Abstract
Human immunodeficiency virus (HIV) persistence during antiretroviral therapy (ART) is associated with heightened plasma interleukin-10 (IL-10) levels and PD-1 expression. We hypothesized that IL-10 and PD-1 blockade would lead to control of viral rebound following analytical treatment interruption (ATI). Twenty-eight ART-treated, simian immunodeficiency virus (SIV)mac239-infected rhesus macaques (RMs) were treated with anti-IL-10, anti-IL-10 plus anti-PD-1 (combo) or vehicle. ART was interrupted 12 weeks after introduction of immunotherapy. Durable control of viral rebound was observed in nine out of ten combo-treated RMs for >24 weeks post-ATI. Induction of inflammatory cytokines, proliferation of effector CD8+ T cells in lymph nodes and reduced expression of BCL-2 in CD4+ T cells pre-ATI predicted control of viral rebound. Twenty-four weeks post-ATI, lower viral load was associated with higher frequencies of memory T cells expressing TCF-1 and of SIV-specific CD4+ and CD8+ T cells in blood and lymph nodes of combo-treated RMs. These results map a path to achieve long-lasting control of HIV and/or SIV following discontinuation of ART.
Similar content being viewed by others
Main
Antiretroviral therapy (ART) does not cure human immunodeficiency virus (HIV) infection1. Viral load (VL) generally rebounds on treatment interruption due to the existence of long-lived cellular reservoirs2,3,4. Heightened plasma interleukin-10 (IL-10) levels are associated with an absence of viral clearance in chronic infections5,6,7, resulting from dysfunctional innate immunity and impaired differentiation of central memory T cells (TCM) into effector T cells. T lymphocytes from IL-10−/− mice exhibit a highly activated phenotype, are oligoclonal, express antiviral cytokines and degranulate in response to cognate antigen (Ag), consistent with Ag-specific immune responses8. IL-10 promotes the survival of memory CD4+ T cell subsets, including effector memory T cells (TEM) that harbor high levels of inducible HIV9. IL-10 blockade improved cytotoxic T cell responses in immunization models10 and cleared chronic viral infections11,12. In HIV infection, IL-10Ra blockade in vitro increased secretion of IFNγ by CD4+ T cells13 and restored the polyfunctionality of HIV-specific T cells14,15. Recently, our work in rhesus macaques (RMs)16 showed the critical role of IL-10 in the maintenance of the simian immunodeficiency virus (SIV) reservoir via multiple mechanisms, including the upregulation of survival signals and heightened expression of co-inhibitory receptors (co-IRs), including PD-1 (refs. 17,18).
PD-1 is upregulated in activated and exhausted T cells19,20. It impedes the development of the differentiation of Ag-specific TCM cells into polyfunctional TEM cells18,21,22 that can control viral infections23. Several studies in cancers24,25,26 and infectious diseases27,28,29,30 used aPD-1 treatment to revert immune exhaustion and restore immune-mediated control of tumors or viruses. HIV preferentially replicates in activated CD4+PD-1+ T cells31. However, engagement of PD-1 and other co-IRs with their respective ligands triggers T cell quiescence32,33. PD-1 expression by myeloid cells and its engagement to PD-L1 leads to increased IL-10 production34 and immune dysfunction35. Collectively, these data suggest that IL-10 and PD-1 synergize to suppress T cell activation, differentiation and effector function in chronic viral infections36. Data presented herein confirm that the combined blockade of both pathways reverts immune exhaustion, leading to long-lasting control of SIV VL in nine of ten combo-treated RMs following analytical treatment interruption (ATI), which was not observed in RMs treated with aIL-10 alone. Most importantly, these results provide a mechanistic framework to understand the immunological events that lead to the control of viral rebound post-ATI.
Results
Experimental design and safety monitoring
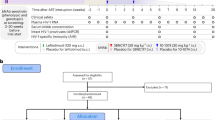
Twenty-eight RMs were infected intravenously (i.v.) with SIVmac239 (day 0, Fig. 1a). After 14 months on ART, RMs were randomized into three groups (Fig. 1a): eight RMs received vehicle and served as controls (group 1, black); ten RMs received deimmunized aIL-10 (ref. 16) (group 2, red) and ten RMs received deimmunized aIL-10 and deimmunized aPD-1 (group 3, blue) (Supplementary Table 1). The pharmacokinetics and pharmacodynamics and the absence of antidrug antibodies (ADA) are described in Extended Data Fig. 1. Complete blood counts (CBC) and blood chemistry (>20 analytes) (Extended Data Fig. 2) were within normal range in all groups over 24 weeks of follow-up. One RM presenting with severe adverse events in the aIL-10-treated group was euthanized 8 weeks post-ATI and removed from the study. One RM in the combo-treated arm also presenting with severe adverse events was euthanized 20 weeks post-ATI. However, the data on this animal were kept in the study (Supplementary Table 2).
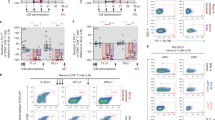
a, Experimental design and dosing. Week 0, intravenous (i.v.) SIVMac239 infection (n = 28). Week 6 postinfection (p.i.), initiation of antiretroviral treatment. Week 66 p.i., i.v. administration of aIL-10 (10 mg kg−1). Week 8 posttreatment (PTX), i.v. administration of aPD-1 (10 mg kg−1). Doses were administered every 3–4 weeks, as illustrated by solid arrows. Week 12 PTX, Week 0 post-ATI. aIL-10 administration (n = 10; red arrows); combo-treatment administration (n = 10; blue arrows); ART control group (n = 8). Collection of blood and LN biopsies for immunological assays are indicated by vertical dotted lines (weeks 1, 7, 12, 21 and 36 PTX). b, SIVmac239 plasma VL was followed at intervals allowed by Institutional Animal Care and Use Committee. Plasma viremia (copies per milliliter of plasma) was measured longitudinally for each animal. Groups: control (black, top panel); aIL-10 (red, middle panel); combo (aIL-10+ aPD-1− blue, bottom panel). Timelines for ART, ATI and redosing (bottom panel) are indicated by vertical dotted lines. Solid lines represent median values for each group over time. RKq17 was withdrawn from the study 20 weeks post-ATI. Thin lines show individual RMs; the thick line shows the median of all RMs from each group. Each animal is identified on the right side of the graph. The y axis shows the log10 SIV RNA copies per milliliter of plasma; the x axis shows weeks p.i. c, VL AUC from week 80–101 post-ATI, Ordinary one-way analysis of variance, Tukey’s multiple comparisons test, P = 0.04. Data are presented as mean values ± s.e.m. d, Cumulative frequencies of RMs presenting with VL < 1,000 copies per milliliter of plasma in each group at least once per time point. The bottom table indicates the number of RMs per time point with VL < 1,000 copies per milliliter of plasma (black, control; red, aIL-10; blue, combo treatment). P values for AUC across groups. e, Absolute CD4+ T cell counts 24 weeks post-ATI as computed based on CBC. Sample size: control n = 8, aIL-10 n = 9, aIL-10 + aPD-1 n = 9. Two-sided Wilcoxon rank sum test control versus aIL-10 + aPD-1 P = 0.027; aIL-10 versus aIL-10 + aPD-1 P = 0.014. f, CA-vRNA levels in LNs 24 weeks post-ATI normalized according to absolute numbers of live CD4+ T cells in LNs. Sample size: control n = 8, aIL-10 n = 9, aIL-10 + aPD-1 n = 10. Two-sided Wilcoxon rank sum test control versus aIL-10 + aPD-1 P = 0.0085; aIL-10 versus aIL-10 + aPD-1 P = 0.0041. g, log10 of numbers of integrated proviruses (IPDA) in LNs over time. Black lines, P values between the indicated time points in the control group; red lines show P values between the indicated time points in the aIL-10 group; AUC between combo-treated versus control or aIL-10 treated RMs (P = 0.008). h, log10 of 2LTR in LNs over time: AUC combo-treated versus aIL-10 (P = 0.07); AUC of combo-treated versus aIL-10 or controls (P = 0.03); combo-treated (blue asterisk) week −13 versus week 9 post-ATI (P = 0.003); combo-treated versus control 9 weeks post-ATI (P = 0.030); combo-treated versus IL-10 (P = 0.07); control group pre-ATI versus 24 weeks post-ATI (P = 0.054, black line). g,h, Data are presented as mean values ± s.e.m. i, CA-vDNA in LNs pre-ATI (circles) and 24 weeks post-ATI (squares). Combo-treated versus aIL-10 alone 24 weeks post-ATI (P = 0.051). Four combo-treated RMs versus all other RMs (n = 23) at 24 weeks post-ATI (P = 0.0002). Comparison of means between groups for e–i was performed with two-sided Wilcoxon rank sum test. Central line in boxplots marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5× IQR (interquartile range) from the hinges. ART, antiretroviral treatment; DTG, dolutegravir; FTC, emtricitabine; NS, not significant; PTX, postimmune treatment; TDF, tenofovir disoproxil fumarate.
Combo-treatment leads to sustained VL control post-ATI
All RMs rebounded similarly within 2 weeks post-ATI (Fig. 1b). However, starting at 4 weeks post-ATI, nine out of ten combo-treated RMs controlled viremia to substantially lower levels than the control group. At 40 weeks post-ATI, eight RMs from the combo-treatment that continued on the study, two still fully controlled viremia, three presented intermediate levels (<8,000 copies per milliliter plasma) and three returned to their initial VL set point (105–106 copies per milliliter) (week 101, Fig. 1b, bottom plot). The three RMs presenting intermediate viremia were redosed with combo treatment (three doses every 3 weeks starting 40 weeks post-ATI). Immediately after redosing, viremia declined to undetectable levels (<40 copies per milliliter) in all three RMs, which was maintained until week 64 post-ATI (6 months after the first redosing (week 40 post-ATI), Fig. 1b, bottom panel). Combo-treatment induced long-term control of viremia (<10,000 copies per milliliter) up to 24 weeks post-ATI, with a significantly lower area under the curve (AUC) than controls (Fig. 1c). We observed that one in eight RMs in the control group, four out of nine RMs in the aIL-10 group and nine out of ten RMs in the combo-treated group presented with VL < 1,000 copies per milliliter at least once during the 24 weeks post-ATI (Fig. 1d). Loss of PD-1 receptor occupancy varied across RMs and started 4 weeks after the last aPD-1 infusion (Extended Data Fig. 1e). Four combo-treated RMs controlled viremia for 24 weeks post-ATI, even after anti-PD-1 receptor occupancy was lost (Extended Data Fig. 1e; RMs: 34,918, Rbv17, RRh17, RWs17). Consistent with lower VL at those time points, combo-treated RMs presented with preserved absolute CD4+ T cell counts (Fig. 1e and Extended Data Fig. 3a) and preserved CD4/CD8 ratios (Extended Data Fig. 3b) 24 weeks post-ATI. Furthermore, cell-associated viral RNA (CA-vRNA) levels in lymph nodes (LNs) of combo-treated RMs were significantly lower than those found in the other two groups (Fig. 1f). Thus, the dual blockade of IL-10 and PD-1 was beneficial both virologically and immunologically, with better control of viral replication and maintenance of the CD4+ T cell compartment in absence of ART. Immunological and virological correlates of VL control were monitored up to 24 weeks post-ATI as, after this time point, RMs from the control and aIL-10 arms were necropsied.
Viral rebound in absence of ART leads to the infection of new target cells and increases the frequency of cells with intact proviral DNA. Longitudinal intact or integrated proviral DNA assay (IPDA) showed no differences in the frequencies of cells with intact proviruses during ART across groups (weeks −13 (before initiation of immune interventions) versus week 0 (during immune interventions and just before ATI)) in both LNs and peripheral blood mononuclear cells (PBMCs) (Fig. 1g and Extended Data Fig. 3c). The frequency of CD4+ T cells harboring intact provirus in LNs from combo-treated RMs remained stable across all time points post-ATI (24 weeks), while control groups presented with a significant ~1 log10 increase. Overall, control and aIL-10-treated RMs presented significantly higher intact proviral DNA levels than combo-treated RMs both in LNs (Fig. 1g) and PBMCs (Extended Data Fig. 3c).
After ATI, lack of seeding of newly infected CD4+ T cells by rebounding virus should result in lower frequencies of cells with two-long terminal repeat circles (2LTRs), a marker of ongoing viral replication37. Indeed, combo-treated RMs presented lower levels of cells with 2LTRs in LNs when compared to control and/or aIL-10-treated RMs (Fig. 1h). There was a significant decay in 2LTR measurements in the combo between week −13 and week 9 post-ATI, which could have resulted from cell loss due to cytopathic effects associated with viral reactivation or immune-mediated cell killing. Control RMs trended to have significantly higher levels of 2LTRs on ATI (Fig. 1h). Assessment of number of cells with 2LTRs in PBMCs showed results comparable to those observed in LNs (Extended Data Fig. 3d).
We next assessed the impact of treatment on the number of cells containing cell-associated viral DNA (CA-vDNA) in LNs pre-ATI and 24 weeks post-ATI. No significant variation in the numbers of CA-vDNA-bearing cells pre-ATI across groups (Fig. 1i, full circles) was observed, thereby confirming that the magnitude of viral replication post-ATI was not directly related to pre-ATI reservoir size. Combo-treated RMs presented significantly lower LN CA-vDNA levels 24 weeks post-ATI as compared to the aIL-10 group at the same time point (Fig. 1i). Of note, four RMs in the combo-treated group presented lower numbers of LN cells with CA-vDNA 24 weeks post-ATI compared to all RMs from other groups at the same time point (framed).
All viral readouts in LNs and PBMCs (VL, CA-vDNA, CA-vRNA, intact proviruses, 2LTRs) were significantly correlated with each other (Extended Data Fig. 3e–i), confirming that lower viral rebound was associated with lower viral reactivation (CA-vRNA) and lower re-seeding of CD4+ T cells by rebounding virus (IPDA and 2LTRs). The protective Mamu-A*01 allele did not influence virologic outcomes, as Mamu-A*01+ RMs were randomized across all three arms of the study (Extended Data Fig. 3i).
Expression of BCL-2 is reduced by combo treatment
Relative expression of BCL-2 in terms of median fluorescence intensity (MFI) was analyzed in CD4+ and CD8+ T cells from PBMCs and LNs, including memory T cell subsets (Extended Data Fig. 4a). Pre-ATI, we observed significantly lower levels of BCL-2 in total LN CD4+ T cells and in CD4+ TCM of combo-treated RMs (Fig. 2a,b, respectively, and Fig. 2c,d, longitudinal evaluation). Lower expression of BCL-2 pre-ATI was associated with lower LN CA-vRNA levels 24 weeks post-ATI (Fig. 2e,f). Treatment of PBMCs from uninfected RMs with BCL-2 inhibitor Venetoclax for 36 hours along with IL-10 and aIL-10 led to significantly higher levels of cell death than observed in cells that were kept in culture media containing IL-10 alone (Extended Data Fig. 4b). The absolute number of dead cells was inversely correlated with the frequencies of CD4+ T cells expressing BCL-2 (Extended Data Fig. 4c). These data support the rationale that aIL-10 makes CD4+ T cells more susceptible to cell death and could result in faster clearance of the reservoir by different mechanisms when combined with aPD-1.
a,b, Quantification of BCL-2 MFI in total CD4+ T cells (a) and TCM (b) in LNs pre-ATI. c,d, Modulation of BCL-2 expression over time in total CD4+ T cells (c) and CD4+ TCM cells (d). Continuous lines represent the natural cubic spline interpolation of medians across weeks. For a–d, groups were compared with a linear regression model. The t-statistics for the regression coefficients were used to assess significance, and multiple test correction was performed with the Holm–Bonferroni method. e,f, Correlation between BCL-2 levels in total (e) and TCM (f) CD4+ T cells pre-ATI and CA-vRNA in LNs 24 weeks post-ATI, respectively. Black, control RMs. Red, RMs treated with aIL-10 alone. Blue, combo-treated RMs. Two-sided Spearman correlation P values and rho are shown in each plot. Missing values for some RMs are due to insufficient amounts of cells to perform flow cytometry or absence of viral readout. Sample size: control n = 7, aIL-10 n = 8, aIL-10 + aPD-1 n = 7. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5× IQR from the hinges. UNS, unstimulated.
Proinflammatory cytokines are elevated pre-ATI in combo-treated RMs
On measurement of the concentrations of 28 cytokines and chemokines in the plasma of RMs, principal component analysis revealed significant differences in the cytokine–chemokine milieu induced by combo treatment pre-ATI (Fig. 3a) compared to the other two groups. Cluster analysis (K-means and Gap statistics) identified three major clusters of cytokines and/or chemokines (Fig. 3b and Extended Data Fig. 5a,b). Clusters 1 and 2 (Extended Data Fig. 5a,b) did not discriminate between treatment groups over time. However, cluster 3, which includes CTACK, I-TAC, IL-10 IL-16, IL-17, IL-18, IL-4, IP-10, MCP-1, MCP-2 and MIP-3b, was significantly elevated in combo-treated RMs pre-ATI and 9 weeks post-ATI (Fig. 3b). Cluster 3 centroid score pre-ATI (week 0 post-ATI) was inversely correlated with LN CA-vRNA levels 24 weeks post-ATI (Fig. 3c). Plasma levels of I-TAC, IL-4 and MIP-3b were inversely correlated with CA-vRNA levels in LNs 24 weeks post-ATI (Fig. 3d). Absolute levels of each cytokine in Cluster 3 pre-ATI are shown in Extended Data Fig. 5c. Of note, IL-10 accumulates in the plasma as it is neutralized by binding to the aIL-10 mAb used in this study (Extended Data Fig. 1c,d).
Longitudinal expression of 28 cytokines and chemokines was performed on plasma from all RMs. a, Principal component analysis (PCA) in plasma cytokines pre-ATI. Arrows indicate the contribution of each cytokine to data variability. PC1, 32.7%; PC2, 15,6%. Sig, significant. b, K-means and Gap statistics identified three cytokine and/or chemokine clusters over time. Cluster 3 is significantly modulated by combo-treatment pre-ATI and 9 weeks post-ATI. Cluster 3 components are shown in the table (left). Sample size: control n = 8, aIL-10 n = 9, aIL-10 + aPD-1 n = 10. Continuous lines represent the natural cubic spline interpolation of medians across weeks. The central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5× IQR from the hinges. Groups were compared with a linear regression model. The t-statistics for the regression coefficients were used to assess significance, and multiple test correction was performed with Holm–Bonferroni method *P-adjusted <0.05. MSD, meso scale discovery. c, Cluster 3 centroid scores pre-ATI are inversely correlated to CA-vRNA 24 weeks post-ATI in LNs. Two-sided Spearman correlation P values and rho are shown. Cluster 3, 9 weeks post-ATI, was not associated with LN CA-vRNA levels 24 weeks post-ATI (P = 0.56; rho, 0.12). d, Correlogram among pre-ATI cytokines and/or chemokines of cluster 3 and LN CA-vRNA 24 weeks post-ATI. Red circles show a positive correlation; blue circles show a negative correlation.
Combo treatment is associated with proliferation and differentiation of CD8+ T cells in LNs
We performed a supervised analysis of unstimulated LNs CD4+ and CD8+ T cells that included markers such as Ki67, T-bet, Granzyme B (GrzB), BCL-2, CX3CR1, CD39, TOX, IRF4, CD101 and TCF-1 (Extended Data Fig. 6a–c). Exhausted T cells were defined by expression of several markers, including TCF-1, T-bet, IRF4, TOX, SLAMF6, CD101, CX3CR1 and GrzB38,39,40. Stem-like progenitors were defined as TCF-1+CD101−, intermediate exhausted T cells were TCF-1−CD101− and expressed effector markers T-bet, GrzB and CX3CR1. The sole expression of CD101 in TCF-1− cells with intermediate to low expression of T-bet, GrzB and CX3CR1 defined terminally exhausted T cells. This analysis revealed that pre-ATI LN CD8+ T cells from combo-treated RMs were significantly enriched in total CD8+ T cells and CD8+ TCM expressing Ki67 (and included significantly higher frequencies of CD8+ TCM expressing GrzB (Extended Data Fig. 6d, top rows of the heatmap). The frequency of these cell subsets was inversely correlated with levels of LN CA-vRNA 24 weeks post-ATI (heatmap top rows). On the other hand, CD4+ T cells (total and TCM) expressing significantly higher per-cell levels of BCL-2 were enriched in RMs that presented with significantly higher viremia 24 weeks post-ATI (Extended Data Fig. 6d, top rows of the heatmap). Taken together, combo treatment led to lower levels of BCL-2 in CD4+ T cells from LNs and PBMCs and promoted a poised immune environment characterized by elevated plasma levels of inflammatory or migratory cytokines or chemokines and heightened frequencies of activated LN CD8+ T cells. These features are essential for the development of an efficient immune response that can intercept and control rebounding virus in absence of ART. Among the 104 immune parameters correlated with LN CA-vRNA levels 24 weeks post-ATI, 39 were also significantly correlated with VL (nominal P < 0.05). Activation or differentiation, BCL-2 expression and exhaustion were positively correlated with VL, while SIV-specific T cells and stemness were negatively associated with VL 24 weeks post-ATI.
Combo treatment leads to the expansion of terminally differentiated effector cells
Supervised analysis revealed that 9 weeks post-ATI, combo-treated RMs presented with significantly higher frequencies of LN CD8+ T cells exhibiting a terminally differentiated phenotype (TCF-1−CD101+) and expressing markers of effector T cells (that is, T-bet, ID2, GrzB, CX3CR1) compared to the two other groups (Extended Data Fig. 6e, bottom). Cells expressing these phenotypes were associated with lower levels of LN CA-vRNA 24 weeks post-ATI (Extended Data Fig. 6e, top row annotation). In contrast, at the same time point, the control group and aIL-10-treated RMs exhibited significantly enriched frequencies of effector CD4+ T cells, exhausted CD4+ T cells and CD8+ T cells expressing Ki67 in LNs (Extended Data Fig. 6e, top). This phenotype was associated with significantly higher levels of LN cells containing CA-vRNA 24 weeks post-ATI.
Combo treatment is associated with the establishment of a T cell stemness program
At 24 weeks post-ATI, RMs in the combo group presented significantly higher frequencies of CD4+ and CD8+ T cells expressing TCF-1 in LNs (Extended Data Fig. 6f, bottom), which were inversely correlated with LN CA-vRNA levels at that same time point (heatmap top row annotation). Additionally, LNs from the combo group were enriched in nonexhausted, TOX−IRF4− (notTOXpIRF4p) CD4+ and CD8+ T cells. The frequency of these cells was inversely correlated with LN CA-vRNA levels 24 weeks post-ATI. At this time point, control and aIL-10-treated RMs presented with significantly higher frequencies of CD4+ and CD8+ T cells in PBMCs that expressed heightened per-cell levels of T-bet, as well as higher frequencies of CD4+ and CD8+ T cells in LNs expressing effector markers (TEM), Ki67 and lacking the expression of TCF-1 (TCF-1−) (Extended Data Fig. 6f, top). These activation markers were positively associated with LN CA-vRNA levels 24 weeks post-ATI.
To validate these findings, we performed an unbiased assessment of unstimulated CD4+ and CD8+ T cells from LNs based on the above markers (Fig. 4a,g)41 followed by Monocle3-based pseudotime analysis42,43 (Fig. 4b,h, respectively). Eighteen and 15 clusters were identified in CD4+ and CD8+ T cells, respectively (Fig. 4c,i). In pseudotime analysis, cells were ordered with the starting node in naive T cells (CD95−CD28+) that progressed to different branches of cells expressing markers that highlight various differentiation steps (stemness, activation and exhaustion; Extended Data Fig. 7a,b). The early stages of pseudotime analysis included T cells with a less differentiated status (that is, that expressed naive and/or stemness markers such as TCF-1), whereas late stages of pseudotime are more heterogeneous and were defined by cells expressing activation (ID2, GrzB, T-bet) and exhaustion markers (TOX, IRF4). In the CD4+ T cell compartment, only cluster 9, enriched in long-lived exhausted T cells (CD95dimCD28+BCL-2hiTCF-1+TOXhiIRF4hiID2+Ki67+, Fig. 4c,d) was present at significantly higher frequencies in controls and aIL-10-treated RMs as compared to combo-treated RMs 24 weeks post-ATI (Fig. 4e). Cluster 9 accumulated importantly in the last half of the pseudotime analysis. The frequency of cells in cluster 9 was positively correlated with LN CA-vRNA levels in LNs 24 weeks post-ATI (Fig. 4f). In contrast, combo-treated RMs presented with significantly higher frequencies of CD8+CD95dimCD28+CCR7+TCF-1+BCL-2+ cells 24 weeks post-ATI (cluster 2, Fig. 4i,j, k). These data confirmed results of supervised analysis (Extended Data Fig. 6), where cells with stem-like markers prevailed in combo-treated RMs at this time point. Cluster 2 accumulated notably in the first half of the pseudotime analysis (Extended Data Fig. 7c), showing a lower frequency of differentiated effector T cells at this time point (Extended Data Fig. 7d). The frequency of cluster 2 was negatively correlated with levels of CA-vRNA in LNs 24 weeks post-ATI (Fig. 4l). Together, our results show that the control of viremia in combo-treated RMs was associated with increased frequencies of activated CD8+ T cells early post-ATI and stem-like memory CD8+ T cells later post-ATI, when VL was controlled to <1,000 copies per milliliter for an extended period of time.
Analysis was performed in unstimulated LN cell suspensions gated on CD4+ (a–f) and CD8+ (g–l) T cells and included all time points used for immune monitoring (−13, −7, 0, 9 and 24 weeks post-ATI). a,g, Uniform manifold approximation and projection for dimension reduction (UMP) plot and PhenoGraph clustering. b,h, Pseudotime analysis starting from naive T cells (CD95−CD28+), dark blue to yellow show less differentiated to more differentiated cells. c,i, Heatmap showing the expression of markers used for clustering (blue, low expression; red, high expression). Markers identity is shown on the left side. Dotted lines (a–c) highlight Louvain cluster 9 (CD4+ T cells), which is significantly decreased in LNs from combo-treated RMs 24 weeks post-ATI. d, Back gating to validate markers that defined cluster 9. e, Frequencies of cells belonging to cluster 9 over time. Sample size: control n = 8, aIL-10 n = 9, aIL-10 + aPD-1 n = 9. f, Two-sided Spearman correlation between frequencies of cells belonging to cluster 9 and LN CA-vRNA levels at 24 weeks post-ATI. Dotted lines (g–i) highlight Louvain cluster 2 (CD8+ T cells), which was significantly increased by combo treatment 24 weeks post-ATI. j, Back gating to validate markers that defined cluster 2. k, Frequencies of cells belonging to cluster 2 over time. Sample size: control n = 8, aIL-10 n = 9, aIL-10 + aPD-1 n = 9. For e and k, continuous lines represent the natural cubic spline interpolation of medians across weeks and groups were compared with a linear regression model. The t-statistics for the regression coefficients were used to assess significance, and multiple test correction was performed with Holm–Bonferroni method *P-adjusted <0.05. l, Two-sided Spearman correlation between frequencies of cells belonging to cluster 2 and LN CA-vRNA levels at 24 weeks post-ATI. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5× IQR from the hinges.
Combo treatment enhances the maintenance of SIV-specific T cells post-ATI
Stem-like memory T cells (TCF-1+) are required to maintain a pool of cells that can rapidly expand on encountering cognate Ag and differentiate into effector cells that can control rebounding virus44. We next evaluated the ability of T cells to produce key cytokines (IFNγ, tumor necrosis factor-α (TNFα), IL-2) when exposed in vitro to a pool of SIV-Gag peptides (Fig. 5a). No differences in SIV-specific immune responses across groups were observed pre-ATI as measured by fold change Gag–unstimulated (Extended Data Fig. 8a–c). However, 24 weeks post-ATI, Gag stimulation induced significantly higher frequencies of SIV-specific CD4+ T cells that produced IFNγ, IL-2 and TNFα in LNs from the combo-treated group (Fig. 5b). Production of IFNγ and IL-2 on SIV-Gag peptide stimulation was also observed in the CD4+ TCM compartment only in combo-treated RMs. SIV-specific CD4+ T cells that produced IFNγ, IL-2 and TNFα in LNs were absent or less potent in the other two groups. SIV-Gag stimulation of LN cells promoted significant production of IFNγ by CD8+ T cells 24 weeks post-ATI in the combo-treated group (Fig. 5c). Specifically, significant induction of IFNγ production was observed in LN CD8+ TCM and TEM in these RMs. In PBMCs, the combo-treated group presented with significantly higher frequencies of CD8+ T cells producing IFNγ and TNFα (Fig. 5d). The longitudinal fold change (Gag–unstimulated) for CD4+ and CD8+ T cells in PBMCs and LNs highlights that both SIV-specific CD4+ and CD8+ T cells expanded significantly in combo-treated RMs (Extended Data Fig. 8a–c) 24 weeks post-ATI, when expression of exhaustion markers was reversed by the immune intervention and viremia was low (Extended Data Fig. 6f, bottom). Induced SIV-specific immune responses (fold change Gag–unstimulated) were inversely correlated with LN CA-vRNA and 2LTRs levels (Fig. 5e) 24 weeks post-ATI. Of note, LN SIV-specific CD4+ T cell responses were positively associated with SIV-specific IFNγ and TNFα responses of CD8+ T cells in LNs and PBMCs, respectively (Fig. 5e), indicating the importance of cognate help for the development of effective CD8+ T cell responses45,46.
a, Gating strategy used on cell suspensions from LN (24 weeks post-ATI) for the evaluation of cytokine production by CD4+ (pink) and CD8+ (green) T cells. The same approach was applied to PBMCs. b, Frequencies of CD4+ T cells from LNs that produced IFNγ, IL-2 or TNFα spontaneously (unstimulated, UNS, shown as full circles) or in response to stimulation with a pool of SIV-Gag peptides (SIV-Gag, diamonds) 24 weeks post-ATI. c, Frequencies of CD8+ T cells from LNs that expressed IFNγ spontaneously (UNS, full circles) or in response to stimulation with a pool of SIV-Gag peptides (SIV-Gag, diamonds) 24 weeks post-ATI. d, Frequencies of CD8+ T cells from PBMCs that expressed IFNγ or TNFα spontaneously (UNS, full circles) or in response to stimulation with a pool of SIV-Gag peptides (SIV-Gag, diamonds) 24 weeks post-ATI. Pairwise comparison between UNS and SIV-gag-specific groups in a–d was performed with two-sided paired t-test *P < 0.05; **P < 0.01. e, Correlogram between the levels of LN CA-vRNA and 2LTR and the fold change in the ratios of frequencies of SIV-Gag-specific T cells over unstimulated T cells 24 weeks post-ATI. Red circles, positive correlation; blue circles, negative correlation. The circle size and color shades represent rho values for the two-sided Spearman correlation. Black, control RMs; red, RMs treated with aIL-10 alone; blue, combo-treated RMs. Missing values are due to insufficient amounts of cells to perform flow cytometry or absence of viral readout. GAG, SIV-Gag-specific; PMA-Iono, phorbol myristate acetate-ionomycin. Sample size: control n = 8, aIL-10 n = 9, aIL-10 + aPD-1 n = 10.
Adaptive immunity poised state pre-ATI confers advantage for VL control post-ATI
We compared high-density flow cytometry data from PBMCs and LNs of combo-treated RMs to those from RMs that received aIL-10 alone pre-ATI, when plasma VL was undetectable in all RMs. We postulated that features observed in combo-treated RMs and not in aIL-10 treated RMs would result from disengagement of PD-1 and posited that these signatures would highlight synergies between aPD-1 and aIL-10. Of 2,987 combinations of different markers, we identified 84 cell subsets that were significantly different between the two groups. We associated these 84 features with CA-vRNA levels in LNs 24 weeks post-ATI and identified 15 that were significantly associated with this outcome (Extended Data Fig. 9). TCF-1− features in CD8+ T cells in LNs of combo-treated RMs or CD8+ T cells expressing GrzB and Ki67 were inversely correlated with CA-vRNA levels in LNs 24 weeks post-ATI. RMs that received aIL-10 alone presented with significantly higher expression of TCF-1 and BCL-2, a feature of TCM cells, in LN CD4+ and CD8+ T cells, indicating that those cells did not transition or differentiated into TEM cells. This phenotype was positively associated with CA-vRNA levels in LNs 24 weeks post-ATI. In contrast, RMs that received combo-treatment showed a transition of TCF-1+ TCM to TCF-1− TEM cells. This feature was associated with control of viral rebound post-ATI. These results are consistent with previous reports from preclinical models and clinical interventions in patients with cancer47,48,49,50,51.
A feature selection analysis was performed to identify the minimum number of features that defined sustained viral control or lack thereof (Fig. 6). The cytokine milieu pre-ATI (IL-4 and MIP-3b), the early post-ATI (9 weeks) induction of CD8+ T cells in the LNs that expressed GrzB and T-bet, and the late enrichment (24 weeks post-ATI) of nonexhausted (IRF4−TOX−) CD4+ and CD8+ T cells in LNs and of CD4+ TEM cells expressing higher levels of TCF-1 in combo-treated RMs were identified as the minimal features necessary to lead to lower CA-vRNA levels post-ATI. In contrast, control and aIL-10-treated RMs presented activation of CD4+ and CD8+ T cells (TEM, Ki67) and reduced numbers of long-lasting T cells (most were TCF-1−) post-ATI, all of which were positively correlated with higher levels of CA-vRNA and 2LTRs in LNs 24 weeks post-ATI. The longitudinal modulation of these selected features is shown in Extended Data Fig. 10.
Heatmap shows the minimal number of features that discriminate between groups and predict lower levels of LN CA-vRNA 24 weeks post-ATI. Correl, correlation; Wk, week.
Discussion
Herein we report, in a stringent model of SIVmac239 infection of RMs with ART starting at 42 days postinfection, similar to early chronic infection in people with HIV (PWH) and maintained for 16 months, that the blockade of IL-10 and PD-1, two independent pathways associated with HIV and SIV reservoir persistence16 and lack of T cell effector function18 led to at least 6 months of control of viral rebound post-ATI in 90% of treated RMs. RMs in the combo-treatment group presented stable frequencies of cells with intact provirus and limited ongoing viral seeding as defined by stable frequencies of cells with 2LTR circles in the absence of ART. Comprehensive analysis of soluble and cellular effectors enabled us to develop a model that shows that long-term control of VL (equal to or more than several weeks post-ATI) is mediated by a triphasic immune response. RMs that controlled VL post-ATI were defined by a poised immune environment pre-ATI (phase 1) that enabled them to develop a prompt and efficient immune response capable of intercepting and rebounding virus (phase 2). Subsequent control of rebounding viremia enabled the development and maintenance of effective SIV-specific CD4+ and CD8+ T cells and the establishment of long-term memory (phase 3). This triphasic process, which defines stages of the development of long-term protective immune responses that lead to the control of viral replication, is well established in several experimental systems52.
Downregulation of BCL-2 was a phase 1 feature associated with controlling rebounding virus. HIV reservoir size and persistence are driven by the upregulation of antiapoptotic molecules3,53,54 such as BCL-2, which acts downstream of IL-10 signaling16. BCL-2 antagonism sensitizes cytotoxic T cell-resistant HIV reservoirs to elimination ex vivo55, which is understandable since the pro-apoptotic activity of GrzB is blocked by BCL-2 over-expression56. BCL-2 downregulation triggered by combo treatment before ATI may increase the susceptibility of infected CD4+ T cells to CD8+ killing and/or cytopathic effect of the virus on viral rebound. Several groups have proposed BCL-2 inhibition as a potential strategy to cure HIV infection55,57.
The heightened levels of proinflammatory cytokines and chemokines in cluster 3 pre-ATI (phase 1) were specific to RMs in the combo treatment arm. The infusion of aIL-10 together with aPD-1 could overcome the immunosuppressive activity of IL-10 on innate immunity58. IL-22 and IL-17A, included in cluster 3, are critical for the restoration of mucosal integrity and differentiation and regeneration of intestinal epithelial cells59,60. While we did not track cellular migration, several chemokines that are present in cluster 3 pre-ATI, including MIP-3b (CCL19) and I-TAC (CXCL11), are known to promote T cell migration and could promote the recruitment of SIV-specific T cells to tissues and contribute to the suppression of viral reactivation and spreading. The levels of IL-4, also increased in combo-treated RMs, were negatively correlated with VL and positively correlated with frequencies of SIV-specific T cells. A Th2 environment is associated with lower levels of integrated HIV DNA61, although the molecular basis of this observation was not explored.
Our results indicate that treatment with aIL-10 and aPD-1 results in significant expansion of SIV-specific CD4+ and CD8+ T cells that can intercept rebounding virus (phase 2). This expansion reflects the effect of aIL-10, which restores Ag presentation62 as IL-10 downregulates CIITA, the master regulator of HIV transcription and expression63. Restoration of MHC II expression helps rejuvenate the polyfunctionality of CD4+ T cells and enhances the development of SIV-specific CD8+ T cell responses. Moreover, blockade of IL-10 will release T cells from the quiescence triggered by this cytokine, as STAT3 signaling is required for stemness. This is consistent with the increased frequencies of Ki67+ cells pre-ATI that were observed in the aIL-10 arm, as previously reported16. Concomitant infusion of aPD-1 promotes the acquisition of effector functions, as illustrated by higher frequencies of GrzB-positive CD8+ T cells. The rejuvenation of CD4+ T cell function and their capacity to provide cognate help to SIV-specific CD8+ T cells boosts effector function and enables the establishment of long-term memory (phase 3). Previous reports on patients with cancer and in the lymphocytic choriomeningitis virus (LCMV) model showed that infusion of aPD-1 leads to the release of stem-like T cells (TCF-1hi) that differentiate into effector cells40,49,64,65,66. Our results show that combo treatment unleashed these cells toward a state of activation and effector function, which was not observed in the aIL-10 arm alone. These features were associated with a poised state of the immune system, including memory CD4+ and CD8+ T cells, and with lower CA-vRNA contents in LNs from combo-treated RMs 6 months post-ATI.
So far, more than 150 clinical studies (with and without interventions other than ART) have incorporated ATI (reviewed in ref. 67) with significant variations in the duration of ATI and indications for reintroducing ART. None of the preclinical studies using monoclonal antibodies (mAbs) specifically directed against PD-1, or in combination with therapeutic vaccination or with antibodies specific for other co-IRs (that is, CTLA-4) and Toll-like receptor-7 or -8 agonists32,33,49,68,69 showed any significant increase in time to viral rebound or any reduction in the magnitude of viral rebound post-ATI. Repeated infusions of anti-PD-1 post-ATI in chronically infected ART-treated RMs led to a 2-week, 1 log10 decline of viremia in a subset of RMs69. Herein we provide evidence for specific mechanisms that enhance the capacity of PD-1 blockade to rescue innate and adaptive immune responses, leading to long-term control of viral rebound. A limitation of the current study is the lack of an aPD-1 alone arm, which restricts the conclusions that can be drawn about the synergistic effects of combo-treatment. However, aPD-1 by itself was shown to be ineffective in inducing sustained control of viral rebound post-ATI33,64,68,69,70. In our study, aIL-10 alone did not induce a significant control of viremia post-ATI, emphasizing the importance of combining aIL-10 and aPD-1 as a therapeutic strategy. The combination of both blockades was essential for long-term control of VL as they act on different stages of innate immune function and T cell differentiation. PD-1 blockade is required to enhance the differentiation of effector cells while aIL-10 releases TCM cells from quiescence, enabling their differentiation into TEM (refs. 66,71). aIL-10 can also restore the proinflammatory function of monocytes and/or macrophages, and both aIL-10 and aPD-1 can act as latency reversal agents32,72.
In summary, nine out of ten of RMs in the combo-treated group were able to control SIV viremia for at least 24 weeks after ATI and 10 weeks after the last aIL-10 and aPD-1 infusion. This immunotherapeutic intervention, performed in ART-treated, SIV-infected RMs, does not directly target the virus, showing dramatic efficacy in controlling viral rebound post-ATI. This unprecedented degree of viral control was associated with a poised immune system pre-ATI, which in turn promoted the development and maintenance of SIV-specific T cell responses post-ATI that were characterized by limited T cell exhaustion and heightened antiviral effector functions. Decreased viremia resulted in the contraction of the effector arm of cell-mediated immunity and the differentiation of effector cells toward a stemness profile. While this intervention cannot be translated to clinical trials at the present time due to potential side effects, several pathways pre-ATI triggered by combo-treatment were identified that are associated with the control of VL following ATI. These findings pave the way for the testing of new interventions designed to induce a poised state of immune responses that are capable of intercepting rebounding virus and controlling HIV and SIV viremia in the long term when ART is discontinued.
Methods
Materials availability
This study uses deimmunized aIL-10 (JES3.12G8) and aPD-1 mAbs (1B8 LC3/HC1), which are proprietary reagents developed by Merck & Co., Inc. Both aIL-10 and aPD-1 are subject to Material Transfer agreement restrictions as the therapeutic is currently under investigation in human clinical trials.
Study approval
This study was approved by the Emory University Institutional Animal Care and Use Committee under permit no. 201800047. Experiments were conducted following guidelines set out by the National Institutes of Health (NIH) and the Animal Welfare Act regarding the housing and welfare of laboratory RMs. All possible efforts were taken to minimize pain experienced by RMs.
Experimental design
Twenty-eight Indian-origin, specific-pathogen-free RMs (Macaca mulatta) were sourced from the Emory National Primate Research Center colony and single-housed in an animal BSL-2 facility. RMs were between 38 and 50 months old at the time of infection and were Mamu-B*07− and Mamu-B*17−. Some RMs were Mamu-A*01+ (Supplementary Table 1). RMs were infected i.v. with 300 TCID50 SIVmac239 and at day 42 postinfection (p.i.) initiated a daily subcutaneous ART regimen composed of dolutegravir (2.5 mg kg−1 d−1), tenofovir disoproxil fumarate (5.1 mg kg−1 d−1) and emtricitabine (40 mg kg−1 d−1). ART was maintained for 14 months before immune therapy was initiated. Peak VL and kinetics during acute SIV infection and protective Mamu-A*01 alleles were used to split RMs across groups. Eight RMs (group 1) received vehicle and served as ART-only controls; ten RMs (group 2) received aIL-10 and ten RMs (group 3) received aIL-10 and aPD-1 (combo treatment). While on ART, group 2 received four doses of the deimmunized form of aIL-10 at 10 mg kg−1 once every 4 weeks for the first two doses and once every 3 weeks for subsequent doses16. Group 3 received four doses of aIL-10 (10 mg kg−1) once every 4 weeks for the first two doses, once every 3 weeks for subsequent doses and two doses of aPD-1 at 10 mg kg−1 per dose once every 3 weeks starting along with the third dose of aIL-10 (Fig. 1a). ART was interrupted in all RMs 77 weeks postinfection, corresponding to 12 weeks postimmune intervention. RMs in group 2 received three additional doses of aIL-10, while RMs in group 3 received five additional doses of aIL-10 and aPD-1. Times of collection of biospecimens are indicated by vertical dotted lines in Fig. 1a. Animal RGr17 from the aIL-10 group was necropsied earlier due to adverse events. LN biopsies, plasma and PBMCs were collected longitudinally and processed as previously described16,73,74. Briefly, blood was centrifuged to separate the plasma, which was aliquoted and frozen at −80 °C until downstream analysis. PBMCs were isolated from whole blood by density gradient centrifugation. For LN biopsies, the skin over the axillary or inguinal region was clipped and surgically prepped. An incision was then made in the skin over the LN, which was exposed by blunt dissection and excised over clamps. Half of the LN was placed in freshly prepared neutral buffered 4% paraformaldehyde for 24 h at room temperature for fixation, followed by transfer to 80% ethanol and subsequent embedding of tissue in paraffin. The remaining LNs were then homogenized and passed through a 70 μm cell strainer to isolate lymphocytes and cryopreserved for downstream analysis. In groups 2 and 3, peripheral blood was drawn immediately before infusion of the mAb for pharmacokinetics and pharmacodynamics evaluations. Biological samples for immune monitoring were collected at weeks −13, −5, 0, 9 and 24 post-ATI and were cryopreserved.
Formulation of aIL-10 and aPD-1 mAbs
The amino acid sequence of IL-10 is >95% identical between humans and RMs. Furthermore, the epitope targeted by the anti-IL-10 mAb is identical between humans and RMs. The aIL-10 mAb (MK-1966/JES3.12G8) is a kappa chain IgG1 with a human variable domain and a rhesus constant domain to minimize the formation of ADA. Before use, mAbs were purified using size-exclusion chromatography and reverse-phase high-performance liquid chromatography, and were confirmed as endotoxin-free (<0.03 EU mg−1). The route, dose and interval for administration of aIL-10 mAb were selected based on a dose ranging pilot conducted by Merck & Co. Pilot data suggested a 10 mg kg−1 dose would be sufficient to capture >90% of the Ag. Historically, no significant adverse events were encountered with 4 weekly administrations at 25 mg kg−1 using a humanized mAb in cynomolgus macaques. Bioactivity of the deimmunized mAb was confirmed by in vitro neutralization of induced rhesus IL-10 and STAT3 phosphorylation using a U937 reporter cell line and primary RMs PBMCs.
Pharmacokinetics of the aIL-10 mAb
Detection of the aIL-10 mAb was performed using a sandwich immunoassay with a lower limit of detection (LOD) of 0.2 ng ml−1. Capture and detection antibodies were in-house mouse mAbs specific to the drug antibody idiotype (Merck & Co.). Briefly, 100 μl of capture antibody at 2 μg ml−1 in pH 9.0 bicarbonate buffer was coated on a 96-microwell plate overnight, washed once with 200 μl of PBS with 0.05% Tween 20 (PBST) for 5 min with plate shaking at 75 rpm and blocked with 200 μl of 3% BSA/PBS for 2 h or longer. The detection antibody was conjugated to AP and stored in 50% glycerol at −20 °C. Next, 100 μl of rhesus serum sample diluted 1:2,000 in 3% BSA and PBS was added to the plate and 50 μl of alkaline phosphatase (AP)-conjugated detection antibodies diluted 1:500 in 0.3% Tween 20/3%BSA/PBS were added sequentially and incubated at 4 °C overnight. Plates were washed five times with 200 μl of PBST. The AP substrate Tropix CDP-Star Sapphire II (Applied Biosystems) was added for 15 min. Luminescent signals were measured using an EnVision Multilabel Reader (PerkinElmer).
Pharmacodynamics of plasma IL-10 levels
Rhesus plasma has low endogenous levels of IL-10 and the concentration of IL-10 changes significantly following administration of aIL-10 and/or aPD-1. Thus, plasma IL-10 can be used as a biomarker of mAb target engagement and pharmacodynamics. The detection of IL-10 in plasma from RMs was performed using a two aIL-10 mAb sandwich for total IL-10 capture. The binding domain of these two antibodies on IL-10 differs from that of the drug mAb. For detection, aIL-10 antibody was labeled with AP. The antibody 13AHJ, mouse × IL-10_H mAb (TC50.2D2.B8) IgG1 was used to measure total IL-10 after AP labeling. 91AOV, rhesus IL-10 (IL-10 MM) was used for quantification of the total IL-10 protein standard. The LOD for total IL-10 protein in plasma was 0.2 pg ml−1.
Pharmacokinetics of the aPD-1 mAb
Measurement of plasma levels of the anti-PD-1 mAb (deimmunized anti-PD-1 mAb 1B8 LC3/HC1) was performed using a sandwich immunoassay. Briefly, 100 μg of recombinant human PD-1-Fc Chimera Protein (R&D Systems) was directly conjugated with AP and stored in 50% glycerol at −20 °C. Next, 100 μl of 0.2 μg ml−1 unlabeled PD-1-Fc protein was coated on a 96-well microtiter plate overnight, washed and blocked as above. Then, 100 μl of diluted rhesus serum was added and 50 μl of AP-conjugated PD-1-Fc protein at 0.1 μg ml−1 was added and incubated at 4 °C with shaking overnight. The LOD of the assay was 0.2 ng ml−1 of drug aPD-1 mAb.
aPD-1 receptor occupancy
To evaluate PD-1 receptor occupancy, a PD-1 competing antibody was used (PD-1 Clone EH12.2H7). PBMCs and LNs from different time points were stained with a simple mAb panel targeting CD3, CD4, CD8 and PD-1. The frequency of PD-1+ cells was tracked over time and compared to the basal levels (week −1 postimmune intervention). RMs were determined to have lost receptor occupancy on observation of PD-1+ cell frequencies similar to those observed pretreatment or in control RMs.
Anti-drug antibodies (ADA)
A competitive sandwich assay was developed using biotin- and AP-labeled antibodies. Plasma from RMs receiving the immune intervention was acid treated and used to dissociate drug–ADA complexes. Briefly, 50 μl of RMs plasma were treated with 200 μl of 0.3% trifluoroacetic acid pH 1.0–2.0 and incubated at 37 °C for 1 h to dissociate plasma. Then 8 μg ml−1 biotin–drug and 4 μg ml−1 AP–drug were added. The mixture was adjusted to pH 7.0 with Tris 1 M pH 9.0, and incubated at room temperature for 3 h with shaking to do competition binding between labeled and unlabeled drug with the dissociated ADA in plasma. After that, the mixture was added into streptavidin-coated 96-well plates, incubated at room temperature for 1 h, and washed and developed with AP substrate. Plasma from uninfected RMs was used as negative control.
CBC and blood biochemistry
The CBC and biochemistry were performed longitudinally in all RMs using well-established procedures75.
VL
Plasma SIV VL (SIV RNA copies per milliliter of plasma) was determined using real-time quantitative PCR (PCR) assays with a lower LOD of 40 copies per milliliter of plasma76.
CA-vRNA and CA-vDNA measurements
SIV reservoir measurements were performed in both LN and PBMC at weeks 9 and 24 post-ATI (Fig. 1). Values were corrected based on absolute CD4+ T cell counts extracted from CBC and converted to copies per 106 CD4+ T cells. PBMCs and LN biopsies were analyzed for CA-vDNA content as previously described76. Briefly, CA-vDNA and CA-vRNA were assessed using hybrid real-time digital PCR and qPCR assays that involved 12 replicate reactions and had a sensitivity of one copy per 850,000 cells76. When all replicates were not positive, reservoir size was computed based on Poisson methods in which values were normalized according to diploid genome equivalents.
IPDA and 2LTR circles
IPDA was performed by Accelevir Diagnostics77. 2LTR circles were quantified using primers and probes adapted from Policicchio et al.78. For the droplet digital PCR (ddPCR) reactions, DNA and a master mix containing ddPCR Supermix for Probes (no dUTP) (Bio-Rad) and primers and probes for the detection of 2LTR circles and an internal pol or env region were used, as described below. Droplets were read using a QX100 Droplet Reader (Bio-Rad). Proviral DNA was quantified using gag qPCR. Briefly, the following primers and/or probes were used for SIV-Gag: SIV21F: 5′-GTC TGC GTC ATC TGG TGC ATT C-3′, SIV22R: 5′-CAC TAG GTG TCT CTG CAC TAT CTG TTT TG-3′, and SIV23: FAM-5′-CTT CCT CAG TGT GTT TCA CTT TCT CTT CTG-3′-BH1 (Integrated DNA Technologies). Amplification conditions were 50 °C for 30 min, 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s, 55 °C for 15 s and 60 °C for 30 s (ref. 79). Intact SIV genomes were quantitated using a method based on HIV-1 IPDA80. Briefly, DNA was added to a master mix containing ddPCR Supermix for Probes (no dUTP, Bio-Rad), primers and probes77. ddPCR output was analyzed using QuantaSoft Analysis-Pro (Bio-Rad). For all SIV ddPCR samples, input cell number was quantitated using a ddPCR assay for a RM gene (RPP30). This assay allowed to exclude samples with excessive DNA shearing. Two RPP30 amplicons spaced at the same distance as the IPDA amplicons were amplified in duplex ddPCR reactions. An aliquot of each DNA sample was added to a master mix consisting of ddPCR Supermix for Probes, primers and probes78. Output of intact and defective genomes was normalized to copies per million cells (determined by RPP30), and samples with excessive DNA shearing between amplicons were excluded from further analysis. Full genome and env sequences were aligned using Clustal W (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and G → A hypermutants were identified with Hypermut v.2.0 (ref. 80). The consensus sequence of the relevant reference virus was used as the outgroup to root the trees (M33262.1). When available, single genome sequences from the relevant viral stock were included in the alignment81. Values were corrected based on absolute CD4+ T cell counts extracted from CBC and converted to copies per 106 live CD4+ T cells.
Downstream analysis strategy
Only plasma cytokines levels and features of CD4+ and CD8+ T cells from LNs and PBMCs that were significantly modulated by combo-treatment at 0, 9- and 24 weeks post-ATI and correlated with LN CA-vRNA 24 weeks post-ATI (used as a surrogate of VL) are presented in this article.
Flow cytometry
Cryopreserved PBMC and LN-derived mononuclear cells were thawed and stained with antirhesus or antihuman mAbs (106 cells per test). The latter were cross-reactive with RMs cells70,73,82,83 and were validated in databases maintained by the Nonhuman Primate Reagent Resource (https://www.nhpreagents.org/). Two panels including 20–28 mAbs were used to evaluate survival, activation and/or differentiation, exhaustion, stemness and antigenic (SIV) specificity of T cells from RMs. Antibodies that were used across the two panels are described in Supplementary Table 3. Expression of intracellular cytokines such as IFNγ, TNFα and IL-2 was assessed following a 6 h stimulation in vitro at 37 °C with a pool of SIV-Gag peptides (NIH reagents program ARP-12364) at 2 μg ml−1, along with purified anti-CD49d mAb (BD cat. no. 555501) and 1/1,000 v/v brefeldin A. Phorbol myristate acetate-ionomycin was used as a positive control and unstimulated cells served to define background cytokine production (Fig. 5a). Frequencies and fold change of SIV-Gag-specific cells over unstimulated conditions (fold change GAG_UNS) were monitored longitudinally in CD4+ and CD8+ T cells from PBMCs and LNs (Extended Data Fig. 8). Surface staining was performed at room temperature for 20 min. Samples underwent fixation and permeabilization with BD Transcription Factor Buffer Set (cat. no. 562574) for 45 min at 4 °C. Intracellular staining was performed for 45 min at 4 °C. Acquisition was performed on a minimum of 100,000 live cells on a A5 Symphony flow cytometer (BD Biosciences) driven by BD FACSDiva software. Acquired data was analyzed using FlowJo v.10.8.1. Representative cytograms are shown for each panel in the respective figures. Analysis of flow cytometry data using manual gating and uniform manifold approximation and projection for dimension reduction generated >1,000 cell subsets. In Figs. 4 and 5 and Extended Data Figs. 1, 4, 6 and 7, subsets generated by manual gating were labeled as follows: Tissue_Subset_Phenotype_Stim. Ex: LN_CD3p_CD4p_MFI_BCL-2_UNS; if more than one time point is shown in the same plot, the population name is preceded by the time point. Ex: Wk36_LN_CD3p_CD4p_MFI_BCL-2_UNS. The letter ‘p’ after the subset means ‘+’ for that marker; the letter ‘n’ after the subset means ‘−’ for that marker. Pseudotime was performed on unstimulated CD4+ and CD8+ T cells from LNs using unsupflowhelper and Monocle3 (https://cole-trapnell-lab.github.io/monocle3/docs/citations) (Fig. 4 and Extended Data Fig. 7). Cells were ordered based on assessment of cluster phenotypes, with a starting node set within naive T cell clusters42,43 (CD95−CD28+). The progression of the analysis was defined by the positioning of cells based on the modulation of the relative expression of markers included in the panel. For example, naiveness and/or stemness (CD95, CD28, TCF-1); activation and/or differentiation (that is, ID2, T-bet, GrzB); exhaustion (TOX, IRF4, SLAMF6). The same analysis was used to identify CD4+ and CD8+ T cells clusters that were modulated by combo-treatment at different time points (week 0, 9 and 24 post-ATI) and were associated with levels of CA-vRNA in LNs 24 weeks post-ATI.
Cytokine assay
The Meso Scale Multi-Array Technology (Meso Scale Discovery) was used for measurement of cytokine levels. A cytokine panel containing the following analytes was screened: CTACK, I-TAC, IL-10, IL-16, IL-17A, IL-18, IL-4, IP-10, MCP-1, MCP-2, GRO-α, IL-22, IL-7, IL-8, TGF-β1, TGF-β2, TGF-β3, Fractalkine, IFNα, IFNγ, IL-15, IL-2, IL-6, IL-9, MIP-1α, MIP-3β and TNFα, using 25 μl of each plasma from each donor in duplicates. Samples were randomized to avoid batch effects. Results were extrapolated from the standard curve from each specific analyte and plotted in picograms per milliliter, using the DISCOVERY WORKBENCH v.4.0 software (Meso Scale Discovery). K-means and Gap statistics84 were computed to identify clusters of cytokines that were modulated by combo-treatment.
BCL-2 validation assay
To validate that BCL-2hi cells are more refractory to cell death, cryopreserved PBMCs from uninfected RMs (n = 6) were thawed and rested at 2 × 106 cells per milliliter in complete Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum for 8 h at 37 °C and 5% CO2. Cells were then counted and plated in 96-well plates at 2 × 106 cells per milliliter in media containing IL-10 (10 ng ml−1; Peprotech), IL-10 (10 ng ml−1) + aIL-10 (10 μg ml−1; Merck clone IOAS9) or left unstimulated. After 24 h, Venetoclax (ABT-199, Selleckchem) was added at different concentrations (10, 100 or 1,000 nM). Cells were kept in culture for an extra 36 h at 37 °C, 5% CO2. Absolute numbers of cells were measured by trypan blue staining and the remaining cells were stained with antibodies known to cross-react with RMs (Nonhuman Primate Reagent Resource, Reactivity Database). The following antibodies were used: anti-CD28 (clone CD28.2; BD cat. no. 612815), anti-CD3 (clone SP34-2; BD cat. no. 564117), anti-CD4 (clone OKT4; BioLegend cat. no. 317412), anti-CD8 (clone RPA-T8; BD cat. no. 612942) and anti-CD95 (clone DX2; BioLegend cat. no. 305628). The LIVE/DEAD Fixable Aqua Dead Cell Stain Kit for 405 nm was used as viability marker (Invitrogen cat. no. L34957). Anti-BCL-2 mAb (clone BCL-2/100; BD cat. no. 563601) was used for intracellular staining. Cell surface staining was performed at room temperature for 20 min. Samples underwent fixation and permeabilization with the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) for 45 min at 4 °C. Intracellular staining for BCL-2 was performed for 45 min at 4 °C. Acquisition and analysis of acquired data were performed as described above. Live–dead stains were not used in downstream analysis to avoid diluting the findings by excluding cells that were rendered apoptotic by the treatment.
Statistical analysis
The identification of features modulated by combo-treatment versus controls and/or the aIL-10 group pre-ATI and at 9 to 24 weeks post-ATI was performed using a linear regression model controlling for group staggering per year. Features with adjusted P < 0.05 (Holm–Bonferroni) were considered significantly different. Once combo-specific features were identified, Spearman correlation tests versus LN CA-vRNA levels 24 weeks post-ATI were performed, resulting in specific features that were positively or negatively associated with this outcome. Features exhibiting statistically significant associations (FDR < 0.1) were also examined for their correlation with plasma VL (copies per milliliter) at 24 weeks post-ATI. AUC was calculated to compare the longitudinal distribution of features (including VL). Modified Kaplan–Meier curves were constructed by using the sum of RMs in each group that achieved a VL < 1,000 copies per milliliter at least once. The minimal set of features modulated by combo-treatment and associated with levels of CA-vRNA 24 weeks post-ATI was identified through feature selection using a least absolute shrinkage and selection operator regression. Data showing averaged statistical outcomes are represented as mean ± s.e.m. and population sizes are listed within the figure legends for each analysis. Statistical analyses were performed using R programming language v.4.2.2 with rstatix package v.0.7.2 and GraphPad Prism v.9.4.0. Plots were generated with the R package ggplot2 or GraphPad Prism.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated and analyzed in the current study for each figure are provided upon request and are also available along with the code. Source data are provided with this paper.
Code availability
The analysis pipeline is available via GitHub at https://cole-trapnell-lab.github.io/monocle3/docs/citations and https://sekalylab.github.io/unsupflowhelper/guides/trajectory_vignette. The code used for analysis is available via GitHub and Zenodo at https://github.com/sekalylab/dual_blockade_IL-10_PD-1_SIV and https://doi.org/10.5281/zenodo.10211327 (ref. 85).
References
Pourcher, V., Gourmelen, J., Bureau, I. & Bouee, S. Comorbidities in people living with HIV: an epidemiologic and economic analysis using a claims database in France. PLoS ONE 15, e0243529 (2020).
Galvez, C. et al. Atlas of the HIV-1 reservoir in peripheral CD4 T cells of individuals on successful antiretroviral therapy. mBio. 12, e0307821 (2021).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15, 893–900 (2009).
Salantes, D. B. et al. HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J. Clin. Invest. 128, 3102–3115 (2018).
Tian, Y., Mollo, S. B., Harrington, L. E. & Zajac, A. J. IL-10 regulates memory T cell development and the balance between Th1 and follicular Th cell responses during an acute viral infection. J. Immunol. 197, 1308–1321 (2016).
Santos, C. O. et al. Blocking IL-10 signaling with soluble IL-10 receptor restores in vitro specific lymphoproliferative response in dogs with leishmaniasis caused by Leishmania infantum. PLoS ONE 16, e0239171 (2021).
Rojas, J. M., Avia, M., Martin, V. & Sevilla, N. IL-10: a multifunctional cytokine in viral infections. J. Immunol. Res. 2017, 6104054 (2017).
Jones, M. et al. IL-10 restricts memory T cell inflation during cytomegalovirus infection. J. Immunol. 185, 3583–3592 (2010).
Kulpa, D. A. et al. Differentiation into an effector memory phenotype potentiates HIV-1 latency reversal in CD4(+) T cells. J. Virol. 93, e00969–19 (2019).
Pitt, J. M. et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J. Immunol. 189, 4079–4087 (2012).
Ejrnaes, M. et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203, 2461–2472 (2006).
Brockman, M. A. et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114, 346–356 (2009).
Saeidi, A. et al. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front. Immunol. 9, 2569 (2018).
Wilson, E. B. & Brooks, D. G. The role of IL-10 in regulating immunity to persistent viral infections. Curr. Top. Microbiol Immunol. 350, 39–65 (2011).
Rigopoulou, E. I., Abbott, W. G., Haigh, P. & Naoumov, N. V. Blocking of interleukin-10 receptor–a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin. Immunol. 117, 57–64 (2005).
Harper, J. et al. Interleukin-10 contributes to reservoir establishment and persistence in SIV-infected macaques treated with antiretroviral therapy. J. Clin. Invest. 132, e155251 (2022).
Fromentin, R. et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 12, e1005761 (2016).
Trautmann, L. et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202 (2006).
Chikuma, S. et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J. Immunol. 182, 6682–6689 (2009).
Li, J. et al. Reversal of the CD8(+) T-cell exhaustion induced by chronic HIV-1 infection through combined blockade of the adenosine and PD-1 pathways. Front. Immunol. 12, 687296 (2021).
Breton, G. et al. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J. Immunol. 191, 2194–2204 (2013).
Kulpa, D. A. et al. The immunological synapse: the gateway to the HIV reservoir. Immunol. Rev. 254, 305–325 (2013).
Ando, M., Ito, M., Srirat, T., Kondo, T. & Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 43, 1–9 (2020).
Seidel, J. A., Otsuka, A. & Kabashima, K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 8, 86 (2018).
Li, P. et al. The third generation anti-HER2 chimeric antigen receptor mouse T cells alone or together with anti-PD1 antibody inhibits the growth of mouse breast tumor cells expressing HER2 in vitro and in immune competent mice. Front. Oncol. 10, 1143 (2020).
Liu, J. et al. Tumoral EHF predicts the efficacy of anti-PD1 therapy in pancreatic ductal adenocarcinoma. J. Exp. Med. 216, 656–673 (2019).
Turnis, M. E., Andrews, L. P. & Vignali, D. A. Inhibitory receptors as targets for cancer immunotherapy. Eur. J. Immunol. 45, 1892–1905 (2015).
Sawant, D. V. et al. Adaptive plasticity of IL-10(+) and IL-35(+) Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 20, 724–735 (2019).
Kwok, G., Yau, T. C., Chiu, J. W., Tse, E. & Kwong, Y. L. Pembrolizumab (Keytruda). Hum. Vaccin Immunother. 12, 2777–2789 (2016).
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Vollbrecht, T. et al. Impact of changes in antigen level on CD38/PD-1 co-expression on HIV-specific CD8 T cells in chronic, untreated HIV-1 infection. J. Med. Virol. 82, 358–370 (2010).
Fromentin, R. et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4(+) T cells from ART-suppressed individuals. Nat. Commun. 10, 814 (2019).
Harper, J. et al. CTLA-4 and PD-1 dual blockade induces SIV reactivation without control of rebound after antiretroviral therapy interruption. Nat. Med. 26, 519–528 (2020).
Dong, H., Zhu, G., Tamada, K. & Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369 (1999).
Said, E. A. et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 16, 452–459 (2010).
Brooks, D. G. et al. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl Acad. Sci. USA 105, 20428–20433 (2008).
Panther, L. A. et al. Unintegrated HIV-1 circular 2-LTR proviral DNA as a marker of recently infected cells: relative effect of recombinant CD4, zidovudine, and saquinavir in vitro. J. Med. Virol. 58, 165–173 (1999).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1(+) stem-like CD8(+) T cells during chronic infection. Immunity 51, 1043–1058 e1044 (2019).
Zander, R. et al. CD4(+) T cell help is required for the formation of a cytolytic CD8(+) T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042 e1024 (2019).
Beltra, J. C. et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841 e828 (2020).
Geraci, J. et al. Exploring high dimensional data with Butterfly: a novel classification algorithm based on discrete dynamical systems. Bioinformatics 30, 712–718 (2014).
Cacchiarelli, D. et al. Aligning single-cell developmental and reprogramming trajectories identifies molecular determinants of myogenic reprogramming outcome. Cell Syst. 7, 258–268 e253 (2018).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Zehn, D., Thimme, R., Lugli, E., de Almeida, G. P. & Oxenius, A. Stem-like’ precursors are the fount to sustain persistent CD8(+) T cell responses. Nat. Immunol. 23, 836–847 (2022).
Beuneu, H., Garcia, Z. & Bousso, P. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J. Immunol. 177, 1406–1410 (2006).
Ryu, S. J. et al. Cognate CD4 help is essential for the reactivation and expansion of CD8 memory T cells directed against the hematopoietic cell-specific dominant minor histocompatibility antigen, H60. Blood 113, 4273–4280 (2009).
Rao, M., Valentini, D., Dodoo, E., Zumla, A. & Maeurer, M. Anti-PD-1/PD-L1 therapy for infectious diseases: learning from the cancer paradigm. Int. J. Infect. Dis. 56, 221–228 (2017).
Ahn, E. et al. Demethylation of the PD-1 promoter is imprinted during the effector phase of CD8 T cell exhaustion. J. Virol. 90, 8934–8946 (2016).
Amancha, P. K., Hong, J. J., Rogers, K., Ansari, A. A. & Villinger, F. In vivo blockade of the programmed cell death-1 pathway using soluble recombinant PD-1-Fc enhances CD4+ and CD8+ T cell responses but has limited clinical benefit. J. Immunol. 191, 6060–6070 (2013).
Balanca, C. C. et al. PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells. JCI Insight 6, e142513 (2021).
Sundahl, N. et al. A phase I/II trial of fixed-dose stereotactic body radiotherapy with sequential or concurrent pembrolizumab in metastatic urothelial carcinoma: evaluation of safety and clinical and immunologic response. J. Transl. Med. 15, 150 (2017).
Badovinac, V. P. & Harty, J. T. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 211, 67–80 (2006).
Fromentin, R. & Chomont, N. HIV persistence in subsets of CD4+ T cells: 50 shades of reservoirs. Semin. Immunol. 51, 101438 (2021).
Chandrasekar, A. P., Cummins, N. W. & Badley, A. D. The role of the BCL-2 family of proteins in HIV-1 pathogenesis and persistence. Clin. Microbiol. Rev. 33, e00107–e00119 (2019).
Ren, Y. et al. BCL-2 antagonism sensitizes cytotoxic T cell-resistant HIV reservoirs to elimination ex vivo. J. Clin. Invest. 130, 2542–2559 (2020).
Goping, I. S., Sawchuk, T., Rieger, A., Shostak, I. & Bleackley, R. C. Cytotoxic T lymphocytes overcome Bcl-2 inhibition: target cells contribute to their own demise. Blood 111, 2142–2151 (2008).
Arandjelovic, P. et al. Venetoclax, alone and in combination with the BH3 mimetic S63845, depletes HIV-1 latently infected cells and delays rebound in humanized mice. Cell Rep. Med. 4, 101178 (2023).
Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S. & Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356, 513–519 (2017).
Douzandeh-Mobarrez, B. & Kariminik, A. Gut microbiota and IL-17A: physiological and pathological responses. Probiotics Antimicrob. Proteins 11, 1–10 (2019).
Kolls, J. K. & Khader, S. A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 21, 443–448 (2010).
Sun, H. et al. Th1/17 polarization of CD4 T cells supports HIV-1 persistence during antiretroviral therapy. J. Virol. 89, 11284–11293 (2015).
Mittal, S. K. & Roche, P. A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 34, 22–27 (2015).
Wright, K. L. & Ting, J. P. Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol. 27, 405–412 (2006).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458, 206–210 (2009).
Khaitan, A. & Unutmaz, D. Revisiting immune exhaustion during HIV infection. Curr. HIV/AIDS Rep. 8, 4–11 (2011).
Ahn, E. et al. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl Acad. Sci. USA 115, 4749–4754 (2018).
Lau, J. S. Y., Smith, M. Z., Lewin, S. R. & McMahon, J. H. Clinical trials of antiretroviral treatment interruption in HIV-infected individuals. AIDS 33, 773–791 (2019).
Bekerman, E. et al. PD-1 blockade and TLR7 activation lack therapeutic benefit in chronic simian immunodeficiency virus-infected macaques on antiretroviral therapy. Antimicrob. Agents Chemother. 63, e01163–19 (2019).
Velu, V. et al. PD-1 blockade following ART interruption enhances control of pathogenic SIV in rhesus macaques. Proc. Natl Acad. Sci. USA 119, e2202148119 (2022).
McGary, C. S. et al. CTLA-4(+)PD-1(−) memory CD4(+) T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 47, 776–788 e775 (2017).
Arra, A., Lingel, H., Pierau, M. & Brunner-Weinzierl, M. C. PD-1 limits differentiation and plasticity of Tc17 cells. Front. Immunol. 14, 1104730 (2023).
Uldrick, T. S. et al. Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy. Sci. Transl. Med. 14, eabl3836 (2022).
Micci, L. et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J. Clin. Invest. 125, 4497–4513 (2015).
Strongin, Z. et al. Distinct SIV-specific CD8(+) T cells in the lymph node exhibit simultaneous effector and stem-like profiles and are associated with limited SIV persistence. Nat. Immunol. 25, 1245–1256 (2024).
Koo, B. S. et al. Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta) anesthetized with ketamine hydrochloride. Lab. Anim. Res. 35, 7 (2019).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104 (2013).
Bender, A. M. et al. The landscape of persistent viral genomes in ART-treated SIV, SHIV, and HIV-2 infections. Cell Host Microbe 26, 73–85 e74 (2019).
Policicchio, B. B. et al. Dynamics of simian immunodeficiency virus two-long-terminal-repeat circles in the presence and absence of CD8(+) cells. J. Virol. 92, e02100–e02117 (2018).
Gama, L. et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 31, 5–14 (2017).
Rose, P. P. & Korber, B. T. Detecting hypermutations in viral sequences with an emphasis on G–>A hypermutation. Bioinformatics 16, 400–401 (2000).
Del Prete, G. Q. et al. Comparative characterization of transfection- and infection-derived simian immunodeficiency virus challenge stocks for in vivo nonhuman primate studies. J. Virol. 87, 4584–4595 (2013).
Pallikkuth, S. et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)−21. PLoS Pathog. 9, e1003471 (2013).
Micci, L. et al. Paucity of IL-21-producing CD4+ T cells is associated with Th17 cell depletion in SIV infection of rhesus macaques. Blood 120, 3925–3935 (2012).
Arima, C., Hakamada, K., Okamoto, M. & Hanai, T. Modified fuzzy gap statistic for estimating preferable number of clusters in fuzzy k-means clustering. J. Biosci. Bioeng. 105, 273–281 (2008).
Pereira Ribeiro, S. et al. Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption. Zenodo https://doi.org/10.5281/zenodo.10211327 (2023).
Acknowledgements
We thank R. Ahmed, M. Abdel Hakeem, S. Deeks and R. Medina for their comments on the paper; the Emory Primate Center for veterinary support; the team from Merck & Co., Inc. for the drug development and PK/PD studies; Gilead for the supply of antiretrovirals agents and BD for their support in developing mAb panels and reagents for flow cytometry. This project was funded in part with federal funds from the National Cancer Institute, NIH, under contract no. 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. This study was supported by research grants including a Merit Award no. R37 AI141258 from NIH/NIAID, grant nos. CFAR P30 AI050409, P51OD011132, 1UM1AI164561-01 and UM1AI164562.
Author information
Authors and Affiliations
Contributions
S.P.R. conceptualized, planned, performed and analyzed the experiments and wrote the paper. Z.S. participated in the conceptualization, conducted the RMs study, performed the analysis of in vivo clinical and virological data. T.H., K.N. and J.H. conducted all the sample processing and the analyses of receptor occupancy. F.t.C., K.G. and R.M.R. performed the bioinformatic analyses. GPS supported the experimental performance. G.X.M. and A.-N.P. developed, implemented, and performed the pseudotime analysis using flow cytometry data. P.M.D.R.E. and G.X.M. performed the BCL-2 validation assays. S.J. and C.W. were the veterinarians who took care of the health and well-being of RMs and conducted drug administration, biospecimen collection and necropsies. R.B. scientifically reviewed the article and provided technological expertise. J.D.L. performed all virologic assays. J.D.E. contributed to experimental planning and data interpretation. G.S. contributed to data interpretation and reviewed the paper. G.R., E.R., G.W., L.M., D.M.G., B.J.H. and D.J.H. developed, tested and provided deimmunized antibodies and performed the PK/PD experiments. G.P.S. generated data and performed analyses. C.P. was involved in animal care and interventions. C.N.C. performed data analysis. H.S. contributed to writing, editing and scientifically reviewing the paper, and assembled figures and figure legends. M.P. conceptualized, planned, led and interpreted the RMs work, and contributed to writing the paper. R.P.S. conceptualized, planned, interpreted, wrote and funded the development of the project.
Corresponding author
Ethics declarations
Competing interests
G.R., Z.S., E.R., G.A., G.W., L.M., D.M.G., B.J.H. and D.J.H. are employed by and/or have financial interests in Merck & Co., Inc. A.-N.P. is a paid consultant, shareholder and board member of RPM Bioinfo Solutions. The other authors declare no competing interests.
Peer review
Peer review information
Primary Handling Editors: Ioana Staicu and Jamie Wilson, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Antibodies, pharmacokinetics, and pharmacodynamics.
a) Representative dot plots for receptor occupancy (RO) as measured by flow cytometry using a competing anti-PD-1 antibody. LN cells were stained longitudinally for T cell markers (CD3, CD4, CD8) and with a competing anti-PD-1 antibody. Decay in frequencies of CD4+PD-1+ and CD8+PD-1+ cells were monitored over time. Blue arrows indicate first and last anti-PD-1 administration in vivo. Numbers on top of each plot represent weeks post-PTX/ATI, respectively; X axis: PD-1 staining; Y axis: CD4 staining; gated on CD3+ cells. b) Frequencies of PD-1 RO in all RMs of the combo-treated group. Dark blue lines represent median RO over time in CD4+ (left panel) and CD8+ T cells (right panel); Week 14 post-ATI: last anti-PD-1 dose. c) Longitudinal plasma IL-10 levels (pg/mL) as measured using the U-PLEX Assay Platform (Meso Scale Discovery). Sample size: Control n = 8, aIL10 n = 9, aIL10 + aPD1 n = 10. Continuous lines represent the natural cubic spline interpolation of medians across weeks. Groups were compared with a linear regression model. The t-statistics for the regression coefficients were used to assess significance, and multiple test correction was performed with Holm–Bonferroni method *p-adjusted < 0.05; **p-adjusted < 0.01; ***p-adjusted < 0.001 d) Longitudinal plasma IL-10 levels (pg/mL) as measured using Ultra-Sensitive Meso Scale assay (Merck & Co., Inc., Rahway, NJ). Sample size: aIL10 n = 10, aIL10 + aPD1 n = 10Comparison of groups means per week was performed with two-sided Wilcoxon rank sum test *p < 0.05; **p < 0.01. Continuous lines represent the polynomial spline interpolation of means across weeks. e) Loss of PD-1 RO in each combo-treated animal shown by red squares in the course of viremia post-ATI (log10 copies/mL plasma). ATI: analytical treatment interruption. LN: lymph nodes. PTX: post-immune intervention. RMs: rhesus macaques. RO: PD-1 receptor occupancy. f) Anti-drug antibody (ADA). Plasma samples from the combo-treated RMs were tested for the induction of ADA. anti-IL-10 and anti-PD-1 ADA at week 0, 8 and 34 PTX (weeks -13, -4 and 22 post-ATI, respectively); g) anti-IL-10 and anti-PD-1 ADA at week 34 PTX (22 post-ATI) for the individual RMs tested. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5 * IQR (inter-quartile range) from the hinges.
Extended Data Fig. 2 Complete blood count (CBC) and blood chemistry over time.
WBC: white blood cells; RBC: red blood cells; HGB: hemoglobin; HCT: hematocrit; PLT: platelets; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RDW-SD: red cell distribution width – standard deviation; RDW-CV: red cell distribution width – cell volume; MPV: mean platelet volume; T-Prot: total protein; ALB: albumin; Glob: globulin; A/G: albumin/globulin; AST: aspartate aminotransferase; ALT: alanine transaminase; ALP: alkaline phosphatase; GGT: gamma-glutamyl transferase; T-BIL: total bilirubin; BUN: blood urea nitrogen; CREAT: creatinine; Bun/Cr: blood urea nitrogen/creatinine; PHOS: phosphorus; GLUC: glucose; CAL: calcium; MAG: magnesium; Sodium: sodium; Potass: potassium; Chloride: chloride; CHOL: cholesterol, TRIG: triglycerides; AMY: amylase; CK: creatine kinase. Top lines represent the time of immune intervention. Grey area: ongoing antiretroviral therapy. Black line: control group; red line: aIL-10 group; blue line: combo treatment group. Sample size: Control n = 8, aIL10 n = 10, aIL10 + aPD1 n = 10. Data are presented as mean values +/− SEM.
Extended Data Fig. 3 All viral readouts are correlated with one another.
a) Absolute CD4+ T cell counts vs log10 VL 24 weeks post-ATI in controls, RMs treated with aIL-10 alone, and combo-treated RMs (two-sided spearman correlation, rho = −0.6652, p = 0.0002). b) CD4/CD8 ratios in PBMCs 24 weeks post-ATI in controls and RMs treated with aIL-10 alone vs combo-treated RMs (two-sided t-test, p = 0.04). c) Log10 of IPDA in PBMCs over time normalized based on live absolute CD4+ T cell counts. Black lines: control RMs; red lines: RMs treated with aIL-10 alone; blue lines: combo-treated RMs; shaded area: AUC. AUC of control/anti-IL-10 treated RMs vs AUC of combo-treated RMs, two-sided t-test test p = 0.04. Data are presented as mean values +/− SEM d) Log10 2LTRs in PBMCs over time. Black lines: control RMs; red lines: RMs treated with aIL-10 alone; blue lines: combo-treated RMs; shaded area: AUC. AUC of control/aIL-10 treated RMs vs AUC of combo-treated RMs, two-sided t-test, p = 0.03; AUC of control RMs at week 9 post-ATI vs AUC of control RMs at week 24 post-ATI, p = 0.04; AUC of RMs treated with aIL-10 alone at week 0 vs AUC of RMs treated with aIL-10 alone at week 9 post-ATI, p = 0.09; AUC of RMs treated with aIL-10 alone at week 0 vs AUC of RMs treated with aIL-10 alone at week 24 post-ATI, p = 0.08; AUC of control RMs vs AUC of combo-treated RMs between week −13 and week 24 post-ATI, p = 0.07 - Anova mixed effect analysis Tukey’s test. Data are presented as mean values +/− SEM e) Two-sided spearman correlation between log10 VL and log10 CA-vRNA levels in LNs from all 3 groups of RMs 24 weeks post-ATI (rho = 0.8, p = 5.3 × 10−7). f) Two-sided spearman correlation between log10 CA-vRNA levels and log10 CA-vDNA levels in LNs from all 3 groups of RMs 24 weeks post-ATI (rho = 0.7, p = 6.5 × 10−5). g) Two-sided spearman correlation between log10 CA-vDNA levels and IPDA in LNs from all 3 groups of RMs 24 weeks post-ATI (rho = 0.78, p = 8.9 × 10−6). h) Two-sided spearman correlation between log10 IPDA and log10 2LTRs in LNs from all 3 groups of RMs 24 weeks post-ATI (rho = 0.97, p = 8.3 × 10−7). i) Heatmap showing the association between all viral readouts 24 weeks post-ATI (complete data are available for 25 RMs). Top row: Mamu-A*01 allele status (white: negative; gray: positive). Second row: group annotations (control RMs: black; RMs treated with aIL-10 alone: red; combo-treated RMs: blue). Values for some RMs are missing due to insufficient amounts of cells to perform the assessment of viral readouts. ATI: analytical treatment interruption. AUC: area under the curve. IPDA: intact proviral DNA assay. LNs: lymph nodes. ns: non-significant. VL: viral load. 2LTR: 2 long terminal repeats.
Extended Data Fig. 4 IL-10 sensitizes CD4+ T-cells to BCL-2-regulated death and is antagonized by anti-IL-10.
a) Representative gating strategy for the evaluation of BCL-2 expression at the per cell level (MFI: median fluorescence intensity) in CD4+ and CD8+ T cells from LNs and PBMCs of all RMs. PBMCs from uninfected RMs (n = 6) were kept in media containing IL-10 (10 ng/mL), IL-10 (10 ng/mL) + anti-IL-10 (10 ug/mL; Merck clone IOAS9) or left unstimulated. After 24 h, Venetoclax (ABT-199) was added at different concentrations (10 nM, 100 nM, or 1,000 nM) for extra 36 h. b) Ratio of dead cells by trypan blue exclusion staining. X axis: Venetoclax dose response (10, 100 and 1,000 nM); Y axis: ratio of the number of dead cells. Red: Treatment with IL-10 alone. Blue: treatment with IL-10 + anti-IL-10. Two-way RM ANOVA, Bonferroni’s multiple comparison test. Data are presented as mean values +/− SEM; *p < 0.05; **p < 0.01; ***p < 0.001 c) Two-sided spearman correlation between the number of dead cells and the relative frequencies of CD4+BCL2+ T cells. The outer edges of the squares refer to the pre-treatment condition: red: IL-10; blue: IL-10 + aIL-10. Shades inside the squares refer to the dose of Venetoclax: white: 10 nM; gray: 100 nM; black: 1,000 nM. rho = −0.6138, p < 0.0001. LNs: lymph nodes. PBMCs: peripheral blood mononuclear cells. RM: rhesus macaques.
Extended Data Fig. 5 Twenty-eight cytokines and chemokines were measured in plasma samples obtained longitudinally from all RMs.
a-b) K-means clustering and Gap statistics identified 3 clusters of cytokines and chemokines over time. Clusters 1 and 2 and their components are shown at different time points before and after ATI. Continuous lines represent the natural cubic spline interpolation of medians across weeks. c) Raw data (pg/mL plasma) for Cluster 3 cytokine and chemokine components of Cluster 3 immediately before ATI (week 0). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 - Kruskal-Wallis test. Black: control RMs; red: RMs treated with aIL-10 alone; blue: combo-treated RMs. ATI: analytical treatment interruption. LLOD: lower limit of detection. RMs: rhesus macaques. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5 * IQR (inter-quartile range) from the hinges.
Extended Data Fig. 6 Analysis of activation, exhaustion and stemness markers in CD4+ and CD8 + T cells.
a-c) Gating strategies for the supervised analysis of activation, exhaustion and stemness markers in CD4+ and CD8+ T cells. CD4+ T cells were classified as « effectors » or « progenitors » based on staining for T-bet, TCF1 and GrzB. CD8+ T cells were classified as « stem-like », « intermediate » or « exhausted » based on staining for CD101, TCF1, T-bet, GrzB, and CX3CR1. Expression of several activation/exhaustion markers was observed based on MFI. These analyses resulted in the identification of hundreds of discrete CD4+ and CD8+ T cell subsets, some of which were differentially modulated pre-ATI and 9- and 24 weeks post ATI in control RMs vs RMs treated with aIL-10 alone vs combo-treated RMs. d-f) Heatmaps illustrate features present pre-ATI, at 9 weeks post-ATI and at 24 weeks post-ATI that were significantly associated with LN CA-vRNA 24 weeks post-ATI. The top row represents log10 CA-vRNA levels, the second row represents group annotation (black: control RMs; red: RMs treated with aIL-10 alone; blue: combo-treated RMs). Arrows indicate the major features that were highlighted in this analysis. ATI: analytical treatment interruption. CA-vRNA: cell-associated viral RNA. LNs: lymph nodes. PBMCs: peripheral blood mononuclear cells. RMs: rhesus macaques.
Extended Data Fig. 7 Pseudotime analysis in unstimulated T cells from LNs at 24 weeks post -ATI.
This analysis was performed as described under Methods, with the earliest trajectory in pseudotime manually defined by naïve T cells (CD95−CD28+). The progression of the pseudotime was defined by the positioning of cells based on the modulation of the relative expression (MFI) of markers of stemness (that is, TCF-1), activation (that is, ID2, GrzB, T-bet) and exhaustion (that is, TOX, IRF4). CD4+ and CD8+ T cell clusters and the analysis of their pseudotime is shown (panels a and c, respectively). X axis: Louvain cluster numbers; Y axis: relative frequency of each cluster in each of the 3 groups: control RMs (black), RMs treated with aIL-10 alone (red), and combo-treated RMs (blue). The bottom bar below the X axis from dark blue to yellow reflects the pseudotime scale from less (dark blue) to more differentiated cells (yellow). The bottom arrows highlight the fact that CD4+ T cells accumulated in the later part of the pseudotime (a), while CD8+ T cells accumulated at the beginning of the pseudotime (c). The MFI of each marker used to define the clusters (Phenograph) and pseudotime progression (Monocle3 pseudotime analysis) are shown for CD4+ (b) and CD8+ (d) T cells. The line going from the bottom to the top in the UMAPs depicts the trajectory of the cells on the pseudotime scale. The color gradient (blue to red) depicts the lowest to the highest per-cell level of expression for each marker, annotated in the bar in the right of each plot with its respective expression (MFI). The squares in b and d represent Cluster 9 and Cluster 2 for CD4+ and CD8+ T cells, respectively. Comparison of means between groups in a and c was performed with two-sided Wilcoxon rank sum test and p-value correction for multiple tests was performed with Benjamini-Hochberg method. *p < 0.05. ATI: analytical treatment interruption; MFI: median fluorescence intensity; RMs: rhesus macaques; UMAP: uniform manifold approximation and projection for dimension reduction. Sample size: Control n = 8, aIL10 n = 9, aIL10 + aPD1 n = 9. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5 * IQR (inter-quartile range) from the hinges.
Extended Data Fig. 8 Modulation of SIV-specific features over time (weeks post-ATI).
a) Fold change (FC) in production of IFN-γ (left panel), IL-2 (middle panel) and TNF-α (right panel) following stimulation with SIV peptides over unstimulated (UNS) in CD4+ cells from LNs of control RMs (black), RMs treated with aIL-10 only (red) and combo-treated RMs (blue) at various time points during follow-up pre- and post-ATI. b) FC in production of IFN-γ following stimulation with SIV peptides over unstimulated (UNS) in CD8+ cells from LNs of control RMs (black), RMs treated with aIL-10 only (red) and combo-treated RMs (blue) at various time points during follow-up pre- and post-ATI. c) FC in production of IFN-γ (left panel) and TNF-α (right panel) following stimulation with SIV peptides over unstimulated (UNS) in CD8+ cells from PBMCs of control RMs (black), RMs treated with aIL-10 only (red) and combo-treated RMs (blue) at various time points during follow-up pre- and post-ATI. For figures a-c, continuous lines represent the natural cubic spline interpolation of medians across weeks and groups were compared with a linear regression model. The t-statistics for the regression coefficients were used to assess significance, and multiple test correction was performed with Holm–Bonferroni method. Sample size a-c: Control n = 8, aIL10 n = 9, aIL10 + aPD1 n = 10. d-f) Two-sided spearman correlation of the FC of each feature 24 weeks post-ATI and CA-vRNA levels in LNs of control RMs (black), RMs treated with aIL-10 only (red) and combo-treated RMs (blue) 24 weeks post-ATI. g-i) Two-sided spearman correlation of the FC of each feature 24 weeks post-ATI and CA-2LTR levels in LNs of control RMs (black), RMs treated with aIL-10 only (red) and combo-treated RMs (blue) 24 weeks post-ATI. Rho and p-values are shown in the plots. *p < 0.05. ATI: analytical treatment interruption; CA-2LTR: cell-associated 2-LTR circles; CA-vRNA: cell-associated viral RNA; FC: fold change; LNs: lymph nodes; PBMCs: peripheral blood mononuclear cells; RMs: rhesus macaques; SIV: simian immunodeficiency virus; UNS: unstimulated. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5 * IQR (inter-quartile range) from the hinges.
Extended Data Fig. 9 Combo and aIL-10 specific markers.
Heatmap representing the top significantly different flow cytometry markers between RMs treated with aIL-10 alone (red; n = 8) and combo-treated RMs (blue; n = 7). Rows represent flow cytometry markers (p < 0.05), and columns represent samples. Flow markers are represented as a variable-wise standardized value (Z-score). Red and blue correspond to up- and downregulated expression of flow cytometry markers, respectively. Levels of CA-vRNA in LNs measured at 24 weeks post-ATI are displayed at the top of the heatmap and Spearman correlation was used to assess the statistical significance of correlations (p < 0.05) between flow cytometry markers and LN CA-vRNA levels. ATI: analytical treatment interruption; CA-vRNA: cell-associated viral RNA; LNs: lymph nodes; RMs: rhesus macaques.
Extended Data Fig. 10 Longitudinal plots of selected features that discriminate groups and predict lower levels of LN CA-vRNA 24 weeks post-ATI.
a) Features that were significantly downregulated and b) upregulated in combo-treated RMs that were associated with levels of CA-vRNA in LNs 24 weeks post-ATI. Black: control RMs; red: RMs treated with aIL-10 alone; blue: combo-treated RMs. ATI: analytical treatment interruption; CA-vRNA: cell-associated viral RNA; LNs: lymph nodes; RMs: rhesus macaques. Continuous lines represent the natural cubic spline interpolation of medians across weeks. Groups were compared with a linear regression model. The t-statistics for the regression coefficients were used to assess significance, and multiple test correction was performed with Holm–Bonferroni method. *p-adjusted < 0.05; **p-adjusted < 0.01. Sample size: Control n = 8, aIL10 n = 9, aIL10 + aPD1 n = 10. In boxplots, the central line marks the median, and the lower and upper hinges represent the first and third quartiles. Whiskers extend to the smallest and largest values within 1.5 * IQR (inter-quartile range) from the hinges.
Supplementary information
Supplementary Table 1
Supplementary Table 1: A*01 status and group staggering per year of the study. Supplementary Table 2: Adverse events. Supplementary Table 3: Antibodies used for flow cytometry assays.
Source data
Source Data Fig. 1
Raw Data Fig. 1.
Source Data Fig. 2
Raw Data Fig. 2.
Source Data Fig. 3
Raw Data Fig. 3.
Source Data Fig. 4
Raw Data Fig. 4.
Source Data Fig. 5
Raw Data Fig. 5.
Source Data Fig. 6
Raw Data Fig. 6.
Source Data Extended Data Fig. 1
Raw Data Extended Data Fig 1.
Source Data Extended Data Fig. 2
Raw Data Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Raw Data Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Raw Data Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Raw Data Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Raw Data Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Raw Data Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Raw Data Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Raw Data Extended Data Fig. 9.
Source Data Extended Data Fig. 10
Raw Data Extended Data Fig. 10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pereira Ribeiro, S., Strongin, Z., Soudeyns, H. et al. Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption. Nat Immunol (2024). https://doi.org/10.1038/s41590-024-01952-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41590-024-01952-4
- Springer Nature America, Inc.