Abstract
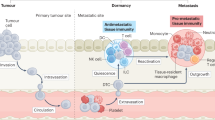
Metastasis, the spread of cancer from a primary site to distant organs, is an important challenge in oncology. This Review explores the complexities of immune escape mechanisms used throughout the metastatic cascade to promote tumor cell dissemination and affect organotropism. Specifically, we focus on adaptive plasticity of disseminated epithelial tumor cells to understand how they undergo phenotypic transitions to survive microenvironmental conditions encountered during metastasis. The interaction of tumor cells and their microenvironment is analyzed, highlighting the local and systemic effects that innate and adaptive immune systems have in shaping an immunosuppressive milieu to foster aggressive metastatic tumors. Effectively managing metastatic disease demands a multipronged approach to target the parallel and sequential mechanisms that suppress anti-tumor immunity. This management necessitates a deep understanding of the complex interplay between tumor cells, their microenvironment and immune responses that we provide with this Review.




Similar content being viewed by others
References
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Conod, A., Silvano, M. & Ruiz, I. A. A. On the origin of metastases: induction of pro-metastatic states after impending cell death via ER stress, reprogramming, and a cytokine storm. Cell Rep. 38, 110490 (2022).
Deng, J. & Fleming, J. B. Inflammation and myeloid cells in cancer progression and metastasis. Front. Cell Dev. Biol. 9, 759691 (2021).
Phan, T. G. & Croucher, P. I. The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020).
Heerboth, S. et al. EMT and tumor metastasis. Clin. Transl. Med. 4, 6 (2015).
Gu, Y., Zhang, Z. & Ten Dijke, P. Harnessing epithelial–mesenchymal plasticity to boost cancer immunotherapy. Cell. Mol. Immunol. 20, 318–340 (2023).
Peinado, H., Olmeda, D. & Cano, A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 (2007).
Eckert, M. A. et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19, 372–386 (2011).
Krebs, A. M. et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518–529 (2017).
Tang, X., Sui, X., Weng, L. & Liu, Y. SNAIL1: linking tumor metastasis to immune evasion. Front. Immunol. 12, 724200 (2021).
Fazilaty, H. et al. A gene regulatory network to control EMT programs in development and disease. Nat. Commun. 10, 5115 (2019).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial–mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Xiao, G. Y. et al. EMT activates exocytotic Rabs to coordinate invasion and immunosuppression in lung cancer. Proc. Natl Acad. Sci. USA 120, e2220276120 (2023).
Taki, M. et al. Tumor immune microenvironment during epithelial–mesenchymal transition. Clin. Cancer Res. 27, 4669–4679 (2021).
Dongre, A. et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 77, 3982–3989 (2017).
Song, W., Mazzieri, R., Yang, T. & Gobe, G. C. Translational significance for tumor metastasis of tumor-associated macrophages and epithelial–mesenchymal transition. Front. Immunol. 8, 1106 (2017).
Grigoriev, I. et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell 13, 305–314 (2007).
Kudo-Saito, C., Shirako, H., Takeuchi, T. & Kawakami, Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15, 195–206 (2009).
Bates, M. et al. Circulating tumour cells: the good, the bad and the ugly. Biochim. Biophys. Acta Rev. Cancer 1878, 188863 (2023).
Mullins, R. D. Z., Pal, A., Barrett, T. F., Heft Neal, M. E. & Puram, S. V. Epithelial–mesenchymal plasticity in tumor immune evasion. Cancer Res. 82, 2329–2343 (2022).
Aiello, N. M. et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 45, 681–695 (2018).
Pastushenko, I. et al. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 (2018).
Li, W. & Kang, Y. Probing the fifty shades of EMT in metastasis. Trends Cancer 2, 65–67 (2016).
Wang, G. et al. The pan-cancer landscape of crosstalk between epithelial–mesenchymal transition and immune evasion relevant to prognosis and immunotherapy response. NPJ Precis. Oncol. 5, 56 (2021).
Subhadarshini, S., Markus, J., Sahoo, S. & Jolly, M. K. Dynamics of epithelial–mesenchymal plasticity: what have single-cell investigations elucidated so far? ACS Omega 8, 11665–11673 (2023).
Parodi, M. et al. Hybrid epithelial–mesenchymal status of lung cancer dictates metastatic success through differential interaction with NK cells. J. Immunother. Cancer 12, e007895 (2024).
Getu, A. A. et al. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 22, 43 (2023).
Kroger, C. et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl Acad. Sci. USA 116, 7353–7362 (2019).
Luond, F. et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 56, 3203–3221 (2021).
Yamamoto, A., Doak, A. E. & Cheung, K. J. Orchestration of collective migration and metastasis by tumor cell clusters. Annu. Rev. Pathol. 18, 231–256 (2023).
Lo, H. C. et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nat. Cancer 1, 709–722 (2020).
Chockley, P. J. et al. Epithelial–mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J. Clin. Invest. 128, 1384–1396 (2018).
Lopez-Soto, A. et al. Epithelial–mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J. Immunol. 190, 4408–4419 (2013).
Boya, M. et al. High throughput, label-free isolation of circulating tumor cell clusters in meshed microwells. Nat. Commun. 13, 3385 (2022).
Chen, Q. et al. A narrative review of circulating tumor cells clusters: a key morphology of cancer cells in circulation promote hematogenous metastasis. Front. Oncol. 12, 944487 (2022).
Lin, D. et al. Circulating tumor cells: biology and clinical significance. Signal Transduct. Target. Ther. 6, 404 (2021).
Chen, M. B. et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl Acad. Sci. USA 115, 7022–7027 (2018).
Najmeh, S. et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer 140, 2321–2330 (2017).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Li, J., Chen, J., Sun, J. & Li, K. The formation of NETs and their mechanism of promoting tumor metastasis. J. Oncol. 2023, 7022337 (2023).
Liu, Q., Liao, Q. & Zhao, Y. Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med. Hypotheses 87, 34–39 (2016).
Sugino, T. et al. Sinusoidal tumor angiogenesis is a key component in hepatocellular carcinoma metastasis. Clin. Exp. Metastasis 25, 835–841 (2008).
Fang, J. H. et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial–mesenchymal transition-independent manner. Hepatology 62, 452–465 (2015).
Renne, S. L. et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology 71, 183–195 (2020).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Xiong, G. et al. Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell–platelet interaction. Proc. Natl Acad. Sci. USA 117, 3748–3758 (2020).
Hapeman, J. D., Carneiro, C. S. & Nedelcu, A. M. A model for the dissemination of circulating tumour cell clusters involving platelet recruitment and a plastic switch between cooperative and individual behaviours. BMC Ecol. Evol. 23, 39 (2023).
Deng, X. & Terunuma, H. Harnessing NK cells to control metastasis. Vaccines 10, 2018 (2022).
Liu, X. et al. Immune checkpoint HLA-E:CD94–NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell 41, 272–287 (2023).
Liu, X. et al. Immune checkpoints HLA-E:CD94–NKG2A and HLA-C:KIR2DL1 complementarily shield circulating tumor cells from NK-mediated immune surveillance. Cell Discov. 10, 16 (2024).
Massague, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7, 1194–1201 (2001).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Gong, Z. et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity 55, 1483–1500 (2022).
Wong, C. C. et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl Acad. Sci. USA 108, 16369–16374 (2011).
Tyagi, A. et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 12, 474 (2021).
Qi, Z., Qi, S., Ling, L., Lv, J. & Feng, Z. Salidroside attenuates inflammatory response via suppressing JAK2–STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int. Immunopharmacol. 35, 265–271 (2016).
Yao, D., Dai, C. & Peng, S. Mechanism of the mesenchymal–epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 9, 1608–1620 (2011).
Del Pozo Martin, Y. et al. Mesenchymal cancer cell–stroma crosstalk promotes niche activation, epithelial reversion, and metastatic colonization. Cell Rep. 13, 2456–2469 (2015).
Ouzounova, M. et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat. Commun. 8, 14979 (2017).
Sceneay, J. et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 72, 3906–3911 (2012).
Strauss, L. et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 5, eaay1863 (2020).
Gao, D. et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 72, 1384–1394 (2012).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Bassler, K., Schulte-Schrepping, J., Warnat-Herresthal, S., Aschenbrenner, A. C. & Schultze, J. L. The myeloid cell compartment—cell by cell. Annu. Rev. Immunol. 37, 269–293 (2019).
LaMarche, N. M. et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature 625, 166–174 (2024).
Millrud, C. R., Bergenfelz, C. & Leandersson, K. On the origin of myeloid-derived suppressor cells. Oncotarget 8, 3649–3665 (2017).
Cassetta, L. et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer 8, e001223 (2020).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Rodriguez-Tirado, C. et al. Interleukin 4 controls the pro-tumoral role of macrophages in mammary cancer pulmonary metastasis in mice. Cancers 14, 4336 (2022).
Jin, Q. et al. IL4/IL4R signaling promotes the osteolysis in metastatic bone of CRC through regulating the proliferation of osteoclast precursors. Mol. Med. 27, 152 (2021).
Ma, R. Y. et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 217, e20191820 (2020).
Luckett, T. et al. Mesothelin secretion by pancreatic cancer cells co-opts macrophages and promotes metastasis. Cancer Res. 84, 527–544 (2024).
Dangaj, D. et al. Mannose receptor (MR) engagement by mesothelin GPI anchor polarizes tumor-associated macrophages and is blocked by anti-MR human recombinant antibody. PLoS ONE 6, e28386 (2011).
Feng, Y., Hu, X., Zhang, Y. & Wang, Y. The role of microglia in brain metastases: mechanisms and strategies. Aging Dis. 15, 169–185 (2024).
Simon, A. et al. Metastatic breast cancer cells induce altered microglial morphology and electrical excitability in vivo. J. Neuroinflammation 17, 87 (2020).
Wu, S. Y. et al. Tamoxifen suppresses brain metastasis of estrogen receptor-deficient breast cancer by skewing microglia polarization and enhancing their immune functions. Breast Cancer Res. 23, 35 (2021).
Xing, F. et al. Loss of XIST in breast cancer activates MSN–c-Met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 78, 4316–4330 (2018).
Zhou, H., Zhao, C., Shao, R., Xu, Y. & Zhao, W. The functions and regulatory pathways of S100A8/A9 and its receptors in cancers. Front. Pharmacol. 14, 1187741 (2023).
Aleckovic, M. & Kang, Y. Welcoming treat: astrocyte-derived exosomes induce PTEN suppression to foster brain metastasis. Cancer Cell 28, 554–556 (2015).
Ma, W. et al. Type I interferon response in astrocytes promotes brain metastasis by enhancing monocytic myeloid cell recruitment. Nat. Commun. 14, 2632 (2023).
Evans, K. T. et al. Microglia promote anti-tumour immunity and suppress breast cancer brain metastasis. Nat. Cell Biol. 25, 1848–1859 (2023).
Correia, A. L. Locally sourced: site-specific immune barriers to metastasis. Nat. Rev. Immunol. 23, 522–538 (2023).
Medaglia, C. et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science 358, 1622–1626 (2017).
Xu, Y. et al. Technological advances in cancer immunity: from immunogenomics to single-cell analysis and artificial intelligence. Signal Transduct. Target. Ther. 6, 312 (2021).
Aouad, P., Quinn, H. M., Berger, A. & Brisken, C. Tumor dormancy: EMT beyond invasion and metastasis. Genesis 62, e23552 (2023).
Borriello, L. et al. Primary tumor associated macrophages activate programs of invasion and dormancy in disseminating tumor cells. Nat. Commun. 13, 626 (2022).
Sosa, M. S. et al. NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nat. Commun. 6, 6170 (2015).
Khalil, B. D. et al. An NR2F1-specific agonist suppresses metastasis by inducing cancer cell dormancy. J. Exp. Med. 219, e20210836 (2022).
Riethmuller, G. & Klein, C. A. Early cancer cell dissemination and late metastatic relapse: clinical reflections and biological approaches to the dormancy problem in patients. Semin. Cancer Biol. 11, 307–311 (2001).
Teng, M. W., Swann, J. B., Koebel, C. M., Schreiber, R. D. & Smyth, M. J. Immune-mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol. 84, 988–993 (2008).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016).
Pantel, K. et al. Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res. 51, 4712–4715 (1991).
Pommier, A. et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science 360, eaao4908 (2018).
Goddard, E. T. et al. Immune evasion of dormant disseminated tumor cells is due to their scarcity and can be overcome by T cell immunotherapies. Cancer Cell 42, 119–134 (2024).
Owen, K. L. et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. 21, e50162 (2020).
Bidwell, B. N. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Reticker-Flynn, N. E. et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 185, 1924–1942 (2022).
Olkhanud, P. B. et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 71, 3505–3515 (2011).
Meyer, M. A. et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 9, 1250 (2018).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Park, S. J. et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 173, 3844–3854 (2004).
Zhao, L. et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 24, 1536–1544 (2018).
Long, H. et al. Tumor-induced erythroid precursor-differentiated myeloid cells mediate immunosuppression and curtail anti-PD-1/PD-L1 treatment efficacy. Cancer Cell 40, 674–693 (2022).
Parker, B. S., Rautela, J. & Hertzog, P. J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer 16, 131–144 (2016).
Yu, R., Zhu, B. & Chen, D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell. Mol. Life Sci. 79, 191 (2022).
Schoenborn, J. R. & Wilson, C. B. Regulation of interferon-γ during innate and adaptive immune responses. Adv. Immunol. 96, 41–101 (2007).
Lazear, H. M., Nice, T. J. & Diamond, M. S. Interferon-λ: immune functions at barrier surfaces and beyond. Immunity 43, 15–28 (2015).
Ye, L., Schnepf, D. & Staeheli, P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 19, 614–625 (2019).
Kotenko, S. V., Rivera, A., Parker, D. & Durbin, J. E. Type III IFNs: beyond antiviral protection. Semin. Immunol. 43, 101303 (2019).
Taffoni, C. et al. DNA damage repair kinase DNA-PK and cGAS synergize to induce cancer-related inflammation in glioblastoma. EMBO J. 42, e111961 (2023).
Antonczyk, A. et al. Direct inhibition of IRF-dependent transcriptional regulatory mechanisms associated with disease. Front. Immunol. 10, 1176 (2019).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Wang, R. W., Vigano, S., Ben-David, U., Amon, A. & Santaguida, S. Aneuploid senescent cells activate NF-κB to promote their immune clearance by NK cells. EMBO Rep. 22, e52032 (2021).
Wang, H. et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl Acad. Sci. USA 114, 1637–1642 (2017).
Hoevenaar, W. H. M. et al. Degree and site of chromosomal instability define its oncogenic potential. Nat. Commun. 11, 1501 (2020).
Liu, J. et al. Epigenetic priming improves salvage chemotherapy in diffuse large B-cell lymphoma via endogenous retrovirus-induced cGAS–STING activation. Clin. Epigenetics 15, 75 (2023).
Lanng, K. R. B., Lauridsen, E. L. & Jakobsen, M. R. The balance of STING signaling orchestrates immunity in cancer. Nat. Immunol. 25, 1144–1157 (2024).
Ni, J. et al. STING signaling activation modulates macrophage polarization via CCL2 in radiation-induced lung injury. J. Transl. Med. 21, 590 (2023).
Wang, Q. et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat. Commun. 13, 3022 (2022).
Du, H., Xu, T. & Cui, M. cGAS–STING signaling in cancer immunity and immunotherapy. Biomed. Pharmacother. 133, 110972 (2021).
Li, Q. et al. cGAS–STING, an important signaling pathway in diseases and their therapy. MedComm 5, e511 (2024).
Hu, J. et al. STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature 616, 806–813 (2023).
Brockwell, N. K. et al. Neoadjuvant interferons: critical for effective PD-1-based immunotherapy in TNBC. Cancer Immunol. Res. 5, 871–884 (2017).
Rautela, J. et al. Loss of host type-I IFN signaling accelerates metastasis and impairs NK-cell antitumor function in multiple models of breast cancer. Cancer Immunol. Res. 3, 1207–1217 (2015).
Touati, N. et al. Correlation between severe infection and breast cancer metastases in the EORTC 10994/BIG 1-00 trial: investigating innate immunity as a tumour suppressor in breast cancer. Eur. J. Cancer 72, 95–102 (2017).
Marks, Z. R. C. et al. Interferon-ε is a tumour suppressor and restricts ovarian cancer. Nature 620, 1063–1070 (2023).
Fung, K. Y. et al. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science 339, 1088–1092 (2013).
Stifter, S. A. et al. Defining the distinct, intrinsic properties of the novel type I interferon, IFNϵ. J. Biol. Chem. 293, 3168–3179 (2018).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Li, J. et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature 620, 1080–1088 (2023).
Qiu, J. et al. Cancer cells resistant to immune checkpoint blockade acquire interferon-associated epigenetic memory to sustain T cell dysfunction. Nat. Cancer 4, 43–61 (2023).
Cole, K., Al-Kadhimi, Z. & Talmadge, J. E. Role of myeloid-derived suppressor cells in tumor recurrence. Cancer Metastasis Rev. 42, 113–142 (2023).
Yofe, I. et al. Spatial and temporal mapping of breast cancer lung metastases identify TREM2 macrophages as regulators of the metastatic boundary. Cancer Discov. 13, 2610–2631 (2023).
Park, M. D. et al. TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat. Immunol. 24, 792–801 (2023).
Fabre, T. et al. Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation. Sci. Immunol. 8, eadd8945 (2023).
Cortese, N. et al. High-resolution analysis of mononuclear phagocytes reveals GPNMB as a prognostic marker in human colorectal liver metastasis. Cancer Immunol. Res. 11, 405–420 (2023).
Parvez, A. et al. PD-1 and PD-L1: architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front. Immunol. 14, 1296341 (2023).
Tarhini, A. A. et al. Tumor associated PD-L1 expression pattern in microscopically tumor positive sentinel lymph nodes in patients with melanoma. J. Transl. Med. 13, 319 (2015).
Klement, J. D. et al. Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment. Cancer Cell 41, 620–636 (2023).
Du, Z. et al. Inhibition of IFN-α signaling by a PKC- and protein tyrosine phosphatase SHP-2-dependent pathway. Proc. Natl Acad. Sci. USA 102, 10267–10272 (2005).
Zanker, D. J. et al. Intratumoral administration of the Toll-like receptor 7/8 agonist 3M-052 enhances interferon-driven tumor immunogenicity and suppresses metastatic spread in preclinical triple-negative breast cancer. Clin. Transl. Immunol. 9, e1177 (2020).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Boydell, E. et al. Neoadjuvant immunotherapy: a promising new standard of care. Int. J. Mol. Sci. 24, 11849 (2023).
Rosner, S., Reuss, J. E. & Forde, P. M. PD-1 blockade in early-stage lung cancer. Annu. Rev. Med. 70, 425–435 (2019).
Topalian, S. L., Taube, J. M. & Pardoll, D. M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367, eaax0182 (2020).
Graff, J. N. et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J. Immunother. Cancer 8, e000642 (2020).
Shah, A. N. et al. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J. Immunother. Cancer 8, e000173 (2020).
Wolchok, J. D. et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 40, 127–137 (2022).
PD-1 blockade falls short (repeatedly) in prostate cancer. Cancer Discov. 13, 1032–1033 (2023).
Walsh, L. A. & Quail, D. F. Decoding the tumor microenvironment with spatial technologies. Nat. Immunol. 24, 1982–1993 (2023).
Mullard, A. Tumour-infiltrating lymphocyte cancer therapy nears FDA finish line. Nat. Rev. Drug Discov. 23, 3–7 (2024).
Zebley, C. C., Zehn, D., Gottschalk, S. & Chi, H. T cell dysfunction and therapeutic intervention in cancer. Nat. Immunol. 25, 1344–1354 (2024).
Lickefett, B. et al. Lymphodepletion — an essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front. Immunol. 14, 1303935 (2023).
Koumprentziotis, I. A. et al. New emerging targets in cancer immunotherapy: the role of B7-H3. Vaccines 12, 54 (2024).
Tao, R. et al. Revolutionizing cancer treatment: enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front. Immunol. 15, 1354825 (2024).
Dagher, O. K. & Posey, A. D. Jr. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat. Immunol. 24, 1994–2007 (2023).
Huang, Y., Wang, H., Yue, X. & Li, X. Bone serves as a transfer station for secondary dissemination of breast cancer. Bone Res. 11, 21 (2023).
Acknowledgements
This work was supported by National Health and Medical Research Council (NHMRC) investigator and ideas grants to B.S.P. (2018167, 2012943), and grant funding from the National Breast Cancer Foundation (IIRS-23-021).
Author information
Authors and Affiliations
Contributions
N.M.H. and B.S.P. conceptualized the manuscript. N.M.H. wrote the manuscript and contributed to the generation of the figures. T.B.C. conceptualized and generated the figures. B.S.P. supervised and contributed to the writing of the manuscript and the preparation of the figures. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Dan Duda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haynes, N.M., Chadwick, T.B. & Parker, B.S. The complexity of immune evasion mechanisms throughout the metastatic cascade. Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01960-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41590-024-01960-4
- Springer Nature America, Inc.