.
Permutation (number: 9999) test for redundancy analysis (RDA) under reduced model of carbon gases emission, PMP, PMO and functional microbial abundance with enviromental biogeochemical properties. TOC: total organic carbon; EC: electrical conductivity; MBC: microbial biomass carbon; PMO: potential methane oxidation; PMP: potential methane production. Lines in black indicate carbon gases emission. PMO and PMP were presented as green color. Functional microbial abundance was marked as italic and red lines. Soil biogeochemical properties were showed in blue lines with arrows.
In our study, comparing with native mangrove KO and AM habitats, S. alterniflora invasion decreased PMO (Fig. 3b), which was negatively correlated with soil CH4 emission (Fig. 5). Generally, the increased PMO activity will directly reduce the CH4 emission44,45. On one hand, high PMO occurred after S. alterniflora invasion due to its highly development of aerenchyma tissue which conducted more O2 into the rhizosphere, inducing more CH4 oxidation20, on the other hand, the aerenchyma structure of S. alterniflora can facilitate the soil CH4 transportation to atmosphere46. In our study, the increases of CH4 produced by S. alterniflora may emit through aerenchyma, finally resulting in high soil surface emission in SA site. S. alterniflora invasion increased soil water content in salt marsh in YNNR47, similar results were also obtained in our study. The high water content may cause an anoxic condition and reduce aerobic CH4 oxidation. In addition, the SO42− concentration showed higher at Mud and KO sites than that at AM and SA sites (Table 1). SO42−, as the electron acceptor, is associated with sulfate-dependent anaerobic CH4 oxidation48. Furthermore, S. alterniflora invasion decreased both pmoA and ANME-pmoA gene abundance, indicating that less CH4 oxidation occurred after S. alterniflora invasion (Fig. 3b). Taken together, comparing with native mangrove species, S. alterniflora invasion significantly (p < 0.05) increased soil EC, MBC, TOC and mcrA gene abundance which collectively enhanced PMP. Correspondingly, S. alterniflora invasion decreased ANME-pmoA and pmoA gene abundance, which decreased PMO.
In our study, S. alterniflora invasion was found to decrease soil CO2 emission (Fig. 1a, Table 2). The CO2 emission at SA sites was significantly (p < 0.01) lower than mangrove KO and AM habitats while was comparable (p = 0.91) to Mud site (Fig. 1a). However, our study did not agree with previous studies which suggested that S. alterniflora had higher CO2 emission than mangrove K. obovata site in JRMR8,26. Besides, there were no changes in CO2 emission after S. alterniflora invasion into Yangtze River estuarine wetland49. It is widely known that CO2 emission is mainly from root respiration and heterotrophic microbial respiration in the soil. Relatively, heterotrophic microbial respiration was found to be the main source of soil CO2 emission50. The effects of S. alterniflora on soil CO2 emission in our study can be explained as following. Firstly, soil CO2 emission was positively correlated with 16S rRNA gene abundance and soil TOC in Tibetan alpine meadow51, similar to our results (Fig. 5). Soil CO2 emission derives from the decomposition of organic substances3. Therefore, soil CO2 emission is at least partially influenced by soil TOC content and its mineralization52. Chen and co-authors found that, in the mangrove ecosystem, soil CO2 emission was positively correlated with TOC12,26, similar as the results in our study (Fig. 5). However, other studies showed that soil CO2 emission was negatively correlated with TOC in North Sulawesi mangrove swamps13. In conclusion, on one hand, as highly productive and biomass-rich ecosystem, mangrove species may provide more TOC for soil microbes (Table 1). On the other hand, high 16S rRNA gene abundance was found at mangrove stands (Fig. 2a). Thus, high abundance of the 16S rRNA gene and high TOC may collectively support the high CO2 emission in mangrove habitats (Fig. 1a). Secondly, a large proportion of produced CH4 was oxidized to CO2, especially in marine soil31,53. A strongly positive relationship between CO2 emission and PMO showed an important role of CH4 oxidation in CO2 emission (Fig. 5). Tong et al. (2012) found that the CH4 oxidation rate was higher at S. alterniflora site than that at C. malaccensis site in Shanyutan wetland20, while we characterized that the PMO decreased after S. alterniflora invasion comparing with mangrove habitats (Fig. 3b). S. alterniflora absorb SO42− for its growth, resulting in low concentration of SO42− (Table 1). High SO42− at mangrove stands may cause more sulfate-dependent anaerobic CH4 oxidation48. In addition, pmoA and ANME-pmoA gene abundance was also higher at mangrove KO stands, indicating that mangrove habitats have higher CH4 oxidation, especially the CH4 oxidation associated with SO42− reduction (Fig. 2c,d). Overall, S. alterniflora invasion decreased CO2 emission compared with native mangrove habitats. The combinations of heterotrophic microbial respiration (16S rRNA) and anaerobic methane oxidation (pmoA and ANME-pmoA) were the main factors in regulating CO2 reduction.
We suggest that S. alterniflora invasion is a double-edged sword. On one hand, S. alterniflora have expanded vastly from 57.94 ha to 116.11 ha during 2003–2015 in Zhangjiang mangrove estuary27. Due to its high productivity, this large-scale of S. alterniflora plant community shift is expected to allocate more atmospheric CO223. Comparing with mangrove ecosystem, S. alterniflora invasion enhanced soil carbon stock (TC, TOC, CN ratio, organic matter content and MBC) (Table 1). On the other hand, S. alterniflora invasion decreased soil CO2 emission while significantly (p < 0.0001) increased soil CH4 emission (Fig. 1, Table 2). The estimation of GWP was drastically increased by S. alterniflora invasion (Fig. 4). Xu et al. (2014) reported that in YNNR, S. alterniflora site has the highest GWP21. In our study, the estimation of GWP may be over-estimated since we calculated only based on the emission data during the growing season. Nevertheless, our results indicated the important effects of S. alterniflora invasion on soil carbon dynamics as well as the regional GWP. Further studies aiming at the impacts of S. alterniflora invasion, particularly by root exudations, on mangrove soil microbial community structure and then C dynamics should be focused.
Materials and Methods
Study site
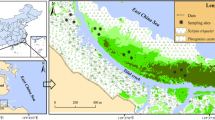
Our study was conducted at Zhangjiang River Estuary Mangrove National Natural Reserve in Yunxiao County, Fujian Province, China (117°24′–117°30′E, 23°53′–23°56′N) (Fig. 6). This region is subjected to subtropical marine monsoon with an annual average temperature of 21.2 °C and annual average precipitation of 1714 mm. This area experiences typical semi-diurnal tide with a mean tidal range of 2.32 m54. In addition, this area situates at the outlet of Zhangjiang River with an average river discharge of about 1,011,220,000 m3 per year27. The total area of this natural reserve is 2360 ha, which consists of 116.11 ha of the invasive plant species S. alterniflora (SA)27. Besides, native mangrove species K. obovata (KO) and A. marina (AM) are widely distributed in this area55. Therefore, four experimental sites with three replicates each were randomly established, including bare mudflat (Mud), KO, AM and SA, representing different vegetation types. The vegetation at each site was monoculture and was at least 20 m away from each other to eliminate the edge effects. All sites were experiencing similar elevation and exposure time to reduce the tidal influences during the field experiment period.
Map description of the study site. Map of Fujian Province and the location of Yunxiao County which marked as black square (a); the selected study area (marked as black square) in Zhangjiang River Estuary Mangrove National Natural Reserve (b); red points indicate the sample site location in Zhangjiang River Estuary Mangrove National Natural Reserve. Mud: bare mudflat; KO: K. obovata; AM: A. marina; SA: S. alterniflora. Base maps (a,b), scale bars and the longitude and latitude were added using ArcGIS for desktop version 10.3 (http://www.esri.com). Image (c) was generated from Google Earth (image source: Google, Landsat/Copernicus, copyright DigitalGlobe). Images were arranged using Adobe Photoshop CC 2017 version 18.0.1 (http://www.adobe.com).
Carbon gases sampling and quantification
In order to obtain daily average soil CO2 and CH4 emission, a pilot experiment was conducted in KO and AM sites during two complete tidal cycles in Oct 2014. Three replicates for each site were applied in carbon gases emission measurements using static closed chamber coupled with gas chromatography12. PVC chambers with the volume of 2 L and the diameter of 20 cm (total area coverage of 0.031 m2) were applied in sampling, similar to Chen et al.12. Before sampling, a foam board was installed at each site to diminish the anthropogenic impacts. Gas samplings were conducted at neap tide during 12:00–14:00 local time as predicted by China Shipping Service (CNSS). During each sampling period, above-ground mangroves or S. alterniflora vegetation were not covered in chambers. The sampling procedures in the present study were similar to Chen et al. (2010) with small modification56. In brief, the open end of the chamber was lightly inserted 3 cm into the soil, then were removed to make a groove, which are used to sealed the chamber in case of the gas leakage. After soil stabilization for 20 mins, the chamber was recovered again over the soil. At 0, 5, 10 and 15 min after closure, headspace air was mixed carefully and 10 ml mixed air was collected by passing a 100 ml gas-tight glass syringe through the sampling outlet. Gas samples were then injected into clean fluorinated ethylene propylene (FEP) Teflon air bags immediately. Temperature inside the chamber was measured simultaneously. In mangrove and S. alterniflora ecosystems, soil GHGs emissions were usually calculated and well represented by using 3 time points6,12,24,57. Although our pilot experiment showed that the obtained gas fluxes by using these approaches were comparable with those data by Jin et al.58, these operations need to be proceed very carefully, or it may cause bias in gases concentrations inside chamber.
Gas samples were analyzed within 24 hours using gas chromatograph (Agilent 7890B, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) with pure nitrogen as the carrier gas. The gas concentrations were determined by comparing the sample peak areas against the standard curves (National Research Center for Certified Reference Materials, Beijing, China). To assure data quality, gradient standard gases were inserted into the gas chromatograph system every hour. All gas concentrations showed confident linear relationship with closure time. Therefore, gas emission rates were calculated using a linear least squares fit6. Data were accepted if the slope of the linear fitting had R2 > 0.90. Based on our observations, the data collected from 2016 and 2017 were comparable for each site. Hence, the obtained data were combined for analysis. Besides, we defined the net soil CO2 flux as soil CO2 emission, which including both plant dark respiration and soil basal respiration.
Soil biogeochemical properties measurements
For each site, three independent soil cores (0–20 cm) were randomly collected using a 6.0 cm diameters PVC tube in Jul 2016. Intact soil cores were then sealed in black plastic bags immediately and stored with ice during the transportation. Before analysis, plant roots and other debris were removed and the remaining soil was completely homogenized. Pore-water salinity was determined after centrifugation of soil using a MASTER-S/MillM salinity refractometer (ATAGO, Japan). Pore-water SO42− concentration was measured using a turbidimetric method according to the methods proposed by Cáceres and colleagues59. Soil inorganic N (NH4+, NO3− and NO2−) concentration were analyzed using AA3 Auto Analyzer 3 (Seal, Germany) after extracted with 2 mol/L KCl as described by Chen et al.12. The chloroform fumigation-extraction method was employed to measure soil MBC47. Traditionally, soil water content was determined by oven-drying of 50 g fresh soil at 30 °C to a constant weight60. After that, dried soil was ground and sieved through a 2 mm sieves. Soil pH was measured at a w (soil): v (water) of 1: 5 using an Orion 3 star digital portable pH meter with a precision level of ±0.01 (Thermo, USA). Organic matter content was measured based on the loss on ignition at 550 °C for 6 hours after 105 °C oven-dried and calculated as OM = ((DW105 °C − DW550 °C)/DW105 °C) * 100, where DW denotes dry weight61. Before TOC measurements, soil samples were decarbonized with 1 mol/L HCl62. Total carbon (TC), TOC and CN ratio were then determined using a Vario EL III Elemental Analyzer with a precision level of ±0.01 for C and N determination (Elementar, Hanau, Germany). In addition, soil invertase activity and β-glucosidase activities were measured according to Shao et al.41. Soil EC was measured at 25 °C using a conductivity meter (Leici DDS-307, Shanghai, China)40.
Soil DNA extraction and quantitative real-time PCR assay
Soil DNA was extracted with FastDNA Spin Kit for Soil (MP Biomedical, Carlsbad, USA) and stored at −20 °C for further experiments. DNA quality and quantity were examined with Nanodrop ND-1000 spectrophotometer (PeqLab, Germany) and agarose gel electrophoresis. A quantitative real-time polymerase chain reaction (qRT-PCR) method was established and applied to determine soil functional microbial abundance based on 16S rRNA63, mcrA64, pmoA44, ANME-pmoA65 and M. oxyfera-pmoA66 (Supplementary Table S1). All qRT-PCR reactions were performed on Bio-Rad CFX96 machine in three replicates for each sample. 20 µL of each reaction mixture contained 10 µL TransStart Tip Green qPCR SuperMix (TransGen Biotech, Beijing), 1 µL DNA, 0.4 µL of each primer and 8.2 µL of nuclease free H2O. PCR products generated using M13 primers from plasmids were used for standard curves. PCR programs: initialization at 95 °C for 5 min, 40 cycles of denaturation at 95 °C for 30 s, annealing for 30 s and final extension at 72 °C for 30 s.
Potential methane production (PMP) and potential methane oxidation (PMO) measurements
PMP and PMO were determined according to Robroek et al.67. In brief, for PMP measurements, 10 g fresh soils were incubated in 120 mL glass vials with a serum cap containing 10 mL sterilized demineralized water. The vials were sealed and the slurry was mixed evenly. All vials were then removed to an incubator under dark after purging with pure N2 for 20 minutes. For PMO measurements, 10 g fresh soils were incubated in 120 mL glass vials containing 10 mL sterilized demineralized water. Headspace air was replaced with 2 mL CH4 (10000 ppmv) to ensure the sufficient methane throughout the experiment. The glass vials were then incubated on a shaker with 150 rpm at 20 °C. Headspace CH4 were sampled every 24 hours for 10 days. For CH4 quantification, headspace gas samples were injected into gas chromatograph (Agilent 7890B, Santa Clara, CA, USA) as described previously. PMP and PMO were calculated from the linear changes of CH4 concentrations and presented as positive values.
Statistics analysis
Figures were performed in GraphPad Prism (Version 6.0c, GraphPad Software, California, USA) or ECharts (http://echarts.baidu.com). Data normality and homogeneity of variance were checked using Shapiro-Wilk normality test and Bartlett test, respectively (R Studio, 1.0.44). The ordinary one-way ANOVA analysis followed by Tukey’s multiple comparisons test was performed to compare the spatial difference in soil carbon gases emission, functional microbial abundance, biogeochemical properties, PMP and PMO when data passed normality and homogeneity of variance tests (SPSS, 22.0). Otherwise, non-parametric (Kruskal-Wallis) analysis followed by Dunn’s multiple comparisons test were used (SPSS, 22.0). In addition, we used a permutation test (permutations number: 9999) for redundancy analysis (RDA) under reduced model to test the primarily environmental factors that drive the variations of soil carbon gases emission, functional microbial abundance, PMP and PMO (R Studio, Version 1.0.44). Significance was accepted if p < 0.05 for all analysis.
Data availability
Additional data can be found in the supplementary material. All data are available upon request by email to the corresponding author.
References
IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Core Writing Team, R.K. Pachauri and L.A. Meyer, https://doi.org/10.1017/CBO9781107415324.004 (2014).
World Meteorological Organization. WMO Greenhouse Gas Bulletin. WMO Bull. 13, 1–8 (2017).
Kuzyakov, Y. & Gavrichkova, O. REVIEW: Time lag between photosynthesis and carbon dioxide efflux from soil: A review of mechanisms and controls. Global Change Biology 16, 3386–3406 (2010).
Allen, D. E. et al. Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol. Biochem. 39, 622–631 (2007).
Kiese, R. & Butterbach-Bahl, K. N2O and CO2 emissions from three different tropical forest sites in the wet tropics of Queensland, Australia. Soil Biol. Biochem. 34, 975–987 (2002).
Yuan, J. J. et al. Exotic Spartina alterniflora invasion alters ecosystem-atmosphere exchange of CH4 and N2O and carbon sequestration in a coastal salt marsh in China. Glob. Chang. Biol. 21, 1567–1580 (2015).
Tiemeyer, B. et al. High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob. Chang. Biol. 22, 4134–4149 (2016).
Wang, H. et al. Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environ. Sci. Pollut. Res. 23, 1873–1885 (2016).
Lu, W. et al. Contrasting ecosystem CO2 fluxes of inland and coastal wetlands: a meta-analysis of eddy covariance data. Glob. Chang. Biol. 23, 1180–1198 (2017).
Donato, D. C. et al. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 4, 293–297 (2011).
Alongi, D. M. Carbon Cycling and Storage in Mangrove Forests. Ann. Rev. Mar. Sci. 6, 195–219 (2014).
Chen, G. C., Tam, N. F. Y. & Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 48, 175–181 (2012).
Chen, G. C. et al. Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci. Total Environ. 487, 91–96 (2014).
Leopold, A., Marchand, C., Deborde, J. & Allenbach, M. Temporal variability of CO2 fluxes at the sediment-air interface in mangroves (New Caledonia). Sci. Total Environ. 502, 617–626 (2015).
Magnoli, S. M., Kleinhesselink, A. R. & Cushman, J. H. Responses to invasion and invader removal differ between native and exotic plant groups in a coastal dune. Oecologia 173, 1521–1530 (2013).
Zhang, Y., Huang, G., Wang, W., Chen, L. & Lin, G. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 93, 588–597 (2012).
Yu, X. Q., Yang, J., Liu, L. M., Tian, Y. & Yu, Z. Effects of Spartina alterniflora invasion on biogenic elements in a subtropical coastal mangrove wetland. Environ. Sci. Pollut. Res. 22, 3107–3115 (2015).
Kulawardhana, R. W. et al. The role of elevation, relative sea-level history and vegetation transition in determining carbon distribution in Spartina alterniflora dominated salt marshes. Estuar. Coast. Shelf Sci. 154, 48–57 (2015).
Cheng, X. et al. Seasonal variation in CH4 emission and its 13C-isotopic signature from Spartina alterniflora and Scirpus mariqueter soils in an estuarine wetland. Plant Soil 327, 85–94 (2010).
Tong, C. et al. Invasive alien plants increase CH4 emissions from a subtropical tidal estuarine wetland. Biogeochemistry 111, 677–693 (2012).
Xu, X. et al. Seasonal and spatial dynamics of greenhouse gas emissions under various vegetation covers in a coastal saline wetland in southeast China. Ecol. Eng. 73, 469–477 (2014).
Yin, S. L. et al. Spartina alterniflora invasions impact CH4 and N2O fluxes from a salt marsh in eastern China. Ecol. Eng. 81, 192–199 (2015).
Zhou, C. F. et al. The invasion of Spartina alterniflora alters carbon dynamics in China’s Yancheng Natural Reserve. Clean-Soil Air Water 43, 159–165 (2015).
Zhang, Y., Ding, W., Cai, Z., Valerie, P. & Han, F. Response of methane emission to invasion of Spartina alterniflora and exogenous N deposition in the coastal salt marsh. Atmos. Environ. 44, 4588–4594 (2010).
Emery, H. E. & Fulweiler, R. W. Spartina alterniflora and invasive Phragmites australis stands have similar greenhouse gas emissions in a New England marsh. Aquat. Bot. 116, 83–92 (2014).
Chen, Y., Chen, G. & Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total Environ. 526, 19–28 (2015).
Liu, M. et al. Monitoring the invasion of Spartina alterniflora using multi-source high-resolution imagery in the Zhangjiang Estuary, China. Remote Sens. 9, 539 (2017).
Chen, Q., Guo, B., Zhao, C. & Xing, B. Characteristics of CH4 and CO2 emissions and influence of water and salinity in the Yellow River delta wetland, China. Environ. Pollut. 239, 289–299 (2018).
Bykova, S., Boeckx, P., Kravchenko, I., Galchenko, V. & Van Cleemput, O. Response of CH4 oxidation and methanotrophic diversity to NH4 + and CH4 mixing ratios. Biol. Fertil. Soils 43, 341–348 (2007).
Dalal, R. C., Allen, D. E., Livesley, S. J. & Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant and Soil 309, 43–76 (2008).
Boetius, A. et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407, 623–626 (2000).
Liu, M. et al. Invasion by cordgrass increases microbial diversity and alters community composition in a mangrove nature reserve. Front. Microbiol. 8, 1–12 (2017).
Inubushi, K. et al. Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Glob. Chang. Biol. 9, 1458–1464 (2003).
Yu, B., Stott, P., Yu, H. & Li, X. Methane emissions and production potentials of forest swamp wetlands in the eastern great Xing’an Mountains, Northeast China. Environ. Manage. 52, 1149–1160 (2013).
Yuan, J. et al. Shifts in methanogen community structure and function across a coastal marsh transect: Effects of exotic Spartina alterniflora invasion. Sci. Rep. 6, 18777 (2016).
Liu, J., Zhou, H., Qin, P. & Zhou, J. Effects of Spartina alterniflora salt marshes on organic carbon acquisition in intertidal zones of Jiangsu Province, China. Ecol. Eng. 30, 240–249 (2007).
Bu, N. et al. Effects of Spartina alterniflora invasion on soil respiration in the Yangtze River Estuary, China. PLoS One 10, 1–16 (2015).
Yang, W. et al. Consequences of short-term C-4 plant Spartina alterniflora invasions for soil organic carbon dynamics in a coastal wetland of Eastern China. Ecol. Eng. 61, 50–57 (2013).
Chmura, G. L., Anisfeld, S. C., Cahoon, D. R. & Lynch, J. C. Global carbon sequestration in tidal, saline wetland soils. Global Biogeochem. Cycles 17, 1111 (2003).
Yang, W. et al. Response of the soil microbial community composition and biomass to a short-term Spartina alterniflora invasion in a coastal wetland of eastern China. Plant Soil 408, 443–456 (2016).
Shao, X., Yang, W. & Wu, M. Seasonal dynamics of soil labile organic carbon and enzyme activities in relation to vegetation types in Hangzhou Bay tidal flat wetland. PLoS One 10, 1–15 (2015).
Zeleke, J. et al. Effects of Spartina alterniflora invasion on the communities of methanogens and sulfate-reducing bacteria in estuarine marsh sediments. Front. Microbiol. 4, 243 (2013).
Liu, D., Ding, W., Jia, Z. & Cai, Z. Influence of niche differentiation on the abundance of methanogenic archaea and methane production potential in natural wetland ecosystems across China. Biogeosciences Discuss. 7, 7629–7655 (2010).
Han, X. et al. Mitigating methane emission from paddy soil with rice-straw biochar amendment under projected climate change. Sci. Rep. 6, 1–10 (2016).
Segarra, K. E. A. et al. High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat. Commun. 6, 1–8 (2015).
Laanbroek, H. J. Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann. Bot. 105, 141–153 (2010).
Yang, W., Jeelani, N., Leng, X., Cheng, X. L. & An, S. Q. Spartina alterniflora invasion alters soil microbial community composition and microbial respiration following invasion chronosequence in a coastal wetland of China. Sci. Rep. 6, 26880 (2016).
Timmers, P. H. A. et al. Anaerobic oxidation of methane associated with sulfate reduction in a natural freshwater gas source. ISME J. 10, 1400–1412 (2016).
Sheng, Q. et al. Greenhouse gas emissions following an invasive plant eradication program. Ecol. Eng. 73, 229–237 (2014).
Poungparn, S. et al. Carbon dioxide emission through soil respiration in a secondary mangrove forest of eastern Thailand. J. Trop. Ecol. 25, 393–400 (2009).
Che, R. et al. 16S rRNA-based bacterial community structure is a sensitive indicator of soil respiration activity. J. Soils Sediments 15, 1987–1990 (2015).
Hu, M. et al. Response of gaseous carbon emissions to low-level salinity increase in tidal marsh ecosystem of the Min River estuary, southeastern China. J. Environ. Sci. (China) 52, 210–222 (2017).
Milucka, J. et al. Methane oxidation coupled to oxygenic photosynthesis in anoxic waters. ISME J. 9, 1991–2002 (2015).
Chen, J. et al. Diurnal variation of nitric oxide emission flux from a mangrove wetland in Zhangjiang River Estuary, China. Estuar. Coast. Shelf Sci. 90, 212–220 (2010).
Li, Z., Wang, W. & Zhang, Y. Recruitment and herbivory affect spread of invasive Spartina alterniflora in China. Ecology 95, 1972–1980 (2014).
Chen, G. C., Tam, N. F. Y. & Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 408, 2761–2767 (2010).
Bauza, J. F., Morell, J. M. & Corredor, J. E. Biogeochemistry of nitrous oxide production in the red mangrove (Rhizophora mangle) forest sediments. Estuar. Coast. Shelf Sci. 55, 697–704 (2002).
Jin, L. et al. Soil respiration in a subtropical mangrove wetland in the Jiulong River Estuary, China. Pedosphere 23, 678–685 (2013).
Cáceres, V. I. et al. Development and validation of an analytical method for the extraction and quantification of soluble sulfates in red clay. Ceramica 61, 277–284 (2015).
Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 32, 1625–1635 (2000).
Heiri, O. & André, F. L. & Gerry Lemcke. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J. Paleolimnol. 25, 101–110 (2001).
Zhang, Y. H., Ding, W. X., Luo, J. F. & Donnison, A. Changes in soil organic carbon dynamics in an Eastern Chinese coastal wetland following invasion by a C-4 plant Spartina alterniflora. Soil Biol. Biochem. 42, 1712–1720 (2010).
Fierer, N., Jackson, J. A., Vilgalys, R. & Jackson, R. B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 71, 4117–4120 (2005).
Rastogi, G., Ranade, D. R., Yeole, T. Y., Patole, M. S. & Shouche, Y. S. Investigation of methanogen population structure in biogas reactor by molecular characterization of methyl-coenzyme M reductase A (mcrA) genes. Bioresour. Technol. 99, 5317–5326 (2008).
Jagersma, C. G. et al. Enrichment of ANME-1 from Eckernförde Bay sediment on thiosulfate, methane and short-chain fatty acids. J. Biotechnol. 157, 482–489 (2012).
Hu, B. -l. et al. Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Natl. Acad. Sci. 111, 4495–4500 (2014).
Robroek, B. J. M. et al. Peatland vascular plant functional types affect methane dynamics by altering microbial community structure. J. Ecol. 103, 925–934 (2015).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2017YFC0506102) and the Natural Science Foundation of China (NSFC) (31570586, 30930076). We are grateful to the Zhangjiang Estuary Mangrove National Natural Reserve for their supports in field works. We appreciate anonymous reviewers and editor for the insightful comments and valuable suggestions.
Author information
Authors and Affiliations
Contributions
G.F. Gao and H.L. Zheng designed the experiment. G.F. Gao and P.F. Li performed the field works. G.F. Gao carried out the gas chromatographic analyses. Z.J. Shen and X.M. Zhang helped to conducted the soil biogeochemical analysis and molecular analysis. Y.Y. Qin and K. Ghoto helped to gather data and performed the artwork. G.F. Gao wrote this manuscript. X.Y. Zhu and H.L. Zheng revised this manuscript. All authors contributed to the discussion of the data and have approved for submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, G.F., Li, P.F., Shen, Z.J. et al. Exotic Spartina alterniflora invasion increases CH4 while reduces CO2 emissions from mangrove wetland soils in southeastern China. Sci Rep 8, 9243 (2018). https://doi.org/10.1038/s41598-018-27625-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27625-5
- Springer Nature Limited
This article is cited by
-
Changes in sediment N mineralization and immobilization in association with Spartina alterniflora invasion in mangrove wetland
Plant and Soil (2024)
-
The fate of carbon resulting from pore water exchange in a mangrove and Spartina alterniflora ecozone
Acta Oceanologica Sinica (2023)
-
Effect of different factors dominated by water level environment on wetland carbon emissions
Environmental Science and Pollution Research (2022)
-
Ecological parameter reductions, environmental regimes, and characteristic process diagram of carbon dioxide fluxes in coastal salt marshes
Scientific Reports (2020)