Abstract
Claudin18.2 (CLDN18.2) is highly expressed with the development of various malignant tumors, especially gastrointestinal cancers, and is emerging as a new target for cancer treatment. Satricabtagene autoleucel (satri-cel)/CT041 is an autologous chimeric antigen receptor (CAR) T cell targeting CLDN18.2, and the interim results of the CT041-CG4006 trial were reported in June 2022. Here we present the final results of this single-arm, open-label, phase 1 trial, which evaluated the safety and efficacy of satri-cel in patients with CLDN18.2-positive advanced gastrointestinal cancers. This trial included a dose-escalation stage (n = 15) and a dose-expansion stage in four different cohorts (total n = 83): cohort 1, satri-cel monotherapy in 61 patients with standard chemotherapy-refractory gastrointestinal cancers; cohort 2, satri-cel plus anti-PD-1 therapy in 15 patients with standard chemotherapy-refractory gastrointestinal cancers; cohort 3, satri-cel as sequential treatment after first-line therapy in five patients with gastrointestinal cancers; and cohort 4, satri-cel monotherapy in two patients with anti-CLDN18.2 monoclonal antibody-refractory gastric cancer. The primary endpoint was safety; secondary endpoints included efficacy, pharmacokinetics and immunogenicity. A total of 98 patients received satri-cel infusion, among whom 89 were dosed with 2.5 × 108, six with 3.75 × 108 and three with 5.0 × 108 CAR T cells. Median follow-up was 32.4 months (95% confidence interval (CI): 27.3, 36.5) since apheresis. No dose-limiting toxicities, treatment-related deaths or immune effector cell-associated neurotoxicity syndrome were reported. Cytokine release syndrome occurred in 96.9% of patients, all classified as grade 1–2. Gastric mucosal injuries were identified in eight (8.2%) patients. The overall response rate and disease control rate in all 98 patients were 38.8% and 91.8%, respectively, and the median progression-free survival and overall survival were 4.4 months (95% CI: 3.7, 6.6) and 8.8 months (95% CI: 7.1, 10.2), respectively. Satri-cel demonstrates therapeutic potential with a manageable safety profile in patients with CLDN18.2-positive advanced gastrointestinal cancer. ClinicalTrials.gov identifier: NCT03874897.



Similar content being viewed by others
Data availability
The data supporting the findings of this trial are available within the manuscript and its Supplementary Information files. All requests for further data sharing will be reviewed by the leading clinical center, the Department of Gastrointestinal Oncology at Peking University Cancer Hospital & Institute, and the trial collaborator, CARsgen Therapeutics Co., Ltd., to verify whether the request is subject to any intellectual property or confidentiality obligations. Requests for access to individual participant-level data from this trial can be submitted via email to shenlin@bjmu.edu.cn with detailed proposals for approval and will be responded to within 60 days. Each participant’s rights and privacy are key subjects taken into consideration when sharing information. A signed data access agreement with the collaborator is required before accessing shared data.
References
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Newick, K., O’Brien, S., Moon, E. & Albelda, S. M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 68, 139–152 (2017).
Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 21, 47–66 (2024).
Wei, J. et al. Clinical development of CAR T cell therapy in China: 2020 update. Cell. Mol. Immunol. 18, 792–804 (2021).
Global Burden of Disease Cancer, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 4, 1553–1568 (2018).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Kubota, Y. et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 8, 100762 (2023).
Qi, C. et al. Clinicopathological significance and immunotherapeutic outcome of claudin 18.2 expression in advanced gastric cancer: a retrospective study. Chin. J. Cancer Res. 36, 78–89 (2024).
Nakayama, I. et al. Claudin 18.2 as a novel therapeutic target. Nat. Rev. Clin. Oncol. 21, 354–369 (2024).
Türeci, O. et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann. Oncol. 30, 1487–1495 (2019).
Shitara, K. et al. Global prevalence of CLDN18.2 in patients with locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: biomarker analysis of two zolbetuximab phase 3 studies (SPOTLIGHT and GLOW). J. Clin. Oncol. 41, 4035 (2023).
Shah, M. A. et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat. Med. 29, 2133–2141 (2023).
Shitara, K. et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 401, 1655–1668 (2023).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
Qi, C. et al. Safety and efficacy of CT041 in patients with refractory metastatic pancreatic cancer: a pooled analysis of two early-phase trials. J. Clin. Oncol. https://doi.org/10.1200/JCO.23.02314 (2024).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454 (2016).
Kang, Y. K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471 (2017).
Sakemura, R. L. et al. CD19 occupancy with tafasitamab increases therapeutic index of CART19 cell therapy and diminishes severity of CRS. Blood 143, 258–271 (2024).
Jacobson, C. A. et al. End of phase 1 results from ZUMA-6: axicabtagene ciloleucel (axi-cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. Blood 132, 4192–4192 (2018).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021).
Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
Hirayama, A. V. et al. Timing of anti-PD-L1 antibody initiation affects efficacy/toxicity of CD19 CAR T-cell therapy for large B-cell lymphoma. Blood Adv. 8, 453–467 (2024).
Evgin, L. et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 14, eabn2231 (2022).
Mackensen, A. et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat. Med. 29, 2844–2853 (2023).
Ma, L. et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell 186, 3148–3165 (2023).
Bobisse, S. et al. A phase 1 trial of adoptive transfer of vaccine-primed autologous circulating T cells in ovarian cancer. Nat. Cancer 4, 1410–1417 (2023).
Pellino, A. et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J. Pers. Med. 11, 1095 (2021).
Wang, S. et al. First-in-human CLDN18.2 functional diagnostic pet imaging of digestive system neoplasms enables whole-body target mapping and lesion detection. Eur. J. Nucl. Med. Mol. Imaging 50, 2802–2817 (2023).
Kamdar, M. et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 399, 2294–2308 (2022).
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Bishop, M. R. et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N. Engl. J. Med. 386, 629–639 (2022).
Rodriguez-Otero, P. et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N. Engl. J. Med. 388, 1002–1014 (2023).
San-Miguel, J. et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N. Engl. J. Med. 389, 335–347 (2023).
Uslu, U. et al. Chimeric antigen receptor T cells as adjuvant therapy for unresectable adenocarcinoma. Sci. Adv. 9, eade2526 (2023).
Lin, X., Lee, S., Sharma, P., George, B. & Scott, J. Summary of US Food and Drug Administration chimeric antigen receptor (CAR) T-cell biologics license application approvals from a statistical perspective. J. Clin. Oncol. 40, 3501–3509 (2022).
Wang-Gillam, A. et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387, 545–557 (2016).
Marin, D. et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 30, 772–784 (2024).
Mailankody, S. et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 29, 422–429 (2023).
Xiang, J. et al. An ‘off-the-shelf’ CD2 universal CAR-T therapy for T-cell malignancies. Leukemia 37, 2448–2456 (2023).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Dickinson, M. J. et al. A novel autologous CAR-T therapy, YTB323, with preserved T-cell stemness shows enhanced CAR T-cell efficacy in preclinical and early clinical development. Cancer Discov. 13, 1982–1997 (2023).
Jiang, H. et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J. Natl Cancer Inst. 111, 409–418 (2019).
Locke, F. L. et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol. Ther. 25, 285–295 (2017).
Corsaro, B. et al. 2020 White Paper on Recent Issues in Bioanalysis: Vaccine Assay Validation, qPCR Assay Validation, QC for CAR-T Flow Cytometry, NAb Assay Harmonization and ELISpot Validation (Part 3 - Recommendations on Immunogenicity Assay Strategies, NAb Assays, Biosimilars and FDA/EMA Immunogenicity Guidance/Guideline, Gene & Cell Therapy and Vaccine Assays). Bioanalysis 13, 415–463 (2021).
Acknowledgements
We thank all the participants in this trial and their families as well as the trial site investigators. This trial was funded, in part, by the National Key Research and Development Program of China (no. 2022YFC2505006 and no. 2023YFC3403700), CARsgen Therapeutics Co., Ltd. and the National Natural Science Foundation of China (no. U22A20327).
Author information
Authors and Affiliations
Contributions
The manuscript was written by the first authors and critically reviewed and revised by the other authors. All authors contributed to the analysis and interpretation of data. The authors affirm the accuracy and completeness of the data and adherence of the trial to the protocol.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Nathan Singh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Trial flow diagram and procedure schema.
(a) Two-phase trial design: a dose escalation/de-escalation phase and a dose-expansion phase to determine the recommended dose (RD). In dose-expansion stage, satri-cel was evaluated in 4 cohorts. (b) Schema of trial procedure. DLT, dose-limiting toxicity; ECOG, Eastern Cooperative Oncology Group performance status.
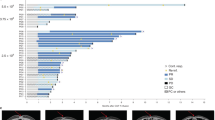
Extended Data Fig. 2 Antitumor activity.
Best percentage changes in target lesions in dose escalation stage and each of the 4 cohorts (n = 90). The patients without tumor type labeled were GC/GEJ.
Extended Data Fig. 3 Survival by subgroups in all patients (n = 98).
(a) Progression-free survival. (b) Overall survival. Note: The mPFS and mOS were estimated by Kaplan-Meier method, and the corresponding two-sided 95% CI was calculated using Brookmeyer-Crowley method. Squares and bars represent the mPFS and mOS with 95% CIs for each subgroup.
Extended Data Fig. 4 Association of CAR T cell expansion, cytokines, CRP, and ferritin with different index or outcomes.
(a) CAR copy numbers after first (n = 98), second and third infusions (n = 59) in all patients. (b) CAR copy numbers after first (n = 59), second and third infusions (n = 35) in GC/GEJ patients with monotherapy. (c) CAR copy numbers of satri-cel monotherapy (n = 83) and satri-cel plus PD-1 inhibitor cohort (n = 15) after the first infusion. (d) The association between Cmax after the first infusion and the tumor response. (e) Cmax after the first infusion and CRS grade. (f) Baseline IL-6 and CRS grade. (g) Baseline TGF-β1 and CRS grade. (h) Baseline CRP and CRS grade. (i) Baseline ferritin and CRS grade. Note: Dots and bars show the median and interquartile ranges (IQR) for CAR copies at each visit in plot a-c; The horizontal lines and the boxes show the medians and IQR in plot d-i, whiskers show the minimum observation above the lower fence (1.5 IQR below the 25th percentile) and the maximum observation below upper fence (1.5 IQR above the 75th percentile). The p-values were calculated using the two-sided Wilcoxon rank sum test.
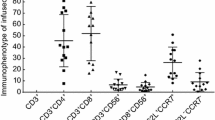
Extended Data Fig. 5 Association between frequencies of T cell subsets and Cmax or AUClast of CAR copies after first infusion in GC/GEJ patients with monotherapy.
The association between Cmax or AUClast of CAR copies after first infusion and the frequencies of, (a) CD3+CD4−CD8+ (cytotoxic T lymphocytes, CTLs) cells, (b) CD3+CD4+CD8− (Th) cells, (c) CD45RA+/CCR7+ (naive T and stem cell-like memory T, TN/SCM) cells, (d) CD45RA−/CCR7+ (central memory T, TCM) cells, (e) CD45RA−/CCR7− (effector memory T, TEM) cells, and (f) CD45RA+/CCR7− (terminally differentiated effector T, TEFF) cells in CT041 product were tested by Spearman Correlation Analysis and p-value was calculated from a Student’s t distribution. Linear regression analyses were performed for the correlation among the frequencies of CD45RA+/CCR7− cells and Cmax (g) or AUClast (h). Shaded bands show the 95% CIs of predicted probability, and lines present the predicted probability of Cmax and AUClast from linear regression analyses. Note: For Fig. 5a–f, Cmax and AUClast were analyzed by quartile of T cell subsets, i.e, Patients were divided into 4 groups based on the quartiles of T cell subset (the 4 groups are the minimum to quartile 1 (Q1), Q1 to median(Q2), median to Q3, and Q3 to maximum). The horizontal lines and the boxes show the medians and IQR, whiskers show the minimum observation above the lower fence (1.5 IQR below the 25th percentile) and the maximum observation below upper fence (1.5 IQR above the 75th percentile). The circular dots represent individual T cell subset level parameters for patients.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Supplementary Tables 1–8
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, C., Liu, C., Gong, J. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial final results. Nat Med 30, 2224–2234 (2024). https://doi.org/10.1038/s41591-024-03037-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-03037-z
- Springer Nature America, Inc.
This article is cited by
-
Targeting Claudin-18.2 for cancer therapy: updates from 2024 ASCO annual meeting
Journal of Hematology & Oncology (2024)