Abstract
Sex differences in Alzheimer’s disease (AD) biology and progression are not yet fully characterized. The goal of this study is to examine the effect of sex on cognitive progression in subjects with high likelihood of mild cognitive impairment (MCI) due to Alzheimer’s and followed up to 10 years in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Cerebrospinal fluid total-tau and amyloid-beta (Aβ42) ratio values were used to sub-classify 559 MCI subjects (216 females, 343 males) as having “high” or “low” likelihood for MCI due to Alzheimer’s. Data were analyzed using mixed-effects models incorporating all follow-ups. The worsening from baseline in Alzheimer’s Disease Assessment Scale-Cognitive score (mean, SD) (9 ± 12) in subjects with high likelihood of MCI due to Alzheimer’s was markedly greater than that in subjects with low likelihood (1 ± 6, p < 0.0001). Among MCI due to AD subjects, the mean worsening in cognitive score was significantly greater in females (11.58 ± 14) than in males (6.87 ± 11, p = 0.006). Our findings highlight the need to further investigate these findings in other populations and develop sex specific timelines for Alzheimer’s disease progression.
Similar content being viewed by others
Introduction
Understanding the role of sex in health and disease is a cornerstone of personalized medicine1,2. The high failure rate of clinical drug trials in Alzheimer’s disease (AD) over the past decade3 has increased the urgency to better dissect the heterogeneity of AD4 in order to facilitate more personalized therapies. Females have been noted to be at the epicenter of the AD epidemic due to the fact that they account for roughly two-thirds of AD patients in the US and also the majority of caregivers1,5,6. However, despite substantial research investment in AD over decades, the biological role of sex in the neurodegenerative process has been relatively understudied. Laboratory research into AD mechanisms is largely done on male rodents - mirroring the sex bias that exists in many areas of biomedical research where findings from male animals are viewed as generalizable to humans of both sexes6.
The higher prevalence of AD in females was largely assumed to be due to their longer life spans compared to men but recent studies are beginning to paint a more complex picture1,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23. In addition to lifespan differences, there are also well known sex differences in other possible AD risk factors such as in genetics, sex hormone changes in midlife, cognitive reserve, and age of onset of comorbid cardio metabolic diseases (reviewed in1,5,6) whose interactive effects remain poorly studied. Emerging evidence suggests that female sex may be linked to a greater effect of apolipoprotein ε4 allele (APOE ε4) on amyloid pathology and dementia risk as well as a faster rate of cognitive decline after onset of mild cognitive impairment (MCI) or AD10,11,12,13,14,15,16,17. In contrast, other studies note that men may have faster verbal memory decline in normal aging17, an earlier onset of cardiovascular disease, greater risk for cerebral micro-hemorrhage10 and a higher risk for incident MCI18. Initial studies of pathological (e.g. beta-amyloid and tau measurements) and neuronal loss (e.g. hippocampal volumetric imaging) biomarkers has also suggested there may be sex differences in the evolution of AD pathophysiology1,13,20,21,22,23, reviewed in1 and6. These findings, while preliminary, raise the possibility of multiple points of interaction between sex and AD progression.
The Alzheimer’s Disease Neuroimaging Initiative (ADNI), a multicenter, prospective, naturalistic study (www.adni-info.org), conducted at sites in the US and Canada, has provided new insights into the timeline of evolution of AD biomarkers24,25,26. New NIA-AA recommendations for defining “MCI due to AD – high likelihood”27, which require positive pathological (molecular imaging or spinal fluid tests of beta-amyloid and tau) and/or neuronal loss (structural MR imaging) biomarkers in addition to clinical criteria, were, in part, based on MCI data from ADNI. However, these data have not yet been fully examined to study potential sex differences in the progression of subjects with MCI due to AD.
The aims of this study were to examine sex differences in the longitudinal cognitive progression of subjects with high likelihood of MCI due to AD.
Materials and Methods
Study Design
The institutional review board at Duke University Health System and at each site reviewed and approved all ADNI protocols. Prior to data collection, all subjects and their legal representatives, when appropriate, gave written informed consent.
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (adni.loni.ucla.edu). ADNI was launched in 2003 as a large-scale public-private partnership with a primary goal to investigate whether the integration of clinical assessments, serial imaging studies, and other biological markers can be used to discover early signs of Alzheimer’s Disease (AD) progression. ADNI (ClinicalTrials.gov identifier: NCT00106899) involved over 60 sites across the United States and Canada. ADNI-1 recruited approximately 400 MCI subjects and followed them up to 5 years. These subjects could then choose to continue in ADNI-2 and hence had total follow up of up to 10 years. ADNI-2 recruited approximately 150 new MCI subjects and followed them up to 5 years. Details of protocols and methods can be found in the procedures manual [www.adni-info.org].
Subjects
Subjects with late MCI enrolled in ADNI-1 and ADNI-2 were eligible for inclusion in this study and were pooled for analyses. All late MCI subjects were between the ages of 55 and 90, had subjective memory complaint, objective memory deficit documented by the Wechsler Memory Scale Logical Memory II, and a Clinical Dementia Rating (CDR) Global of 0.5, did not meet criteria for dementia and had a Geriatric Depression Scale score of less than 6. The diagnostic criteria for late MCI were identical between ADNI-1 and ADNI-2 (http://adni.loni.usc.edu/methods/documents). All subjects met criteria for late MCI. All ADNI-1 and ADNI-2 MCI subjects with at least one post-baseline visit data were eligible for inclusion. In addition to demographic data, for subject inclusion, data for all the following parameters were required: Alzheimer’s Disease Assessment Scale-Cognitive subscale 11 item (ADAS-Cog11) for at least two different time points, APOE ε4 genotyping results, and biomarker data. The term “baseline” is used to indicate data collected first at either screening or baseline. The definition of the subset of subjects with a high likelihood of “MCI due to AD” is described under Cerebrospinal fluid (CSF) methods.
Demographic and Clinical Variables
Demographic variables included were age, sex, education level. Cognitive variables included the ADAS-Cog11 and Mini Mental State Examination (MMSE) [http://www.adni-info.org/].
APOE ε4 Genotyping
APOE ε4 allele genotyping of all subjects was completed using DNA extracted from peripheral blood cells as detailed previously28.
MRI Hippocampal Volume Measures
Hippocampal volumes for each subject at baseline were extracted from structural MRI brain scans acquired using a standardized protocol and an automated pipeline using FreeSurfer software (https://surfer.nmr.mgh.harvard.edu/)25,29. For this report, only baseline total (right plus left) hippocampal volumes (mm3) were used25.
Cerebrospinal fluid (CSF) Assay
Baseline CSF total tau (t-tau), phosphorylated tau181P (p-tau), and amyloid-beta1–42 (Aβ42) were analyzed by the ADNI Biomarker Core Laboratory at the University of Pennsylvania Medical Center using the multiplex xMAP Luminex platform (Luminex Corp) with Innogenetics (INNO-BIA AlzBio3, for research use–only reagents) immunoassay kit–based reagents (www.adni-info.org). CSF data was available for approximately one-half of ADNI-1 subjects and most of ADNI-2 subjects. Based on t-tau/Aβ42 ratio values, MCI subjects were sub-classified as having “high” (>0.395 cut-off) or “low” (<0.394 cut-off) likelihood of meeting criteria for MCI due to Alzheimer’s26.
Longitudinal Cognitive tests
MCI subjects were monitored in both ADNI-1 and ADNI-2 at 12 month intervals for up to 5 years. In addition, ADNI-1 MCI subjects could be followed for an additional 5 years if they chose to continue into ADNI-2 thus had a maximum possible follow up of 10 years. At each annual follow visit, subjects underwent cognitive assessments [http://www.adni-info.org/].
Statistical Analysis
We pooled MCI subjects from ADNI-1 and ADNI-2 studies. Sex-differences in baseline demographic and cognitive variables were tested using either analysis of variance (ANOVA) or analysis of covariance (ANCOVA).
Next we ran three mixed-effect models to examine the effect of sex on change from baseline in ADAS-Cog11. The first model adjusted for age, education, baseline ADAS-Cog11, and APOE ε4 allele status as follows:
In this model, the follow-up time (month) was centered with the median follow-up time and covariates were centered i.e. a 75 years old APOE ε4- male with 16 years of education and an ADAS-Cog11 score of 11. We included both random slope and random intercept of each subject in this mixed effect model to account for subject-specific variability in each baseline dependent variable and rate of change, respectively, as reported previously14. Square root transformations were used for all dependent variables in all models to obtain approximate normality of estimated error distribution and homoscedasticity (constant variance) of the errors across fitted values of each dependent variable. In the models, APOE ε4 status was treated as a categorical variable while age, education, cognitive scores were treated as continuous variables. In the equation, APOE ε4 ++ indicates carriers of two APOE ε4 alleles (homozygous) while APOE ε4 + indicates carriers of one APOE ε4 allele (heterozygous). We also ran a second mixed effect model replacing APOE ε4 with biomarkers (baseline t-tau, Aβ42 or hippocampal volume) as covariates. Next, we ran a mixed effects model in subjects with high likelihood of MCI due to AD selected two ways. The first was based on high t-tau/Aβ42 ratio. In this model, APOE ε4 status was not included as a term in this last model due to its collinearity with Aβ42. We also ran a mixed effects model in MCI subjects who are APOE ε4 positive. The model terms and covariates are all described in the Tables. Not all analyses had the same sample sizes due to missing values or drop outs. All statistical analyses were conducted in the R (www.r-project.org); mixed-effect models for longitudinal analyses were conducted using the nlme package in the R. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Table 1 summarizes the baseline demographics of the 559 MCI subjects included in this study. Female subjects were younger than male MCI subjects (p = 0.002). There was no statistically significant difference in APOE ε4 carrier status between males and females.
Effect of sex on longitudinal cognitive decline
Mean follow up duration (months) for males (44.8 ± 30.9) did not significantly differ from that of females (42.4 ± 27.3). The mean (±SD) change from baseline in ADAS-Cog11 in females (8.7 ± 12.6) was greater than in males (5.8 ± 10.1, p = 0.001). The mean change from baseline in ADAS-Cog11 in female APOE ε4 carriers (10 ± 14) and non-carriers (7 ± 10) was greater than in male APOE ε4 carriers (8 ± 11) (p = 0.04) and non-carriers (3 ± 9) (p = 0.003), respectively.
Table 2 depicts the mixed effects model testing for sex and APOE ε4 effects on longitudinal change in ADAS-Cog11 in MCI subjects. In this model, sex had a significant effect on ADAS-Cog11 slope (p = 0.003) with the cognitive decline being greater in females than males. Baseline cognition, education and APOE ε4 status also had a significant effect. Subjects with worse baseline cognition and higher education declined faster. APOE ε4 heterozygotes and homozygotes declined faster, and APOE ε4 had a significant effect on both slope and curvature of ADAS-Cog11 change (compared to non-carriers). The effect of interaction between sex and APOE ε4 on ADAS-Cog11 change was not significant (Supplementary Table 1).
Table 3 depicts the mixed effects model testing the effect of sex and baseline CSF Aβ42 (as a continuous measure) on longitudinal change in ADAS-Cog11 in MCI subjects. In this model, sex (p = 0.027), baseline CSF Aβ42 (p < 0.001) had a significant effect on ADAS-Cog11 change. Females and subjects with lower CSF Aβ42 declined faster. Aβ42 also had a significant effect on curvature of ADAS-Cog11 change (p < 0.001). The effect of age, education, baseline cognition was not significant. Supplementary Table 2 depicts the effect of sex and baseline CSF t-tau on ADAS-Cog11 change. In this model, the effect of sex was not significant but patients with higher CSF t-tau had greater ADAS-Cog11 worsening (p < 0.001). Supplementary Table 3 depicts the effect of sex and baseline total hippocampal volume on ADAS-Cog11 change. Baseline total hippocampal volumes were smaller in females (than males). However, when normalized as a ratio to intracranial volume, they were significantly larger than that of males. In the mixed model of ADAS-Cog11 change, the effect of sex was not significant but the effect of baseline total hippocampal volume was significant (p < 0.0001).
Sex differences in Subjects with High Likelihood of MCI due to AD
As described in Methods, we used t-tau/Aβ42 ratio cut-off to identify subjects with “MCI due to AD – high likelihood”. 70% of all MCI subjects, 73% of females and 67% of males met this criterion for MCI due to AD (Table 1). The mean (±SD) change from baseline in ADAS-Cog11 (9 ± 12) in subjects with “MCI due to AD – high likelihood” subjects was significantly greater than that in MCI subjects who did not meet such criteria (1 ± 6, p < 0.0001). Among subjects with “MCI due to AD – high likelihood”, the mean worsening in ADAS-Cog11 was significantly greater in females (12 ± 14) than in males (7 ± 11, p = 0.006) (Figs 1 and 2). Table 4 depicts the mixed effects model testing the effect of sex on ADAS-Cog11 change in subjects with MCI due to AD – in this model, the effect of sex was significant on ADAS-Cog11 slope (p = 0.021). In this model, age, education and baseline cognition did not have a significant effect.
ADAS-Cog11 change in subjects with high or low probability of MCI due to AD. Y-axis depicts the mean (SE) change from baseline in ADAS-Cog11 of MCI subjects by sex. X-axis depicts the grouping by CSF t-tau/Aβ42 ratio into “high” or “low” likelihood of having MCI due to AD. MCI due to AD high probability subjects had greater change than those with low probability. Among subjects with high probability of MCI due to AD, females showed greater change than males. Data comprises pooled MCI subjects from ADNI-1 and ADNI-2.
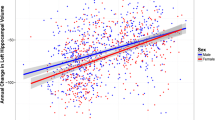
ADAS-Cog11 Slopes in subjects with high or low probability of MCI due to AD. X-axis depicts maximum duration of follow up. Y-axis depicts ADAS-Cog11 scores. MCI subjects have been grouped using CSF t-tau/Aβ42 ratio as having “high” or “low” probability of MCI due to AD. Slopes and confidence intervals are derived from a simple quadratic model (polynomial regression) by sex over time without any other covariates. Data comprises pooled MCI subjects from ADNI-1 and ADNI-2. Female subjects with high probability of MCI due to AD showed greater decline than the other groups.
Sex differences in APOE ε4 positive MCI subjects
Table 5 depicts results of a mixed effect model testing for sex differences in ADAS-Cog11 change over time in APOE ε4 positive MCI subjects (including both heterozygotes and homozygotes). In this model, sex had a near significant effect on ADAS-Cog11 slope (p = 0.05) with the cognitive decline being greater in females than males.
ADNI-1 versus ADNI-2
Supplementary Figures 1 and 2 depict the mean change from baseline to last observation as well as the slopes (derived from a simple quadratic model) of ADAS-Cog11 change in males and females in ADNI-1 and ADNI-2 separately for subjects with MCI due to AD high probability. Of the overall MCI sample, there were 397 from ADNI-1 and 162 from ADNI-2. The mean follow up of ADNI-1 subjects was 48.2 and for ADNI-2 subjects was 33.2. In both ADNI-1 and ADNI-2, MCI due to AD high probability subjects declined much faster than biomarker negative subjects (Supplementary Figures 1 and 2). Among MCI due to AD subjects, significant longitudinal sex differences were seen in ADNI-1 MCI but not in ADNI-2.
Discussion
Understanding the potential underpinnings of sex-related differences in the risk for dementia is an important research priority for the field1,2,3,4,5,6,7. Our study systematically examined sex differences in longitudinal cognitive outcomes using pooled MCI data from two multicenter studies, ADNI-1 and ADNI-2. To our knowledge, this is also the first report to examine sex differences in outcomes of MCI subjects defined using pathological CSF biomarkers to have a high likelihood for MCI due to Alzheimer’s.
Several interesting findings emerged from this study. Our longitudinal cognitive analyses of the pooled dataset found that MCI females showed greater cognitive decline than males. Our study also found that APOE ε4 has an effect on both slope and curvature of ADAS-Cog11 decline and with both heterozygotes and homozygotes declining faster than non-carriers. Further among MCI APOE ε4 carriers, we found that females declined faster than males. We found no interaction effect between sex and APOE ε4 suggesting these variables may potentially have additive but not multiplicative effects. Lastly, we used CSF biomarkers to identify subjects with “high” or “low” probability of having MCI due to AD. Although not a perfect classifier, the tau/Aβ42 ratio cut-off we used has been validated in a clinic-pathological study25, used in published studies e.g.30,31, and cited in the NIA-AA guideline report on diagnosing MCI due to AD27. Approximately 70% of the ADNI MCI sample met these surrogate criteria for “MCI due to AD – high likelihood”. MCI due to AD subjects showed a markedly greater cognitive decline (almost 9-fold) than MCI subjects not meeting these criteria. This further supports the utility of the CSF ratio as a potential prognostic marker for selecting subjects at high risk for decline in clinical trials. Further, among the “MCI due to AD – high likelihood” group, females showed greater cognitive decline than males. This finding extends prior reports of sex differences in MCI13,14,23 and to our knowledge is the first study to examine sex differences in subjects with “MCI due to AD – high likelihood”.
There are some strengths and limitations to our study. A major strength of the ADNI study data is that it represents a multicenter biomarker study that recruited subjects from over 60 sites in the US and Canada and performed longitudinal clinical and biomarker assessments using a highly standardized protocol24. ADNI results have, in part, formed the basis for entry criteria in many prevention trials and hence ADNI is a highly relevant dataset. Our analyses tried to mimic the emerging new criteria for MCI due to AD – high likelihood using pathological CSF biomarkers. There is as such no definitive binary marker for neuronal loss and hence we relied on pathological markers. The relatively large sample size and long duration of follow up are other strengths of the analyses. There are also some limitations. ADNI subjects were recruited largely at clinic-based research sites for a biomarker study and as such may not reflect milder subjects seen in general practice, especially in primary care settings. While we relied on a pathologically validated25 tau/Aβ42 ratio cut-off as a surrogate to identify biomarker positive MCI subjects, there is as yet no perfect in-vivo method to identify MCI due to AD. While the diagnostic utility of CSF tau/Aβ42 ratio may differ by setting and laboratory32, it still remains useful for identifying a subset of rapid decliners. While baseline sex differences were seen in both studies, longitudinal sex differences were driven primarily by ADNI-1 data and were not significant in ADNI-2. We do not know why, but one possibility may be that ADNI-1 recruited twice as many MCI subjects as ADNI-2 and ADNI-1 subjects could have a 120 month maximum follow up (versus 60-month maximum follow up in ADNI-2). Although entry criteria were the same for late MCI between ADNI-1 and ADNI-2, we cannot rule out the possibility of selection bias since the two studies were done 5 years apart. Differences in comorbid conditions and concomitant medications between ADNI studies may also have contributed. Since the follow up period was roughly the same between males and females, the observed differences are less likely to be due to attrition or survival biases but we cannot rule them out. We studied sex effects on the ADAS-Cog11 as it is frequently used in clinical trials. However, it is not necessarily perfectly balanced across all cognitive domains and there is some evidence that females may show a slightly different profile (as a group) than males on this test. To rule out potential testing bias, it is important to also examine sex differences on other cognitive and functional domains. Hence, these issues must be kept in mind while interpreting the data and our findings must be viewed as initial pending replication in population samples.
Our study does not directly address mechanisms that may underlie potential sex differences in MCI progression. Theories proposed have included a greater potency of the AD risk associated with the APOE ε4 allele and the BDNF Met66 allele in females, differences in sex hormones, smaller head size, lower cognitive reserve, as well as the possibility of differential expression of a variety of genes (reviewed in1,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23). The disappearance of sex effects after co-varying for total hippocampal volumes and total tau levels suggest these may be somehow related. However, because AD involves multiple biochemical alterations, systems biology approaches to examine sex differences at a network and pathway level are warranted and may yield deeper insights28,33,34. In summary, our findings of sex differences in both baseline biomarkers and cognitive progression in biomarker defined MCI subjects highlight the need to further investigate sex specific biomarker evolution and disease progression in AD.
References
Snyder, H. M. et al. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimer’s Dement. 12, 1186–1196 (2016).
Cure Alzheimer’s Fund. Women and Alzheimer’s. http://womenandalzheimers.org/ (2016).
Cummings, J. L., Morstorf, T. & Zhong, K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 6, 37 (2014).
Lam, B., Masellis, M., Freedman, M., Stuss, D. T. & Black, S. E. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res. Ther. 5, 1 (2013).
Mielke, M. M., Vemuri, P. & Rocca, W. A. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. J. Clin. Epidemiol. 6, 37–48 (2014).
Lin, K. A. & Doraiswamy, P. M. When Mars versus Venus is not a cliche: gender differences in the neurobiology of Alzheimer’s disease. Front. Neurol. 5, 288 (2014).
Beery, A. K. & Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 (2011).
Seshadri, S. et al. Lifetime risk of dementia and Alzheimer’s disease The impact of mortality on risk estimates in the Framingham Study. Neurology 49, 1498–1504 (1997).
Paganini-Hill, A. & Henderson, V. W. Estrogen deficiency and risk of Alzheimer’s disease in women. Am. J. Epidemiol. 140, 256–261 (1994).
Finch, C. E. & Shams, S. Apolipoprotein E and sex bias in cerebrovascular aging of men and mice. Trends Neurosci. 39, 625–637 (2016).
Bretsky, P. et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 13, 216–221 (1999).
Altmann, A., Tian, L., Henderson, V. W. & Greicius, M. D. Sex modifies the APOE‐related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573 (2014).
Holland, D., Desikan, R. S., Dale, A. M. & McEvoy, L. K. Higher rates of decline for women and apolipoprotein E ε4 carriers. AJNR. Am. J. Neuroradiol. 34, 2287–2293 (2013).
Lin, K. A. et al. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s & dementia: translational research & clinical interventions 1, 103–110 (2015).
Barnes, L. L. et al. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 62, 685–691 (2005).
Irvine, K., Laws, K. R., Gale, T. M. & Kondel, T. K. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J. Clin. Exp. Neuropsychol. 34, 989–998 (2012).
Maylor, E. A. et al. Gender and sexual orientation differences in cognition across adulthood: Age is kinder to women than to men regardless of sexual orientation. Arc. Sex. Behav. 36, 235–249 (2007).
Roberts, R. O. et al. The incidence of MCI differs by subtype and is higher in men The Mayo Clinic Study of Aging. Neurology WNL. 0b013e3182452862 (2012).
Berchtold, N. C. et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 105, 15605–15610 (2008).
Fleisher, A. et al. Sex, apolipoprotein E ε4 status, and hippocampal volume in mild cognitive impairment. Arch. Neurol. 62, 953–957 (2005).
Skup, M. et al. Sex differences in grey matter atrophy patterns among AD and aMCI patients: Results from ADNI. Neuroimage 56, 890–906 (2011).
Hua, X. et al. Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiol. Aging 31, 1463–1480 (2010).
Koran, M. E. I., Wagener, M. & Hohman, T. J. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 11, 205–213 (2017).
Weiner, M. W. et al. Impact of the Alzheimer’s disease neuroimaging initiative, 2004 to 2014. Alzheimer’s Dement. 11, 865–884 (2015).
Shaw, L. M. et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 (2009).
Jack, C. R. et al. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27, 685–691 (2008).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 270–279 (2011).
Saykin, A. J. et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans. Alzheimer’s Dement. 11, 792–814 (2015).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. Neuroimage 61(4), 1402–1418 (2012).
Lampert, E. J., Choudhury, K. R., Hostage, C. A., Petrella, J. R. & Doraiswamy, P. M. Prevalence of Alzheimer’s pathologic endophenotypes in asymptomatic and mildly impaired first-degree relatives. PLoS One 8, e60747 (2013).
Li, X. et al. Ratio of Aβ42/P-tau181p in CSF is associated with aberrant default mode network in AD. Scientific reports 3 (2013).
Ritchie, C. et al. CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). The Cochrane Library (2017).
Atluri, G. et al. Complex biomarker discovery in neuroimaging data: Finding a needle in a haystack. NeuroImage Clin. 3, 123–131 (2013).
Rollo, J. L. et al. Unraveling the mechanistic complexity of Alzheimer’s disease through systems biology. Alzheimer’s Dement. 12, 708–718 (2016).
Acknowledgements
This analysis was supported by the Cure Alzheimer’s Fund and the Karen L. Wrenn Trust. Data collection and sharing for the underlying studies was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2–0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Contributions
P.M.D. and N.S. conceptualized and designed the study with advice from R.E.T., A.J.S., J.R.P., D.S., K.S., and J.L. P.M.D. and J.R.P. oversaw the clinical and biomarker assessments and N.S. oversaw data management. D.S. and K.S. did the data extraction and D.S. did data analyses with advice from P.M.D., J.L. and N.S. D.S. and P.M.D. did manuscript drafting. P.M.D., N.S., R.E.T., A.J.S., J.R.P., D.S., K.S., and J.L. assisted with interpretation and editing.
Corresponding author
Ethics declarations
Competing Interests
P.M.D., R.E.T., A.J.S. and J.R.P. have served as an advisor to and/or received grants from foundations and companies for other work in this field. P.M.D. owns stock in several companies whose products are not discussed here.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sohn, D., Shpanskaya, K., Lucas, J.E. et al. Sex Differences in Cognitive Decline in Subjects with High Likelihood of Mild Cognitive Impairment due to Alzheimer’s disease. Sci Rep 8, 7490 (2018). https://doi.org/10.1038/s41598-018-25377-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25377-w
- Springer Nature Limited
This article is cited by
-
Lifestyle and behavioural changes in older adults during the Covid-19 pandemic are associated with subjective cognitive complaints
Scientific Reports (2024)
-
Cerebrovascular dysregulation and postoperative cognitive alterations after carotid endarterectomy
GeroScience (2024)
-
Neurobehavioral dysfunction in a mouse model of Down syndrome: upregulation of cystathionine β-synthase, H2S overproduction, altered protein persulfidation, synaptic dysfunction, endoplasmic reticulum stress, and autophagy
GeroScience (2024)
-
Associations between Sedentary Duration and Cognitive Function in Older Adults: A Longitudinal Study with 2-Year Follow-Up
The Journal of nutrition, health and aging (2023)
-
Differential predictability of cognitive profiles from brain structure in older males and females
GeroScience (2023)