Abstract
Sponges are the most basal metazoan phylum1 and may have played important roles in modulating the redox architecture of Neoproterozoic oceans2. Although molecular clocks predict that sponges diverged in the Neoproterozoic era3,4, their fossils have not been unequivocally demonstrated before the Cambrian period5,6,7,8, possibly because Precambrian sponges were aspiculate and non-biomineralized9. Here we describe a late-Ediacaran fossil, Helicolocellus cantori gen. et sp. nov., from the Dengying Formation (around 551–539 million years ago) of South China. This fossil is reconstructed as a large, stemmed benthic organism with a goblet-shaped body more than 0.4 m in height, with a body wall consisting of at least three orders of nested grids defined by quadrate fields, resembling a Cantor dust fractal pattern. The resulting lattice is interpreted as an organic skeleton comprising orthogonally arranged cruciform elements, architecturally similar to some hexactinellid sponges, although the latter are built with biomineralized spicules. A Bayesian phylogenetic analysis resolves H. cantori as a crown-group sponge related to the Hexactinellida. H. cantori confirms that sponges diverged and existed in the Precambrian as non-biomineralizing animals with an organic skeleton. Considering that siliceous biomineralization may have evolved independently among sponge classes10,11,12,13, we question the validity of biomineralized spicules as a necessary criterion for the identification of Precambrian sponge fossils.
Similar content being viewed by others
Main
Morphologically diverse animal fossils have been recognized in fossil assemblages of the late-Ediacaran period (around 575–539 million years ago (Ma)) and include examples of total-group eumetazoans14, cnidarians15,16 and bilaterians17. These fossils, along with molecular clock estimates2,3 and contentious biomarker data18,19,20, demand an Ediacaran existence of sponges, which are probably the most basal animal phylum1. However, few sponge fossils have been found from the Ediacaran period or earlier5,7,21. The absence of Precambrian sponge fossils has been attributed to the low preservation potential of siliceous sponge spicules due to low Al3+ concentrations in Precambrian porewaters22 or to the possibility that early sponges were aspiculate and entirely non-biomineralizing animals9. Here we report a crown-group sponge fossil, Helicolocellus cantori gen. et sp. nov., from the late-Ediacaran Shibantan limestone in South China (Extended Data Fig. 1). This new fossil is characterized by an organic latticework skeleton that is compositionally different from, but architecturally similar to and probably related to, spiculate hexactinellid sponges. It thus fills the late Neoproterozoic gap in sponge evolution and indicates that Precambrian sponges may have been aspiculate and non-biomineralizing animals, particularly if biomineralized skeletons evolved independently among sponge classes10,11,12,13.
Systematic palaeontology
Phylum Porifera Grant, 1836
Helicolocellus cantori gen. et sp. nov.
Etymology. Genus name from Greek/Latin helix, helix; and Latin locellus, small box. Species epithet in honour of the mathematician Georg Cantor (1845–1918), with reference to the Cantor set, which describes the regular, self-similar pattern of subdivided rectangular lattices as observed in this fossil.
Holotype. NIGP-176531 (Figs. 1 and 2) part and counterpart, deposited in the Nanjing Institute of Geology and Palaeontology (NIGP).
a,b, Positive relief on bed top: photographed under reflected light directed from the upper right (a) and topographic elevation map from laser scanning microscopy (b). White arrows mark transition from bottom of conical body to the stem. The specimen is broken into two pieces along the yellow dashed line in a. c, Fracture surface along the breakage in a exposes a cross-section through the holotype specimen, showing a three-dimensional outline (dashed ellipse) infilled with coarser sparry calcite cement, in contrast to fine-grained micritic matrix. f, fringe; ff, first fringe; sf, second fringe. Scale bars, 50 mm.
a, Upper part of the body preserved in positive epirelief, viewed under reflected light directed from the upper right. Arrow marks fringe-like structure. b–d, Negative hyporelief counterpart impression with a different lighting angle. b, First-order rectangle in b corresponds to box in a. c, Second-order rectangle in c corresponds to box in b. d, Third-order rectangle in d corresponds to box in c, with dashed box marking a faintly preserved fourth-order rectangle. e, Schematic diagram of hierarchical rectangles. Black box marks first-order rectangle. Yellow, blue and red lines represent grooves that divide first-, second- and third-order rectangles, respectively. Scale bars, 10 mm (a), 5 mm (b), 2 mm (c), 1 mm (d).
Referred material. Paratype: NIGP-176532. Other specimens: NIGP-176533–176538 (Fig. 3 and Extended Data Fig. 2).
a, Paratype, NIGP-176532, with the body preserved in negative epirelief and the associated stem and disc in positive epirelief. b, NIGP-176533, body preserved in positive relief, with irregular arrangement of rectangular boxes towards the base of the specimen. Stratigraphic orientation uncertain. Arrows indicate two regions where the stem width expands from 32 mm to 48 mm. c, Presumed juvenile specimen, NIGP-176536, showing a truncated distal end. The body is preserved in positive relief, whereas the stem is a negative relief impression. Stratigraphic orientation uncertain. Scale bars, 50 mm (a,b), 20 mm (c).
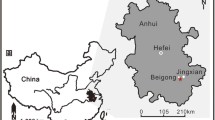
Locality and horizon. From the upper-Ediacaran Shibantan Member of the Dengying Formation at Wuhe, Yangtze Gorges area, Hubei Province, South China.
Diagnosis. A conical to subcylindrical body connected by means of a stem to a basal discoidal structure. The outer surface of the conical body is characterized by regularly arranged rectangles each subdivided into at least three orders of smaller rectangles, forming a hierarchical quadrate reticulation. Rectangles are orthogonally arranged in the upper portion of specimens and become helically twisted around the longitudinal axis towards the base. Rectangles are outlined by grooves which represent an organic cruciform skeletal structure.
Description. The holotype is preserved in positive relief on a bed top (Fig. 1), with a fragmentary impression of the corresponding negative relief preserved on a bed sole (Fig. 2b–d). H. cantori gen. et sp. nov. has a goblet-like morphology with a holdfast, a stem and a conical body, indicating a probable erect benthic lifestyle. The conical body is 291 mm in length (or height in reconstructed life orientation), with a maximum width of 108 mm, tapering basally to a connection with the stem. The stem is partially preserved as a flat and smooth impression, with a width of 23 mm, but the basal disc of the holotype is not preserved. The positive-relief specimen is broken into two parts (along the yellow dashed line in Fig. 1a and perpendicular to the bedding plane), allowing the observation of an elliptical cross-section through the partially flattened body, which is infilled with sparry calcite cement (Fig. 1c), implying an originally conical body with a central cavity.
The body wall exhibits a hierarchical latticework of rectangles, each of which bulges outwardly with a convex surface on the top bedding surface (Fig. 1b). The largest (first-order) rectangles (Fig. 2a,b) are subdivided into smaller rectangles, here termed second- and third-order rectangles (Fig. 2b–e). There are eight first-order rectangles per half-circumference along the distal end of the body. First-order rectangles are 12 ± 1.8 (1σ) mm long and 8.9 ± 1.2 (1σ) mm wide on average (n = 41 rectangles). They are separated from each other by grooves which are 1.1 mm wide and approximately 0.4 mm deep. Finer grooves (0.61 mm wide and about 0.2 mm deep) subdivide the first-order rectangles into four equal second-order rectangles, which have an average length and width of 5.3 ± 0.9 (1σ) mm and 3.8 ± 0.7 (1σ) mm, respectively (n = 164 rectangles). The second-order rectangles are further subdivided into third-order rectangles, which are 2.55 mm long and 1.8 mm wide (n = 146 rectangles), by successively finer grooves (0.3 mm wide and about 0.1 mm deep). Fourth-order rectangles are faintly preserved (Fig. 2d,e).
In the distal region, rectangles are oriented in transverse rows parallel to the arched top edge of the body. Rectangles become diagonally aligned in the middle part of the body. The basal part of the conical body is often poorly preserved, consisting of vaguely defined but poorly aligned rectangles without fine structure, possibly resulting from postmortem distortion, as evidenced by a separate specimen showing regularly and diagonally arranged rectangles at the basal part (Extended Data Fig. 2d). The holotype exhibits two fringes on one side of the body (the right-hand margin in Fig. 1a,b). The fringes each consist of a single row of rectangles, although one of them (labelled ‘ff’ in Fig. 1a,b; hereafter, first fringe) has more sharply defined rectangles than the other (labelled ‘sf’ in Fig. 1a,b: hereafter, second fringe). It is possible that the first fringe represents a longitudinal furrow or suture in the body wall. Alternatively, the two fringes may be attributed to the conical body splitting along a single seam due to compaction. The alignment of neighbouring rectangles in the main body seems to be congruent with those that comprise the fringes (Figs. 1a,b and 2a).
In addition to the holotype, seven other specimens are known (Fig. 3 and Extended Data Fig. 2), two of which are complete (Fig. 3a,c). The paratype NIGP-176532 (Fig. 3a) is 447 mm long and up to 93 mm wide. It possesses a basal disc (Fig. 3a; 57 mm in diameter), which is connected to the conical body (284 mm long) by means of a stem (163 mm long, 30 mm wide). The stem is smooth, with two broader regions where its width expands from 32 mm to 48 mm (arrows in Fig. 3a). The basal disc and stem are both preserved in positive relief on the bed top, whereas the conical body is a negative relief impression. The first-order rectangles have an average length of 12.2 mm (σ = 1.7) and a width of 9 mm (σ = 1.8) (n = 15 measurements). They are subdivided into second-order rectangles, which are approximately 5.2 mm long and 4 mm wide. The second complete specimen, NIGP-176536 (Fig. 3c), is only 113 mm long. It comprises a conical body that is 65 mm long and 38 mm wide, and a stem that is 48 mm long and 11 mm wide. It has a truncated distal end possibly representing an artefact of breakage. A basal disc is not present. The poorly defined and irregularly arranged first-order rectangles are about 7.3 mm long and 6.6 mm wide. The irregular arrangement of rectangles is also observed in specimens NIGP-176536 (Fig. 3b) and NIGP-176534 (Extended Data Fig. 2a). By contrast, specimen NIGP-176538 exhibits well-preserved, diagonally arranged rectangles in the lower part of the conical body (Extended Data Fig. 2d). One of the incomplete specimens, NIGP-176535, shows a single marginal fringe (Extended Data Fig. 2c) rather than double fringes as observed in the holotype.
The preservation style of Helicolocellus is identical to that of other non-biomineralized macrofossils in the Shibantan Member, including Arborea23, Flabellophyton24 and Wutubus25. Specifically, the distinction between the fossils and the sedimentary matrix is defined by lithological contrast, with proportionally more carbonate cement in the sediment filling the central cavity of the conical body (compare Fig. 1c with Fig. 7.2 of ref. 23, Fig. 9E of ref. 24 and Fig. 6g,h of ref. 25). The fossils are preserved as casts and moulds, but the body walls and their constituent elements are not preserved. This taphonomic style differs markedly from that of biomineralized tubular fossils such as Cloudina26 and Sinotubulites27 from the Shibantan Member, which preserve well-defined although secondarily replaced tests. This difference is probably because organic walls are more easily degraded and partially compacted (Fig. 1c), whereas a biomineralized test would be expected to retain its original three-dimensional morphology.
Discussion
The presence of a stem and a discoidal holdfast suggests an erect benthic lifestyle (Fig. 4a), but these features are not phylogenetically informative because they are also present in several distantly related benthic Ediacaran taxa, such as the putative cnidarian Haootia15, the macroalga Discusphyton28, arboreomorphs29 and some rangeomorphs (for example, Charnia14 and Primocandelabrum30), as well as many extant sponges31, cnidarians32, fungi and various algae. Instead, the stem and holdfast probably represent a convergent adaptation to a benthic lifestyle on microbially bound, firm substrates, which were prevalent during the Ediacaran period and are inferred to be present in the Shibantan assemblages on the basis of dark, organic-rich crinkly laminations observed in thin sections from this unit33. Common anchored benthic Ediacaran taxa such as Charnia34 and Arborea35 are leaf-like and constructed by branching modules, whereas H. cantori has a conical body consisting of a regular hierarchical latticework, evidencing a very different body plan and functional morphology.
a, Reconstructed life position of H. cantori on the Ediacaran seafloor. Artwork by D. Yang. b, Bayesian phylogenetic analysis of metazoans, based on character matrix modified from refs. 14,54,68. H. cantori is resolved as a stem-group hexactinellid along with Eiffelia, Diagoniella, Protospongia, Cyathophycus and Minitaspongia. The total-group hexactinellid clade shows a basal polytomy of three branches: 1, Helicolocellus and reticulosans; 2, Eiffelia; 3, crown-group or extant hexactinellids. Numbers are posterior probabilities for nodes. See Supplementary Information for morphological data matrix.
The main body of Helicolocellus shares a conical to cylindrical morphology with late-Ediacaran taxa, which have previously been likened to sponges, but possesses sufficient morphological differences to justify the establishment of a new taxon based on the Shibantan material. The putative sponge Thectardis36,37 from Newfoundland is inferred to have possessed a conical body which is similar to Helicolocellus, but Thectardis typically shows a featureless external surface and lacks a stem or basal disc. Another cylindrical fossil, the putative sponge Ausia from the Nama Group of Namibia38, has millimetre-scale pore-like structures on its surface, albeit notably smaller than the first-order rectangles observed in H. cantori and lacking second-order divisions. Palaeophragmodictya, a discoidal fossil found in South Australia and the White Sea region of Russia, was initially interpreted as a sponge39 and shares reticulate surface patterns which are smaller than those of H. cantori. However, it differs markedly in shape, being discoidal rather than conical, and more recent research indicates that it may represent an attachment disc of frondose organisms or a microbially produced texture5. The Ediacaran fossil Gibbavasis40,41 from Iran and Moldova shares a lattice-like appearance with Helicolocellus but possesses only a single order of orthogonally arranged ‘boxes’. Furthermore, Gibbavasis is much smaller (4–14 mm long, 2–7 mm wide) than Helicolocellus. Perpendicular cross-hatched structures have also been described in an unnamed fossil from Nevada (Fig. 3a,b in ref. 42). However, its surface ornamentation can be readily distinguished from the reticulation observed in H. cantori by the absence of secondary latticework.
The box-like latticework pattern of Helicolocellus is superficially similar to the transverse and longitudinal furrows on the body walls of some living hexacorallians (Cnidaria: Anthozoa), such as stony corals (Scleractinia) and sea anemones (Actiniaria). In these hexacorallians, the furrows correspond to a latticework of internal muscle fibres known as a muscle field43. However, the box-like structures in the Hexacorallia are not subdivided into finer units, nor are they arranged diagonally as in Helicolocellus and sponges. Furthermore, the irregular arrangement of boxes observed in some Helicolocellus specimens (for example, Figs. 1a and 3b) cannot be easily explained by disturbance of the contractile muscle field seen in the Hexacorallia. Contractile muscle fields are typically highly variable in length-to-width ratio, inconsistent with the relatively stable ratio of Helicolocellus specimens (around 3.0, measured on three specimens) in Helicolocellus. More importantly, tentacles are considered a synapomorphy (shared derived characteristic) of the Anthozoa44 but are not present in Helicolocellus, thus excluding Helicolocellus from the total-group Hexacorallia. Similarities with the longitudinal and transverse muscle bundles of extant tunicates can also be refuted by the ability of Helicolocellus rectangles to behave as discrete individual blocks, which can result in irregular arrangement, particularly in the lower part of the conical body (Figs. 1a and 3b). The enigmatic Cambrian fossil Paramackenzia, which has previously been compared to modular Ediacaran organisms and exhibits potential three-dimensional box-like compartmental structures45, has passing similarity, but its compartmental structures neither subdivide nor show a helical arrangement.
The body plan of Helicolocellus, with its goblet-like shape and especially the hierarchical rectangular ornament, is highly similar to the overall morphology and skeletal grid of Palaeozoic hexactinellid sponges, in which the pattern results from the presence of regularly arranged biomineralized spicules. For example, stem-group hexactinellids such as the Protospongiidae (Extended Data Fig. 3a,b) and the Dictyospongiidae (Extended Data Fig. 3c,d) are characterized by similar regular and uniformly divided meshes of spicules46. Dictyospongiids, which are funnel-shaped or cylindrical sponges, can reach considerable sizes, with genera such as Hydnoceras growing larger than 250 mm (ref. 46). Clathrospongia and Minitaspongia exhibit first-order meshes that are 1.5–15 mm wide and 2–15 mm long47,48, which are comparable in size to those of Helicolocellus. In some cases, the orthogonal rays of spicules that form the skeletal grids and outline the ‘boxes’ in Clathrospongia may appear as deep impressions as a result of the dissolution of spicules during diagenesis48, resulting in a preservational style virtually identical to Helicolocellus. The protrusion of sediment through the spaces between the orthogonal rays may also result in a rectangular pattern47 similar to that of Helicolocellus. Some other Cambrian sponges, for example, the demosponge Vauxia and the ascosponge Leptomitus, also exhibit a grid pattern49. However, these patterns differ significantly from the regular and hierarchical grid pattern found in Helicolocellus and other Palaeozoic hexactinellid sponges in having less hierarchy in their organization and less regularity in the spicule arrangements.
The hierarchical skeletal grid in these Palaeozoic hexactinellid sponges consists of multiscale bundles of spicules, typically either fused together or loosely assembled to form a latticework. Although Helicolocellus lacks direct evidence for a biomineralized skeleton, it probably had a somewhat rigid skeleton consisting of discrete cruciform elements, considering the presence of both regularly and irregularly arranged reticulate patterns in observed specimens. It is thus taphonomically similar to the Devonian fossil Pontagrossia50 (Extended Data Fig. 3f), which has been compared to a sponge and is inferred to have possessed a largely organic skeleton characterized by a reticulate pattern. The skeleton of Helicolocellus could similarly have been originally organic, considering its taphonomic style similar to other non-biomineralized macrofossils such as Arborea23, Flabellophyton24 and Wutubus25 but different from the biomineralized tubular fossil Cloudina26 in the Shibantan Member. Additionally, the outwardly bulging upper surface of the rectangles in Helicolocellus (as evidenced by convex-up positive epirelief preservation, seen in the holotype) is consistent with a flexible wall or membrane which was pushed outward during sediment infilling and compaction and impressed against a more rigid external framework.
Some clusters of rectangles in Helicolocellus are irregularly arranged, particularly in the lower part of the conical body (Figs. 1a and 3b). These irregularly arranged rectangles may result from the dislocation of some rectangular elements during either degradation or compaction. Similar irregular arrangement is also observed in protospongiid sponges (Extended Data Fig. 3b), which are constructed of loosely articulated cruciform units known as stauractine spicules which are prone to postmortem dislocation51. This observation indicates that the skeleton of Helicolocellus was constructed of unfused skeletal elements. Considering the possibility that early Palaeozoic sponge spicules were weakly biomineralized and contained large proportions of organic matter, with full biomineralisation only seen in later sponges9, we might expect early sponges such as Helicolocellus to have organic, unfused skeletal elements prone to dislocation.
The attachment strategy of Helicolocellus can also find analogues among younger sponges. The Jurassic protospongiid-like sponge Ammonella, for example, shows a regular and hierarchical meshwork of stauractines49, resembling the pattern observed in Helicolocellus, and was also anchored to a potentially microbially stabilized substrate by a discoid root plate52. This attachment strategy bears similarity to the way Helicolocellus adheres to the (presumably microbially stabilized53) Ediacaran substrate.
To more rigorously test the possible hexactinellid affinity of Helicolocellus, a Bayesian phylogenetic analysis was conducted using a dataset consisting of 79 taxa (including 8 fossil taxa) and 235 characters, with 67 of these characters scored for Helicolocellus (see Supplementary Information for methods and details of the character matrix). Notably, Helicolocellus was assigned to have a highly regular hierarchical reticulate skeleton (character 176). The analysis recovers Helicolocellus as a crown-group sponge and a stem-group hexactinellid (Fig. 4b and Extended Data Fig. 4). This result is unsurprising given that all crown-group hexactinellids have biomineralized spicules54 and Helicolocellus does not. Moreover, the Bayes factor (15.82) indicates a strong statistical support for a crown-group sponge placement of Helicolocellus over a stem-group Porifera alternative (Supplementary Information). The results remain stable in several sensitivity tests: when ctenophores are constrained as the sister-group to all other animals55 (Extended Data Fig. 5a) and when the relationships of the classes in Porifera and the internal relationships in Hexactinellida are constrained to follow recent molecular phylogenies10,54 (Extended Data Fig. 5b).
The latticework of Helicolocellus may have facilitated feeding or mechanical stability. As in modern sponges and diploblastic animals, early sponges probably lacked specialized internal organs and may have depended on diffusion for gas exchange and osmotrophic or filter feeding32,56. A simple and effective strategy to achieve these functions is to increase the ratio of surface area to volume, as occurs in several extant shallow-water sponges, in which organic particles and dissolved organic matter serve as a chief food source57. Modern hexactinellid sponges possess a syncytial pinacoderm and a water canal system lined with choanocytes58 (branched choanoderm). The various forms (for example, folding, branching and anastomosis) of hexactinellid skeletons have been interpreted as an adaptation to maximize the choanoderm surface area in the limited space occupied by the sponge59. The repeatedly divided box pattern in Helicolocellus may represent a similar strategy to increase surface area. This pattern resembles a self-similar fractal known as the Cantor dust set, which is generated through a recursive process of repeatedly inserting a central cross in rectangles, resulting in successively smaller rectangles which are geometrically similar to one another (Fig. 2). Fractals are ubiquitous in biology but appear almost exclusively in the form of branching tubes60, such as in the lungs, leaf veins and plant roots. Non-branching fractals are exceedingly rare in organisms. The Cantor dust set is unique to Helicolocellus and some Palaeozoic sponges. This multiscale hierarchical structure may also provide mechanical benefits; broadly similar skeletal systems in modern hexactinellid sponges, such as Euplectella, have been shown to contribute to mechanical stability61.
The overall morphology and regular grid-like pattern on the body wall of Helicolocellus are consistent with an affinity with sponges, particularly hexactinellids. However, there are two key features of modern hexactinellids that are not observed in Helicolocellus. First, the reticulation of modern hexactinellid sponges is typically constructed by cruciform stauract spicules bounded by soft tissue or by fused spicules, whereas no mineralized spicules have been found in Helicolocellus. This apparent absence is probably original, with any cruciform skeletal elements in Helicolocellus being non-biomineralized. The last common ancestor of sponges may have been aspiculate11,12,13, and the siliceous spicules of the modern sponge classes—Hexactinellida, Demospongiae and Homoscleromorpha—have been shown not to be homologous13. Those sponge classes may have independently acquired mineralized skeletons along with other metazoan lineages in the early Cambrian10,11,12,13. Palaeontological and molecular phylogenetic analyses have not arrived at a conclusive resolution with regard to the origin(s) of biomineralization in sponges12, with some studies advocating the origin of biomineralized spicules in the last common ancestor of the Silicea62 or even Porifera6 and others entertaining the possibility of independent origins of siliceous spicules in the Hexactinellida, Demospongiae and Homoscleromorpha9,13,63. Our phylogenetic placement of Helicolocellus as a stem-group hexactinellid that possesses some (for example, a reticulate skeleton) but not all (for example, biomineralized spicules) features of the crown-group Hexactinellida is consistent with either independent origins or secondary loss of siliceous spicules. Second, the surface of Helicolocellus lacks evidence for ostia (inhalant pores). It is possible that the minute size of ostia, such as those observed in extant hexactinellid sponges (for example, 4–30 µm; ref. 64) may not have been preserved in this deposit. The smallest resolvable features preserved in the Shibantan limestone are tertiary branches of Charnia, which have a submillimeter minimum dimension (ref. 65), an order of magnitude larger than would be predicted for ostia. Our ability to determine the presence of an osculum-like structure in Helicolocellus is hampered by the lateral compression of all specimens at hand, but the three-dimensional cement-filled cross-section through the holotype (Fig. 1c) suggests the likely presence of a central cavity in Helicolocellus, which could be homologous to the spongocoel in modern sponges.
It is also worth commenting on the phylogenetic placement of the hexactine-bearing Reticulosa, which has traditionally been assigned to the hexactinellids66 but has since been proposed to be paraphyletic to the Hexactinellida, Demospongiae, Calcarea and Homoscleromorpha and even the entire Porifera6. Our phylogenetic analysis indicates that at least some extinct reticulosan taxa, that is, Diagoniella, Protospongia, Eiffelia, Cyathophycus and Minitaspongia, along with the heteractinid Eiffelia, are grouped with extant hexactinellids (Fig. 4b). Therefore, reticulosan and heteractinid sponges may represent stem-group hexactinellids.
To conclude, H. cantori represents an Ediacaran crown-group sponge with an organic skeleton that is architecturally similar to the Hexactinellida. If siliceous biomineralization evolved independently in the Hexactinellida, Demospongiae and Homoscleromorpha13, then a pre-existing organic scaffold with a regular hierarchical reticulate skeleton (as present in H. cantori) may have served as a template for subsequent acquisition of biomineralized spicules. An important ramification is that we should broaden our search image of Precambrian sponge fossils, not only because they may have been aspiculate if sponge biomineralization evolved several times (for example, Tonian candidate keratose sponge material from Canada7) but also because stem-group representatives necessarily lacked some features diagnostic of their crown-group counterparts67. This emphasizes the phylogenetic importance of the fossil record in the search of the evolutionary root of sponges and indeed all animals.
Methods
Fossil specimens were collected from a single stratigraphic horizon about 2–2.5 m above the base of the Shibantan Member (around 551–543 Ma) at Wuhe village in the Yangtze Gorges area, South China (Extended Data Fig. 1). All specimens are preserved on limestone bedding surfaces and are deposited at the NIGP, Nanjing, China. Photographs were taken using a Nikon D850 DSLR camera and a Zeiss Axio Zoom V16 microscope. Measurements were carried out on fossil images using ImageJ 1.52a and analysed using Microsoft Excel 2013. Laser scanning data (Fig. 1b) were obtained using the Faro Design ScanArm and processed using the software Geomagic Warp 2017 and CloudCompare 2.13 to capture surface details and to generate elevation maps.
Phylogenetic analysis was conducted on the basis of a previously published character matrix for metazoans14,54,68 with modifications. No statistical methods were used to predetermine sample size. A total of 235 morphological characters were coded for 79 taxa. Only characters that are parsimony-informative were included in the analysis, to accentuate shared derived characters (synapomorphies). We scored H. cantori for 67 of these morphological characters (see Supplementary Information for details). Bayesian phylogenetic analysis was run using MrBayes 3.2.7 (ref. 69) on the CIPRES Science Gateway70. Analyses were run for 6,000,000 generations, sampled with a frequency of every 1,000 generations, discarding the first 25% samples as burn-in. The average standard deviation of split frequencies was about 0.01 in all runs. The effective sample size, calculated using Tracer 1.7 (ref. 71), indicated that all parameters had effective sample size scores above 200.
We compared the Mk model with gamma and lognormal distributions, considering both symmetric and asymmetric transition frequencies. Additionally, we evaluated the topological hypotheses, specifically whether Helicolocellus is a stem-group sponge or a stem-group hexactinellid. To assess the strength of support for different models and hypotheses, we calculated marginal likelihoods, which were computed using stepping-stone sampling with 50 steps and 20,000,000 generations. The marginal likelihoods for each model were used to calculate Bayes factors and to determine the best-fit model72. Hard constraints were applied to all nodes during the stepping-stone sampling analysis for the two hypotheses. The results revealed that the model with a lognormal distribution and asymmetrical transition frequencies and the topology of the stem-group hexactinellid hypothesis were better supported (Supplementary Information and Supplementary Table 1). Therefore, the model with a lognormal distribution and asymmetrical transition frequencies was used in downstream analyses, as presented in Fig. 4b and Extended Data Fig. 4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Fossils illustrated in this paper are accessioned in the NIGP (catalogue nos. NIGP-176531 to NIGP-176538, NIGP-155870, NIGP-201942). Data collected or generated during this study are included in this article and its Supplementary Information. The nomenclature of H. cantori gen. et sp. nov. is registered in zoobank and the Life Science Identifier for this publication is urn:lsid:zoobank.org:pub:06F779B0-BA00-41AF-A6F7-A552BA8F6BF1.LSID.
References
Redmond, A. K. & McLysaght, A. Evidence for sponges as sister to all other animals from partitioned phylogenomics with mixture models and recoding. Nat. Commun. 12, 1783 (2021).
Lenton, T. M., Boyle, R. A., Poulton, S. W., Shields-Zhou, G. A. & Butterfield, N. J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 7, 257–265 (2014).
dos Reis, M. et al. Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Curr. Biol. 25, 2939–2950 (2015).
Dohrmann, M. & Wörheide, G. Dating early animal evolution using phylogenomic data. Sci. Rep. 7, 3599 (2017).
Antcliffe, J. B., Callow, R. H. T. & Brasier, M. D. Giving the early fossil record of sponges a squeeze. Biol. Rev. 89, 972–1004 (2014).
Botting, J. P. & Muir, L. A. Early sponge evolution: a review and phylogenetic framework. Palaeoworld 27, 1–29 (2018).
Turner, E. C. Possible poriferan body fossils in early Neoproterozoic microbial reefs. Nature 596, 87–91 (2021).
Neuweiler, F. et al. Keratose sponges in ancient carbonates—a problem of interpretation. Sedimentology 70, 927–968 (2023).
Tang, Q., Wan, B., Yuan, X., Muscente, A. D. & Xiao, S. Spiculogenesis and biomineralization in early sponge animals. Nat. Commun. 10, 3348 (2019).
Aguilar-Camacho, J. M., Doonan, L. & McCormack, G. P. Evolution of the main skeleton-forming genes in sponges (phylum Porifera) with special focus on the marine Haplosclerida (class Demospongiae). Mol. Phylogenet. Evol. 131, 245–253 (2019).
Murdock, D. J. E. The ‘biomineralization toolkit’ and the origin of animal skeletons. Biol. Rev. 95, 1372–1392 (2020).
Xiao, S. Ediacaran sponges, animal biomineralization and skeletal reefs. Proc. Natl Acad. Sci. USA 117, 20997–20999 (2020).
Shimizu, K. et al. Silica-associated proteins from hexactinellid sponges support an alternative evolutionary scenario for biomineralization in Porifera. Nat. Commun. 15, 181 (2024).
Dunn, F. S. et al. The developmental biology of Charnia and the eumetazoan affinity of the Ediacaran rangeomorphs. Sci. Adv. 7, eabe0291 (2021).
Liu, A. G., Matthews, J. J., Menon, L. R., McIlroy, D. & Brasier, M. D. Haootia quadriformis n. gen., n. sp., interpreted as a muscular cnidarian impression from the Late Ediacaran period (approx. 560 Ma). Proc. R. Soc. B 281, 20141202 (2014).
Dunn, F. S. et al. A crown-group cnidarian from the Ediacaran of Charnwood Forest, UK. Nat. Ecol. Evol. 6, 1095–1104 (2022).
Chen, Z., Zhou, C., Yuan, X. & Xiao, S. Death march of a segmented and trilobate bilaterian elucidates early animal evolution. Nature 573, 412–415 (2019).
Love, G. D. et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457, 718–721 (2009).
Nettersheim, B. J. et al. Putative sponge biomarkers in unicellular Rhizaria question an early rise of animals. Nat. Ecol. Evol. 3, 577–581 (2019).
Zumberge, J. A. et al. Demosponge steroid biomarker 26-methylstigmastane provides evidence for Neoproterozoic animals. Nat. Ecol. Evol. 2, 1709–1714 (2018).
Muscente, A. D., Marc Michel, F., Dale, J. G. & Xiao, S. Assessing the veracity of Precambrian ‘sponge’ fossils using in situ nanoscale analytical techniques. Precambrian Res. 263, 142–156 (2015).
Sperling, E. A., Robinson, J. M., Pisani, D. & Peterson, K. J. Where’s the glass? Biomarkers, molecular clocks and microRNAs suggest a 200-Myr missing Precambrian fossil record of siliceous sponge spicules. Geobiology 8, 24–36 (2010).
Wang, X. et al. The Ediacaran frondose fossil Arborea from the Shibantan limestone of South China. J. Paleontol. 94, 1034–1050 (2020).
Wan, B. et al. A tale of three taphonomic modes: the Ediacaran fossil Flabellophyton preserved in limestone, black shale and sandstone. Gondwana Res. 84, 296–314 (2020).
Chen, Z. et al. New Ediacara fossils preserved in marine limestone and their ecological implications. Sci. Rep. 4, 4180 (2014).
Chen X., Zhou P., Zhang B., Wei, K. & Zhang, M. Lithostratigraphy, biostratigraphy, sequence stratigraphy and carbon isotope chemostratigraphy of the upper Ediacarian in Yangtze Gorges and their significance for chronostratigraphy. South China Geol. 32, 87–105 (2016).
Yang, B., Warren, L. V., Steiner, M., Smith, E. F. & Liu, P. Taxonomic revision of Ediacaran tubular fossils: Cloudina, Sinotubulites and Conotubus. J. Paleontol. 96, 256–273 (2022).
Wang, Y., Wang, Y. & Du, W. A rare disc-like holdfast of the Ediacaran macroalga from South China. J. Paleontol. 91, 1091–1101 (2017).
Laflamme, M., Gehling, J. G. & Droser, M. L. Deconstructing an Ediacaran frond: three-dimensional preservation of Arborea from Ediacara, South Australia. J. Paleontol. 92, 323–335 (2018).
Hofmann, H. J., O’Brien, S. J. & King, A. F. Ediacaran biota on Bonavista Peninsula, Newfoundland, Canada. J. Paleontol. 82, 1–36 (2008).
Mitchell, E. G. & Harris, S. Mortality, population and community dynamics of the glass sponge dominated community “The Forest of the Weird” from the Ridge Seamount, Johnston Atoll, Pacific Ocean. Front. Mar. Sci. 7, 565171 (2020).
Brusca, R. C., Moore, W. & Shuster, S. M. Invertebrates (Sinauer Associates, 2016).
Xiao, S., Shen, B., Zhou, C., Xie, G. & Yuan, X. A uniquely preserved Ediacaran fossil with direct evidence for a quilted bodyplan. Proc. Natl Acad. Sci. USA 102, 10227–10232 (2005).
Ford, T. D. Pre-Cambrian fossils from Charnwood Forest. Proc. Yorks. Geol. Soc. 31, 211–217 (1958).
Glaessner, M. F. & Daily, B. The geology and Late Precambrian fauna of the Ediacara fossil reserve. Rec. South Aust. Mus. 13, 369–401 (1959).
Clapham, M. E., Narbonne, G. M., Gehling, J. G., Greentree, C. & Anderson, M. M. Thectardis avalonensis: a new Ediacaran fossil from the Mistaken Point biota, Newfoundland. J. Paleontol. 78, 1031–1036 (2004).
Sperling, E. A., Peterson, K. J. & Laflamme, M. Rangeomorphs, Thectardis (Porifera?) and dissolved organic carbon in the Ediacaran oceans. Geobiology 9, 24–33 (2011).
Hahn, G. & Pflug, H. D. Polypenartige organismen aus dem Jung-Präkambrium (Nama-Gruppe) von Namibia. Geol. Palaeontol. 19, 1–13 (1985).
Gehling, J. G. & Rigby, J. K. Long expected sponges from the Neoproterozoic Ediacara fauna of South Australia. J. Paleontol. 70, 185–195 (1996).
Francovschi, I., Grădinaru, E., Li, H., Shumlyanskyy, L. & Ciobotaru, V. U–Pb geochronology and Hf isotope systematics of detrital zircon from the late Ediacaran Kalyus Beds (East European Platform): palaeogeographic evolution of southwestern Baltica and constraints on the Ediacaran biota. Precambrian Res. 355, 106062 (2021).
Vaziri, S. H., Majidifard, M. R. & Laflamme, M. Diverse assemblage of Ediacaran fossils from Central Iran. Sci. Rep. 8, 5060 (2018).
Smith, E. F., Nelson, L. L., Tweedt, S. M., Zeng, H. & Workman, J. B. A cosmopolitan late Ediacaran biotic assemblage: new fossils from Nevada and Namibia support a global biostratigraphic link. Proc. R. Soc. B 284, 20170934 (2017).
McMahon, S., Tarhan, L. G. & Briggs, D. E. G. Decay of the sea anemone Metridium (Actiniaria): implications for the preservation of cnidarian polyps and other soft-bodied diploblast-grade animals. Palaios 32, 388–395 (2017).
Ou, Q. et al. Dawn of complex animal food webs: a new predatory anthozoan (Cnidaria) from Cambrian. Innovation 3, 100195 (2022).
Zhao, Y. et al. An early Cambrian mackenziid reveals links to modular Ediacaran macro-organisms. Pap. Palaeontol. 8, e1412 (2022).
Hall, J. & Clarke, J. M. A Memoir on the Palaeozoic Reticulate Sponges: Constituting the Family Dictyospongidae (Wynkoop Hallenbeck Crawford Company, 1898).
Carrera, M., Rustan, J., Vaccari, N. & Ezpeleta, M. A Mississippian hexactinellid sponge from the Western Gondwana: taxonomic and paleobiogeographic implications. Acta Palaeontol. Pol. 63, 63–70 (2018).
Rigby, J. K. & Keyes, R. First report of hexactinellid dictyosponges and other sponges from the Upper Mississippian Bangor Limestone, northwestern Alabama. J. Paleontol. 64, 886–897 (1990).
Finks, R. M., Reid, R. E. H. & Rigby, J. K. Treatise on Invertebrate Paleontology Part E (Revised) (Geological Society of America and the University of Kansas, 2004).
Chahud, A. & Fairchild, T. R. A new invertebrate from the Ponta Grossa Formation (Devonian), Paraná Basin, Brazil. Rev. Bras. Paleontol. 23, 279–282 (2020).
Wulff, J. in Coral Reefs at the Crossroads (eds Hubbard, D. K. et al.) 103–126 (Springer, 2016).
Keupp, H. & Schweigert, G. Neochoiaella n. gen. (Demospongeae, Choiaellidae)—a second poriferan Lazarus taxon from the Solnhofen Plattenkalk (Upper Jurassic, Southern Germany)? Paläontol. Z. 86, 269–274 (2012).
Bottjer, D. J., Hagadorn, J. W. & Dornbos, S. Q. The Cambrian substrate revolution. GSA Today 10, 1–7 (2000).
Dohrmann, M., Janussen, D., Reitner, J., Collins, A. G. & Wörheide, G. Phylogeny and evolution of glass sponges (Porifera, Hexactinellida). Syst. Biol. 57, 388–405 (2008).
Dunn, C. W., Leys, S. P. & Haddock, S. H. D. The hidden biology of sponges and ctenophores. Trends Ecol. Evol. 30, 282–291 (2015).
Schlichter, D. in Biology of the Integument: Invertebrates (eds Bereiter-Hahn, J. et al.) 79–95 (Springer, 1984).
de Goeij, J. M., Lesser, M. P. & Pawlik, J. R. in Climate Change, Ocean Acidification and Sponges: Impacts Across Multiple Levels of Organization (eds Carballo, J. L. & Bell, J. J.) 373–410 (Springer, 2017).
Leys, S. P., Mackie, G. O. & Reiswig, H. M. The biology of glass sponges. Adv. Mar. Biol. 52, 1–145 (2007).
Finks, R. M. in Series in Geology, Notes for Short Course (ed. Broadhead, T. W.) 101–115 (Univ. Tennessee, 1983).
Nonnenmacher, T. F., Losa, G. A. & Weibel, E. R. Fractals in Biology and Medicine (Birkhäuser, 2013).
Weaver, J. C. et al. Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum. J. Struct. Biol. 158, 93–106 (2007).
Riesgo, A., Maldonado, M., López-Legentil, S. & Giribet, G. A proposal for the evolution of cathepsin and silicatein in sponges. J. Mol. Evol. 80, 278–291 (2015).
Hill, M. S. et al. Reconstruction of family-level phylogenetic relationships within Demospongiae (Porifera) using nuclear encoded housekeeping genes. PLoS ONE 8, e50437 (2013).
Mackie, G. O., Singla, C. L. & Smith, J. E. Studies on hexactinellid sponges. I. Histology of Rhabdocalyptus dawsoni (Lambe, 1873). Philos. Trans. R. Soc. Lond. B 301, 365–400 (1983).
Wu, C. et al. The rangeomorph fossil Charnia from the Ediacaran Shibantan biota in the Yangtze Gorges area, South China. J. Paleontol. https://doi.org/10.1017/jpa.2022.97 (2022).
Reid, R. E. H. A monograph of the Upper Cretaceous Hexactinellida of Great Britain and Northern Ireland Part I. Monogr. Palaeontogr. Soc. 111, 1–46 (1958).
Xiao, S. Extinctions, morphological gaps, major transitions, stem groups and the origin of major clades, with a focus on early animals. Acta Geol. Sin. Engl. Ed. 96, 1821–1829 (2022).
Manuel, M. et al. Phylogeny and evolution of calcareous sponges: monophyly of calcinea and calcaronea, high level of morphological homoplasy and the primitive nature of axial symmetry. Syst. Biol. 52, 311–333 (2003).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001).
Miller, M. A., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE) https://doi.org/10.1109/GCE.2010.5676129 (IEEE, 2010).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904 (2018).
Ronquist, F., Huelsenbeck, J., Teslenko, M., Zhang, C. & Nylander, J. Draft MrBayes version 3.2 manual: tutorials and model summaries. GitHub https://github.com/NBISweden/MrBayes/blob/develop/doc/manual/Manual_MrBayes_v3.2.pdf (2020).
Xiao, S., Chen, Z., Pang, K., Zhou, C. & Yuan, X. The Shibantan Lagerstätte: insights into the Proterozoic–Phanerozoic transition. J. Geol. Soc. 178, 2020–2135 (2021).
Condon, D. et al. U-Pb Ages from the Neoproterozoic Doushantuo Formation, China. Science 308, 95–98 (2005).
Huang, T., Chen, D., Ding, Y., Zhou, X. & Zhang, G. SIMS U-Pb zircon geochronological and carbon isotope chemostratigraphic constraints on the Ediacaran–Cambrian boundary succession in the Three Gorges area, South China. J. Earth Sci. 31, 69–78 (2020).
Okada, Y. et al. New chronological constraints for Cryogenian to Cambrian rocks in the Three Gorges, Weng’an and Chengjiang areas, South China. Gondwana Res. 25, 1027–1044 (2014).
An, Z. et al. Stratigraphic position of the Ediacaran Miaohe biota and its constrains on the age of the upper Doushantuo δ13C anomaly in the Yangtze Gorges area, South China. Precambrian Res. 271, 243–253 (2015).
Xiao, S., Bykova, N., Kovalick, A. & Gill, B. C. Stable carbon isotopes of sedimentary kerogens and carbonaceous macrofossils from the Ediacaran Miaohe Member in South China: implications for stratigraphic correlation and sources of sedimentary organic carbon. Precambrian Res. 302, 171–179 (2017).
Zhou, C. et al. The stratigraphic complexity of the middle Ediacaran carbon isotopic record in the Yangtze Gorges area, South China and its implications for the age and chemostratigraphic significance of the Shuram excursion. Precambrian Res. 288, 23–38 (2017).
Liu, Q., Huang, D. & Gong, Y. Sponge fossils from the Cambrian Mantou Formation of Hebi, Henan, Central China. J. China Univ. Geosci. 37, 129–135 (2012).
Virtual Collection (Digital Atlas of Ancient Life, accessed 23 April 2024); www.digitalatlasofancientlife.org/vc/.
Acknowledgements
This research was supported by Science Fund for Creative Research Groups of National Natural Science Foundation of China (41921002, 42130207, 41972005, 42272005), National Key R&D Program of China (2022YFF0800100, 2022YFF0802700) and the US National Science Foundation (EAR-2021207 to S.X.). We thank J. Li and W. Yang for help in fossil excavation; W. Yuan and Y. Chen for assistance with laser scanning; G. Mussini for assistance with phylogenetic analyses; and N. Butterfield, Z. Zhao and X. Xian for useful discussions.
Author information
Authors and Affiliations
Contributions
X.Y., S.X., Z.C., X.W. and B.W. designed the study. X.W., S.X., A.G.L., Z.C., X.Y. and B.W. interpreted the data. Z.C. coordinated the fieldwork. X.W. performed the phylogenetic analyses, compiled data and figures and composed the first draft of the manuscript with substantial contributions from S.X., A.G.L. and all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Marc Laflamme, Lucy Muir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Geological map and stratigraphic column.
Star in a marks fossil locality on the southern margin of the Huangling anticline. Star in the inset map marks the location of the Huangling anticline on the South China block. Star in b shows the stratigraphic level from which Helicolocellus was discovered. Reproduced from Xiao, S., Chen, Z., Pang, K., Zhou, C. & Yuan, X. The Shibantan Lagerstätte: Insights into the Proterozoic–Phanerozoic transition. J. Geol. Soc. London 178, jgs2020-135 (2020) https://doi.org/10.1144/jgs2020-135 (ref. 73). Sources of geochronometric data: 551.1 ± 0.7 Ma, 632.5 ± 0.5 and 635.2 ± 0.6 Ma from Condon et al.74; 543.4 ± 3.5 Ma from Huang et al.75; and 526.4 ± 5.4 Ma from Okada et al.76. Dashed arrows indicate alternative correlations of the radiometric date from the Miaohe Member77,78,79. Cam. = Cambrian; Cry. = Cryogenian; Fm. = Formation; HMJ = Hamajing Member; Mbr. = Member.
Extended Data Fig. 2 Additional specimens of Helicolocellus cantori gen. et sp. nov.
a, Positive relief of NIGP-176534. Stratigraphic orientation uncertain. Note irregular arrangement of boxes. b, Thin section perpendicular to bedding plane and along dashed line in a, showing boundaries of first order rectangles (arrowed). c, Positive relief on bed sole, NIGP-176535, showing fine grooves along the fringe of specimen. d, Positive relief on bed sole, NIGP-176538. f, fringe. Scale bars, 30 mm (a, d), 10 mm (b), 50 mm (c).
Extended Data Fig. 3 Palaeozoic sponges and candidate sponges with skeletons organized in hierarchical latticework.
a, Pyritized protospongiid Diagoniella, NIGP-155870, from the Mantou Formation of Henan Province, Wuliuan Stage (Cambrian)80. b, Magnification of box in a. Box in b marks dislocated spicules. c. Devonian Hydnoceras, PRI 76741 (Digital Atlas of Ancient Life of the Paleontological Research Institution, Ithaca, New York81; license CC0 1.0), showing helically arranged skeletal tracts. d, Hydnoceras, NIGP-201942, from the Upper Devonian Chemung Formation of New York. e, Magnification of the box in d, showing impressions of spicules. f, Devonian sponge-like fossil Pontagrossia50, from the Ponta Grossa Formation of Paraná State (image provided by Artur Chahud and Thomas Fairchild). Scale bars, 1 mm (a, b), 40 mm (c), 20 mm (d), 10 mm (e), 5 mm (f).
Extended Data Fig. 4 Phylogenetic position of Helicolocellus cantori gen. et sp. nov.
All taxa coded in the Bayesian analysis are included in this figure. Numbers are posterior probabilities for nodes.
Extended Data Fig. 5 Additional phylogenetic topologies run as sensitivity analyses.
a, Ctenophores constrained as sister-group to all other animals58. b. Relationships of Porifera classes constrained by recent molecular phylogenies10 (see Supplementary Information for further details). Numbers are posterior probabilities for nodes.
Supplementary information
Supplementary Information
Information about the phylogenetic database, a list of characters, topological constraints and the data matrix.
Supplementary Data
All measurements of Helicolocellus specimens reported in the main text, with specimen numbers and types of measurements identified in the titles of the sheets.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Liu, A.G., Chen, Z. et al. A late-Ediacaran crown-group sponge animal. Nature 630, 905–911 (2024). https://doi.org/10.1038/s41586-024-07520-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07520-y
- Springer Nature Limited