Abstract
Today oxygenic photosynthesis is unique to cyanobacteria and their plastid relatives within eukaryotes. Although its origin before the Great Oxidation Event is still debated1,2,3,4, the accumulation of O2 profoundly modified the redox chemistry of the Earth and the evolution of the biosphere, including complex life. Understanding the diversification of cyanobacteria is thus crucial to grasping the coevolution of our planet and life, but their early fossil record remains ambiguous5. Extant cyanobacteria include the thylakoid-less Gloeobacter-like group and the remainder of cyanobacteria that acquired thylakoid membranes6,7. The timing of this divergence is indirectly estimated at between 2.7 and 2.0 billion years ago (Ga) based on molecular clocks and phylogenies8,9,10,11 and inferred from the earliest undisputed fossil record of Eoentophysalis belcherensis, a 2.018–1.854 Ga pleurocapsalean cyanobacterium preserved in silicified stromatolites12,13. Here we report the oldest direct evidence of thylakoid membranes in a parallel-to-contorted arrangement within the enigmatic cylindrical microfossils Navifusa majensis from the McDermott Formation, Tawallah Group, Australia (1.78–1.73 Ga), and in a parietal arrangement in specimens from the Grassy Bay Formation, Shaler Supergroup, Canada (1.01–0.9 Ga). This discovery extends their fossil record by at least 1.2 Ga and provides a minimum age for the divergence of thylakoid-bearing cyanobacteria at roughly 1.75 Ga. It allows the unambiguous identification of early oxygenic photosynthesizers and a new redox proxy for probing early Earth ecosystems, highlighting the importance of examining the ultrastructure of fossil cells to decipher their palaeobiology and early evolution.
Similar content being viewed by others
Main
Cyanobacteria have played an important part in the evolution of early life and Earth, as one of the major actors in the Great Oxidation Event (GOE) around 2.4 billion years ago (Ga) and possible but disputed earlier low-oxygenation events, based on the geochemical record2,3,4. Phylogeny of enzymes involved in the use or production of O2 suggests an early availability of oxygen at around 3.1 Ga (ref. 14), whereas phylogenetic analyses of PSII genes suggest that an early form of oxygenic photosynthesis was established by 3.0 Ga (refs. 15,16). However, the appearance of oxygenic photosynthesis does not need to coincide with the origin of crown cyanobacteria, which diversified before17,18 or after19 the GOE. Cyanobacteria diverged from non-photosynthetic relatives between 3.37 and 2.54 Ga based on molecular clock estimates, and crown group cyanobacteria diverged from their stem relatives before or after the GOE (3.67–2.02 Ga)1. These early redox fluctuations led to the development of new oxygenated ecological niches in which aerobe life, including eukaryotes, diversified, leading to complex ecosystems. Cyanobacteria are also the ancestors of the chloroplast20, the organelle in which oxygenic photosynthesis takes place within eukaryotes, which enabled the diversification of photosynthetic protists, multicellular algae and, later, plants. Despite their importance, their unambiguous fossil record remains to be evidenced. Although simple filamentous and coccoidal fossils that are abundant in Proterozoic shales and cherts are usually—and probably rightly—attributed to cyanobacteria, their simple morphology and occurrence in the photic zone of aquatic deposits are not unique to these microorganisms5. To date, only four microfossil taxa are interpreted with confidence as cyanobacteria, including 2.018–1.854 Ga (ref. 13) Eoentophysalis belcherensis, the oldest fossil interpreted as a cyanobacterium5,12,21,22; the endolithic Pleurocapsales 1.35–1.01 Ga Polybessurus23 and 1.63 Ga Eohyella24; and a 1.040–1.006 Ga Nostocales Stigonemataceae cyanobacterium Polysphaeroides filiformis (C.F.D. et al., manuscript in preparation). These microfossils are identified based on their particular morphology and division pattern, and their habitat, except for P. filiformis, unambiguously identified as a cyanobacterium thanks to a combination of morphological, chemical and ultrastructural features (C.F.D. et al., manuscript in preparation).
In combination with morphology, ecology and chemistry, one criterion that is scarcely used to identify microfossils—although it may provide crucial palaeobiological information—is the ultrastructure of cell walls and intracellular organelles (C.F.D. et al., manuscript in preparation)25,26. Thylakoids represent direct ultrastructural evidence for oxygenic photosynthesis metabolism. Thylakoid membranes are dense, mostly galactolipid, protein-containing bilayers in which photosynthesis occurs in photosynthetic organisms. They have a tridimensional lamellar organization in cyanobacterial cells or in the chloroplast of algae and plants. When present in cyanobacteria, thylakoids are found in different arrangements in the cell lumen, whereas in eukaryotes they are compacted within chloroplasts in two types of structure, the grana and stroma lamellar domains27. Thylakoid arrangement in cyanobacteria can be parietal, radial, fascicular, irregular or parallel to the cell membrane28,29. Their origin postdates the emergence of crown cyanobacteria. Indeed, the earliest diverging extant cyanobacterial lineage, the genus Gloeobacter, characterized by the absence of thylakoids6,30, is placed at the most basal position in phylogenetic reconstructions17,31, which suggests that this lineage may have conserved traits inherited from the ancestral lineage of cyanobacteria32. The recent discovery of a second thylakoid-less cyanobacterial genus, Anthocerotibacter, was used to estimate divergence with Gloeobacter around 1.4 Ga (ref. 10).
One recent hypothesis suggested that the early emergence of thylakoid-forming cyanobacteria may partly explain the span of the GOE by ‘multiplier effect’33. This proposition holds on the expansion of the photosynthetic surface by the multilayered photosynthetic membranes inside the cells that allow a higher production of O2 in comparison with the smaller surface of the sole cell membrane that hosts the photosynthetic machinery in Gloeobacter-type cyanobacteria32. In contrast with this hypothesis, other authors have suggested the emergence of crown group cyanobacteria (which includes both thylakoid-less and thylakoid-forming cyanobacteria) after the GOE9, illustrating the debates around the early evolution of cyanobacteria.
Fossil thylakoids
In the fossil record, thylakoids were identified in 0.152–0.157 Ga microfossils from Kimmeridgian bituminous shales34. These structures interpreted as thylakoids are linear or contorted, with sharp outlines and a thickness of 35–70 nm (ref. 34). Thylakoidal structures were also reported in potential algal aggregates from the Late Ediacaran (0.55 Ga)35. In younger samples, chloroplasts have been identified in various fossil plants of the Cenozoic36,37. The preservation of thylakoids extracted by acid demineralization was also reported in 600-year-old cyanobacterial mats from Antarctic lacustrine sediments38.
Here we report the presence of thylakoids in the microfossil Navifusa majensis, an enigmatic, ellipsoidal, organic-walled microfossil known in Mesoproterozoic to Neoproterozoic assemblages, although other species of the genus range up to the Carboniferous39. Its taxonomic identity is unresolved and probably polyphyletic, as such a simple shape is known in diverse clades. The specimens studied here are preserved as carbonaceous compressions in shales of the 1.78–1.73 Ga McDermott Formation, Australia, the 1.01–0.90 Ga Grassy Bay Formation, Arctic Canada and the 1.040–1.006 Ga BIIc6 Formation, Democratic Republic of the Congo (DRC). This mode of fossilization as thin carbonaceous compressions is common throughout the geological record for organic-walled microfossils that are flattened within fine-grained, clay-rich, layered sediments5,22,25,26,37. The presence of thylakoids in the Proterozoic fossil record was previously indirectly inferred from the record of microfossil taxa identified unambiguously as late Palaeoproterozoic and younger cyanobacteria or late Mesoproterozoic algae, but never observed. Here we present direct evidence for the preservation of thylakoids within fossil cells and suggest a minimum age for the emergence of thylakoid-bearing cyanobacteria before 1.75 Ga, consistent with the fossil record of unambiguous cyanobacteria.
Ultrastructure of N. majensis
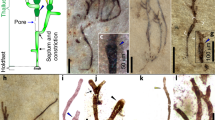
Specimens of N. majensis from (1) laminated grey shales of the 1.78–1.73 Ga, shallow-water, marine-to-estuarine/fluviatile McDermott Formation of the Tawallah Group, McArthur basin, northern Australia40, (2) the 1.01–0.90 Ga, shallow-water estuarine Grassy Bay Formation of the Shaler Supergroup, from the Brock Inlier in the Northwest Territories of Arctic Canada26 and (3) the 1.040–1.006 Ga (ref. 41), shallow-water marine BIIc6 Formation of the Mbuji-Mayi Supergroup, DRC42 were analysed in this study. These consist of organic-walled, unornamented, non-septate, ellipsoidal vesicles with rounded, closed ends43 (Fig. 1). Their size range overlaps, with McDermott specimens (n = 16) that are 57–177 µm in length and 17–40 µm in width; Grassy Bay specimens (n = 22) that are 84–264 µm in length and 23–53 µm in width; BIIc6 Mbuji-Mayi specimens (n = 13) that are 75–183 µm in length and 34–60 µm in width; and specimens from the Bylot Supergroup measured in Hofmann and Jackson44. The ultrastructure of unstained, resin-embedded specimens was observed with transmission electron microscopy (TEM) through transversal ultrathin sections. Because these organic-walled microfossils are preserved as thin carbonaceous compressions in fine-grained sediments, the length of transversal TEM sections corresponds to the width of the microfossils, whereas the thickness corresponds to that of the compressed microfossils (Extended Data Fig. 4). N. majensis specimens from the Tawallah Group show a set of intracellular membranes with sharp, darker edges that appear to be either parallel to the cell wall or locally contorted (Fig. 2a,b). Each membrane comprises one medium-electron-dense layer surrounded by two electron-dense layers (Fig. 2a,b). These membranes have a thickness ranging from 10 to 20 nm (Extended Data Fig. 3 and Extended Data Table 1). The cell wall consists of one electron-lucent layer and one thin electron-dense layer with a total thickness of up to 70 nm (Fig. 2b). The cell wall has an irregular thickness all along the section and is sometimes poorly preserved. The inner membranes are not stored in compartments such as chloroplasts and do not show grana or stroma lamellae-like structures, implying that these fossils are not eukaryotic45. Indeed, in the chloroplast, stacked concentric layers are all surrounded by the plastid wall, a concentric bilayered structure45, which is not the case here (Fig. 2a,b). The intracellular membranes have a thickness consistent with those of modern thylakoids (two membranes separated by a lumen (measured at 12–16 nm in Fig. 4d in ref. 46 and at 14–18 nm in Figs. 1 and 2 in ref. 29)). These are interpreted as compressed, stacked, thylakoidal membranes preserved intracellularly and may represent a parallel and locally contorted arrangement29.
a, N. majensis (n = 16) from the McDermott Formation, Tawallah Supergroup, northern Australia. b, N. majensis (n = 22) from the Grassy Bay Formation, Shaler Supergroup, Arctic Canada. c, N. majensis (n = 13) from the BIIc6 Formation, Mbuji-Mayi Supergroup, DRC. One representative specimen for each formation. n, number of measured specimens. Scale bars, 50 µm.
a,b, Specimen from the McDermott Formation, Australia (n = 2). a, Well-defined contorted layers are interpreted as thylakoids. Each thylakoid comprises one medium-electron-dense layer surrounded by two electron-dense layers. b, Another part of the section, showing the electron-light outer layer (red arrow) overlying the very thin dark layers (yellow arrow), interpreted as the cell wall. c,d, Specimen from the Grassy Bay Formation, Canada (n = 2). End of the specimen (c) and intermediate region of the specimen (d), both showing well-preserved layers separated by white linear spaces (lumen) and interpreted as thylakoids with their concentric and parietal arrangement. The hole in the centre is the intracellular space. Possible partitions are visible between thylakoids, some possibly merged during diagenesis and burial compression (see also Extended Data Fig. 1b). The electron-light outer layer (red arrow in c) overlying the very thin dark layers (yellow arrow in c) is interpreted as the cell wall. n, number of specimens observed by TEM. Scale bars, 200 nm (a,b), 500 nm (c,d).
Ultrathin sections of N. majensis from the Grassy Bay Formation show an ultrastructure that consists of an outer, electron-tenuous layer and a thin, dark layer surrounding a set of four to six uninterrupted, electron-dense concentric layers with sharp edges (Fig. 2c,d). The outer, electron-tenuous layer is sometimes poorly preserved and is underlain by a thin, dark layer, both forming the cell wall for a total thickness of up to 42 nm (Fig. 3a). The inner layers have thickness ranging between 18 and 97 nm (Extended Data Figs. 1 and 2 and Extended Data Table 1), except for the larger, outermost, inner layer with a thickness of approximately 200 nm (Fig. 3a). Larger layers (over 20 nm) probably correspond to merging of several membranes during burial, compression and diagenesis (Extended Data Fig. 1b). Moreover, larger layers have been observed in the modern cyanobacterium Spirulina major (PCC 6313), for which thylakoids may fill almost the whole cell with a 4.75–6.25-fold thicker subperipheral layer consisting of tightly stacked membranes29. All of these intracellular layers are concentric, uninterrupted and show a parietal arrangement (Fig. 2c,d). Such a lamellar architecture is similar to the arrangement of thylakoids in some cyanobacterial strains29 and potentially represents the most primitive architecture of thylakoids33. Here again, the more complex ultrastructure of the chloroplast is not observed for these specimens. The combination of thickness, electron density and parietal arrangement of these inner layers is consistent with the presence of thylakoids preserved intracellularly within these fossil cells.
a,b, Specimens from the Grassy Bay Formation (n = 2). a, The electron-light outer layer (red arrows) overlying the very thin dark layer (yellow arrows), both forming the cell wall; and the partition between stacked thylakoids, seen as lines of small, white holes. b, The complete transversal ultrathin section of a specimen, its length corresponding to microfossil width and its thickness corresponding to that of the flattened microfossil (Extended Data Fig. 4). c,d, Specimens from the BIIc6 Formation (n = 2). c, A homogeneous, medium-dense, inner layer surrounded by the outer wall. d, The outer wall comprises an electron-light outer layer (red arrow) that appears to be fibrous (black arrow) overlying the very thin dark layer (yellow arrow). Scale bars, 200 nm (a,d), 5 µm (b), 500 nm (c).
Raman microspectroscopic geothermometry shows that temperatures undergone by fossils in the diagenetic window were around 181 °C for the Shaler fossils and about 188 °C for the Tawallah fossils, consistent with the respective geological contexts and evidencing their syngenicity (Fig. 4)26,40,47. Despite the darker brown colour of the organic wall of N. majensis from the Shaler Group (Fig. 1c,d) compared with the light brown Tawallah specimens (Fig. 1a,b), their outer wall thicknesses and ultrastructures are similar and thus differences in colour do not result from differences in wall thickness, ultrastructure or temperature. The low burial temperature, together with their taphonomy in clay-rich shales and their recalcitrant lipidic composition, may explain the preservation of intracellular structures such as thylakoids within recalcitrant cell walls. Organic-walled microfossils can be preserved compressed in shales as old as 3.2 Ga and withstand acid demineralization48. The ultrastructure of cell walls can be variably preserved within single Proterozoic specimens (for example, ref. 25) as observed here, and thylakoid membranes also withstand acid demineralization of fossil Holocene mats38 and may show sharp edges compared with ill- to well-defined cell walls following acetolysis of modern and Mesozoic fossil cyanobacteria34. Such exquisite preservation and arrangement of intracellular membranes provide direct evidence for oxygenic photosynthesis, and the absence of chloroplast implies that N. majensis specimens from both formations represent cyanobacteria rather than algae.
a, Representative Raman spectra of N. majensis specimens from the three geological formations, representing the mean spectrum for each map. Palaeothermometry temperatures were calculated using the Raman reflectance method59 for each geological context (McDermott Formation, n = 2,801, mean 187.9 ± 11.3 °C; Grassy Bay Formation, n = 604, mean 180.9 ± 13.1 °C; BIIc6 Formation, n = 3,112, mean 206.8 ± 4.5 °C. n, number of spectra for each map. b, One representative spectrum deconvolution for one specimen from each formation. Intensities are in arbitrary units. G, graphite band; D1–D4, disordered carbon bands.
By contrast, N. majensis specimens from the Mbuji-Mayi Supergroup differ markedly in their ultrastructure (Fig. 3c,d). These specimens show a homogeneous, electron-tenuous inner layer filling the vesicle, the cell lumen is not visible between the compressed walls and there are no distinctive inner layers such as in N. majensis specimens from the two other formations. This inner layer is 43–114 nm thick and is surrounded by one thin, electron-dense layer that, itself, is surrounded by an electron-lucent layer. These two layers are interpreted as the cell wall, which shows variable thickness all around the section, ranging from 3 to 66 nm, and appears locally fibrous or laminated where it is thicker (Fig. 3d). These observations are not consistent with the presence of inner stacked thylakoidal membranes. Raman palaeothermometry shows that the temperature undergone by these fossils was roughly 207 °C, similar to or only slightly higher than that for the other studied localities, suggesting that these DRC specimens either might not have preserved thylakoids or might not have possessed them originally, or they may represent another microbial clade (Fig. 4).
Other interpretations of Navifusa spp.
Navifusa morphospecies gather dozens of microfossils with similar morphology and variable size, with a stratigraphic range from the Palaeoproterozoic to the Carboniferous39,49. Such ellipsoidal or cylindrical, smooth-walled, unornamented form is very simple and widespread in a large number of clades such as bacteria, microalgae and other protists, or fragmented eukaryotic multicellular organisms. This makes the interpretation of Navifusa microfossils ambiguous22 because it could represent distinct lineages among prokaryotes and eukaryotes. The morphogenus Navifusa presents a morphology similar to that of other microfossils called Archaeoellipsoides39 that are preserved silicified tridimensionally in chert50. These silicified microfossils are commonly interpreted as akinetes when associated with short trichomes interpreted as resulting from akinete germination51,52, but see ref. 22. Akinetes are specialized dormant cells of nostocalean cyanobacteria produced under harsh environmental conditions53. One Devonian Navifusa species shows a longitudinal and trochospiral excystment structure and was interpreted as a eukaryotic alga49. Some larger Proterozoic specimens (300–550 μm long and 190–375 μm wide) were interpreted as probable eukaryotes54. Although the morphology and size of Navifusa spp. are coherent with several interpretations, the cellular ultrastructure permits discrimination of these hypothetic taxonomic placements. Indeed, modern cyanobacterial akinetes show an ultrastructure different from that observed in our fossil material55, with a cell wall surrounded by a multilayered extracellular envelope of varying electron density56 and reduced thylakoids55. However, the layers of the envelope may differ between species of cyanobacteria because in some strains the akinete envelope is an extracellular polysaccharidic matrix covering a thin glycolipid layer55,56. Such akinete ultrastructure clearly differs from that exhibited by the fossil specimens of the Shaler Supergroup and Tawallah Group studied here, which do possess well-developed inner layers interpreted as thylakoids with similar electron density and thickness but no multilayered extracellular envelope. Moreover, some modern cyanobacterial species have an ellipsoidal morphology similar to N. majensis with large dimensions, such as Cyanothece major (30–70 × 28–52 µm2) or Cyanothece aeruginosa (10–50 × 10–38 µm2)7.
Implications of preserved thylakoids
Although thylakoid arrangements do not permit pinpointing of a specific clade of cyanobacteria due to convergence within this clade, a parietal arrangement was proposed as the earliest to appear in thylakoid-bearing clades29. The discovery of preserved thylakoids in N. majensis from both the Shaler Supergroup and Tawallah Group provides direct evidence for oxygenic photosynthesis, for a cyanobacterial affinity and for a metabolically active vegetative cell rather than a cyst (akinete) stage for these specimens. As illustrated by the difference in ultrastructure of Navifusa specimens from the three geological successions despite similar morphologies, ultrastructural analyses of enigmatic microfossils, although scarcely applied, is a powerful tool for deciphering their palaeobiology, metabolism and taxonomic identity.
The fossiliferous levels of the (approximately) 1.75 Ga McDermott Formation studied here were deposited in anoxic, shallow-marine-to-evaporitic coastal environments based on sedimentology and trace elements and mineralogical palaeoredox proxies40,47. Our study provides direct evidence for the presence of metabolically active cyanobacteria performing oxygenic photosynthesis. It implies that the well-preserved microfossil record might capture low-concentration or local or short-term oxygenation events that are difficult to detect by geochemical proxies. Indeed, the detection limit of the latter, or the lack of temporal and spatial resolution of the averaging sedimentary record or balance between the biological source and sink of oxygen, might impede the recognition of oxygen traces in the rock record. Our approach thus offers a new, highly sensitive and complementary redox proxy for probing micro-oxic oases on the early Earth, where eukaryogenesis may have taken place close to oxygen-producing cyanobacteria and where early eukaryotes diversified57,58. Our study findings also imply that ultrastructural analyses of early fossil protists might show the presence of thylakoids enclosed in chloroplast and help to constrain the timing of plast endosymbiosis and the early evolution of eukaryotic algae.
The discovery of preserved thylakoids within N. majensis reported here provides direct evidence for a minimum age of about 1.75 Ga for the divergence between thylakoid-bearing and thylakoid-less cyanobacteria. By probing the older fossil record, it may also allow testing of the hypothesis that the emergence of thylakoid membranes may have contributed to the rise in oxygen around the GOE, and to the permanent oxygenation of the early Earth. We predict that similar ultrastructural analyses of well-preserved microfossils might expand the geological record of oxygenic photosynthesizers, and of early, weakly oxygenated ecosystems in which complex cells developed.
Methods
Sample preparation and extraction of microfossils
Fossil specimens were obtained from (1) the Kanshi SB13 drill core, BIIC Formation, DRC (detailed log and geology in ref. 42; datings in ref. 41); (2) outcrop sample 15RAT-021A1, Grassy Bay Formation, Shaler Supergroup, Canada (detailed log and geology in ref. 26); and (3) the GSD7 core, McDermott Formation, Tawallah Group, Northern Territories, Australia (detailed log and geology in ref. 40). Microfossils of N. majensis were extracted from their shale matrix following a procedure modified from Grey60. This modified procedure avoids centrifugation that could damage microfossils. Rock samples were cleaned and crushed before undergoing acid treatment. First, crushed samples were bathed in HCl 35% to remove carbonates then in a HF 60% bath to remove silicates and finally in hot HCl to remove neoformed fluorides. Organic residues were filtered and stored in Milli-Q water. These macerates were then used to prepare samples for electron microscopy and Raman microspectroscopy. All figures in the manuscript were created using Inkscape software 1.1.2.
TEM
Six isolated microfossils (two each from the Shaler and Mbuji-Mayi Supergroups and the Tawallah Group) were pipetted under a Nikon Eclipse Ts2 inverted microscope and then embedded in 1% agarose. Agarose cubes containing microfossils were dehydrated in a graded ethanol series of 70, 90 and 100%. Following dehydratation, microfossils were progressively included within Spi-Pon 812 resin (ARALDITE/EMbed EMBEDDING KIT, Mollenhauer Formula, catalogue no. 13940). Resin inclusion started with two successive baths of propylene oxide, followed by a bath in a mix of propylene oxide and Spi-Pon 812 resin (1:1) and then a final bath in pure resin. Finally, microfossils in pure resin were heated over 2 days at 60 °C. No difference was observed between stained and unstained specimens. The resulting blocks were cut into transversal, ultrathin sections with an ultramicrotome Leica EM UC7 and these were then deposited on formvar-covered copper grids for observation. Samples were observed using a TEM Tecnai Spirit T12 with a voltage of 120 kV (M4I Division of Nanoscopy, University of Maastricht, The Netherlands).
Raman microspectroscopy
Three microfossils (one specimen from each geological formation) were pipetted under a Nikon Eclipse Ts2 inverted microscope, deposited on ZnSe plates and air-dried for 24 h. They were then analysed using a Renishaw Invia Raman microspectrometer with an Air-ion-40 mW monochromatic 514 nm laser source (Early Life Traces & Evolution–Astrobiology Laboratory, University of Liège, Belgium). The laser was focused using an objective of ×100 to obtain a spot size of 1–2 µm. Spectra were acquired in static mode, enabling a range from 1 to 2,000 cm−1 with a spectral resolution of 4 cm−1 and centred at 1,150 cm−1. Acquisitions were acquired at a laser power of 0.1%, an integration time of 1 s and using a 1,800 l mm−2 grating that illuminates a charge-coupled device array detector of 1,040 × 256 pixels. Map spectra were finally processed with Renishaw Wire 4.2 software and RStudio 4.1.1 software. The thermal maturity of the kerogenous walls of specimens was estimated using the Raman reflectance method previously described59. Basic statistics (means and s.d.) were calculated using Microsoft Excel 2016.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All raw data are deposited in ULiege institutional open archive ORBi and can be accessed at https://hdl.handle.net/2268/308458. The folder ‘morphometry’ contains a table with measurements of microfossil lengths and widths; the folder ‘Raman_RawData’ contains raw maps; the folder ‘Raman_TreatedData_Temperatures’ contains tables with the treated data used to obtain temperatures (palaeothermometry - Raman reflectance T°C Rmc Ro); the folder ‘Raw_TEM_images’ contains raw TEM images of microfossil ultrastructure with scales. All tables are in .txt format.
References
Sánchez-Baracaldo, P., Bianchini, G., Wilson, J. D. & Knoll, A. H. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 30, 143–157 (2022).
Ostrander, C. M., Johnson, A. C. & Anbar, A. D. Earth’s first redox revolution. Annu. Rev. Earth Planet. Sci. 49, 337–366 (2021).
Wilmeth, D. T. et al. Evidence for benthic oxygen production in Neoarchean lacustrine stromatolites. Geology 50, 907–911 (2022).
Slotznick, S. P. et al. Reexamination of 2.5-Ga “Whiff” of oxygen interval points to anoxic ocean before GOE. Sci. Adv. 8, eabj7190 (2022).
Demoulin, C. F. et al. Cyanobacteria evolution: insight from the fossil record. Free Rad. Biol. Med. 140, 206–223 (2019).
Rippka, R., Waterbury, J. & Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 100, 419–436 (1974).
Komarek, J. & Anagnostidis, K. in Freshwater Flora of Central Europe Vol. 19, (ed. Moltmann, U. G.) 34–36 (Spektrum Akademischer, 2008).
Cavalier-Smith, T. The neomuran origin of archaebacterial, the negibacterial root of the universal tree and bacterial megaclassification. Int. J. Syst. Evol. Microbiol. 52, 7–76 (2002).
Shih, P. M., Hemp, J., Ward, L. M., Matzke, N. J. & Fischer, W. W. Crown group Oxyphotobacteria postdate the rise of oxygen. Geobiology 15, 19–29 (2017).
Rahmatpour, N. et al. A novel thylakoid-less isolate fills a billion-year gap in the evolution of cyanobacteria. Curr. Biol. 31, 2857–2867 (2021).
Fournier, G. P. et al. The Archean origin of oxygenic photosynthesis and extant cyanobacterial lineages. Proc. R. Soc. Lond. B Biol. Sci. 288, 20210675 (2021).
Hofmann, H. J. Precambrian microflora, Belcher Islands, Canada: significance and systematics. J. Paleontol. 50, 1040–1073 (1976).
Hodgskiss, M. S. et al. New insights on the Orosirian carbon cycle, early Cyanobacteria, and the assembly of Laurentia from the Paleoproterozoic Belcher Group. Earth Planet. Sci. Lett. 520, 141–152 (2019).
Jabłońska, J. & Tawfik, D. S. The evolution of oxygen-utilizing enzymes suggests early biosphere oxygenation. Nat. Ecol. Evol. 5, 442–448 (2021).
Cardona, T., Sánchez-Baracaldo, P., Rutherford, A. W. & Larkum, A. W. D. Early Archean origin of Photosystem II. Geobiology 17, 127–150 (2019).
Sánchez-Baracaldo, P. & Cardona, T. On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 225, 1440–1446 (2020).
Blank, C. E. & Sánchez-Baracaldo, P. Timing of morphological and ecological innovations in the cyanobacteria a key to understand the rise in atmospheric oxygen. Geobiology 8, 1–23 (2010).
Schirrmeister, B. E., Gugger, M. & Donoghue, P. C. Cyanobacteria and the Great Oxidation Event: evidence from genes and fossils. Palaeontology 58, 769–785 (2015).
Shih, P. M. et al. Biochemical characterization of predicted Precambrian RuBisCO. Nat. Commun. 7, 10382 (2016).
Schwartz, R. M. & Dayhoff, M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science 199, 395–403 (1978).
Golubic, S. & Hofmann, H. J. Comparison of Holocene and mid-Precambrian Entophysalidaceae (Cyanophyta) in stromatolitic algal mats: cell division and degradation. J. Paleontol. 50, 1074–1082 (1976).
Butterfield, N. J. Proterozoic photosynthesis – a critical review. Palaeontology 58, 953–972 (2015).
Sergeev, V. N. Microfossils in cherts from the middle riphean (mesoproterozoic) Avzyan Formation, southern ural Mountains, Russian federation. Precambrian Res. 65, 231–254 (1994).
Zhang, Y. Proterozoic stromatolitic micro-organisms from Hebei, North China: cell preservation and cell division. Precambrian Res. 38, 165–175 (1988).
Javaux, E. J., Knoll, A. H. & Walter, M. R. TEM evidence for eukaryotic diversity in mid-Proterozoic oceans. Geobiology 2, 121–132 (2004).
Loron, C. C., Rainbird, R. H., Turner, E. C., Greenman, J. W. & Javaux, E. J. Organic-walled microfossils from the late Mesoproterozoic to early Neoproterozoic lower Shaler Supergroup (Arctic Canada): diversity and biostratigraphic significance. Precambrian Res. 321, 349–374 (2019).
Shimoni, E., Rav-Hon, O., Ohad, I., Brumfeld, V. & Reich, Z. Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17, 2580–2586 (2005).
Gonzalez-Esquer, C. R. et al. Cyanobacterial ultrastructure in light of genomic sequence data. Photosynth. Res. 129, 147–157 (2016).
Mareš, J., Strunecký, O., Bučinská, L. & Wiedermannova, J. Evolutionary patterns of thylakoid architecture in cyanobacteria. Front. Microbiol. 10, 277 (2019).
Mareš, J. et al. The primitive thylakoid-less cyanobacterium Gloeobacter is a common rock-dwelling organism. PLoS ONE 8, e66323 (2013).
Nelissen, B., Van de Peer, Y., Wilmotte, A. & De Wachter, R. An early origin of platids within the cyanobacterial divergence is suggested by evolutionary trees based on complete 16S rRNA sequences. Mol. Biol. Evol. 12, 1166–1173 (1995).
Raven, J. A. & Sànchez-Baracaldo, P. Gloeobacter and the implications of a freshwater origin of cyanobacteria. Phycologia 60, 402–418 (2021).
Guéguen, N. & Maréchal, E. Origin of cyanobacterial thylakoids via a non-vesicvular glycolipid phase transition and their impact on the Great Oxygenation Event. J. Exp. Bot. 73, 2721–2734 (2022).
Pacton, M., Gorin, G. E. & Fiet, N. Unravelling the origin of ultralaminae in sedimentary organic matter: the contribution of bacteria and photosynthetic organisms. J. Sediment. Res. 78, 654–667 (2008).
Kremer, B., Kaźmierczak, J. & Środoń, J. Cyanobacterial-algal crusts from Late Ediacaran paleosols of the East European Craton. Precambrian Res. 305, 236–246 (2018).
Schoenhut, K., Vann, D. R. & LePage, B. A. Cytological and ultrastructural preservation in Eocene Metasequoia leaves from the Canadian High Arctic. Am. J. Bot. 91, 816–824 (2004).
Wang, X., Liu, W., Du, K., He, X. & Jin, J. Ultrastructural of chloroplasts in fossil Nelumbo from the Eocene of Hainan Island, South China. Plant Syst. Evol. 300, 2259–2264 (2014).
Lepot, K. et al. Organic and mineral imprints in fossil photosynthetic mats of an East-Antarctic lake. Geobiol. 12, 424–450 (2014).
Miao, L., Moczydłowska, M., Zhu, S. & Zhu, M. New record of organic-walled, morphologically distinct microfossils from the late Paleoproterozoic ChangCheng Group in the Yanshan Range, North China. Precambrian Res. 321, 172–198 (2019).
Spinks, S. C., Schmid, S. & Pagès, A. Delayed euxinia in Paleoproterozoic intracontinental seas: vital havens for the evolution of eukaryotes. Precambrian Res. 287, 108–114 (2016).
François, C. et al. Multi-method dating constrains the diversification of early 2 eukaryotes in the Proterozoic Mbuji-Mayi Supergroup of the D.R.Congo and the geological evolution of the Congo Basin. J. Afr. Earth Sci. 198, 104785 (2023).
Baludikay, B. K., Storme, J. Y., François, C., Baudet, D. & Javaux, E. J. A diverse and exquisitely preserved organic-walled microfossil assemblage from the Meso–Neoproterozoic Mbuji-Mayi Supergroup (Democratic Republic of Congo) and implications for Proterozoic biostratigraphy. Precambrian Res. 281, 166–18 (2016).
Pyatiletov, V. G. Yudoma complex microfossils from southern Yakutia. Geol. Geofiz. 7, 8–20 (1980).
Hofmann, H. J. & Jackson, G. D. Shale-facies microfossils from the Proterozoic Bylot Supergroup, Baffin Island, Canada. J. Paleontol. 68, 1–35 (1994).
Kirchhoff, H. Chloroplast ultrastructure in plants. New Phytol. 223, 565–574 (2019).
Meng, L. et al. Measuring the dynamic response of the thylakoid architecture in plant leaves by electron microscopy. Plant Direct. 4, e00280 (2020).
Spinks, S. C., Schmid, S., Pagés, A. & Bluett, J. Evidence for SEDEX-style mineralization in the 1.7 Ga Tawallah Group, McArthur basin, Australia. Ore Geol. Rev. 76, 122–139 (2018).
Javaux, E. J., Marshall, C. P. & Bekker, A. Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463, 934–938 (2010).
Fatka, O. & Brocke, R. Morphological variability and method of opening of the Devonian acritarch Navifusa bacilla. Rev. Palaeobot. Palynol. 148, 108–123 (2008).
Horodyski, R. J. & Donaldson, J. A. Microfossils from the middle Proterozoic Dismal Lakes Groups, Arctic Canada. Precambrian Res. 11, 125–159 (1980).
Golubic, S., Sergeev, V. N. & Knoll, A. H. Mesoproterozoic Archaeoellipsoides: akinetes of heterocystous cyanobacteria. Lethaia 28, 285–298 (1995).
Tomitani, A., Knoll, A. H., Cavanaugh, C. M. & Ohno, T. The evolutionary diversification of cyanobacteria: molecular–phylogenetic and paleontological perspectives. Proc. Natl Acad. Sci. USA 103, 5442–5447 (2006).
Kaplan-Levy, R. N., Hadas, O., Summers, M. L., Rücker, J. & Sukenik, A. in Dormancy and Resistance in Harsh Environments (eds Lubzens, E. et al.) 5–27 (Springer, 2010).
Sergeev, V. N., Knoll, A. H., Vorob’eva, N. G. & Sergeeva, N. D. Microfossils from the lower Mesoproterozoic Kaltasy Formation, East European Platform. Precambrian Res. 278, 87–107 (2015).
Sukenik, A., Rücker, J. & Maldener, I. in Cyanobacteria from Basic Science to Applications (eds Mishra, A. K. et al.) 65–77 (Academic, 2019).
Perez, R., Forchhammer, K., Salerno, G. & Maldener, I. Clear differences in metabolic and porphological adaptations of akinetes of two Nostocales living in different habitats. Microbiology 162, 214–223 (2016).
López-García, P. & Moreira, D. The Syntrophy hypothesis for the origin of eukaryotes revisited. Nat. Microbiol. 5, 655–667 (2020).
Javaux, E. J. in Encyclopedia of Astrobiology (eds Gargaud, M. et al.), Ch. 538–4, 1–5 (Springer, 2021).
Baludikay, B. K. et al. Raman microspectroscopy, bitumen reflectance and illite crystallinity scale: comparison of different geothermometry methods on fossiliferous Proterozoic sedimentary basins (DR Congo, Mauritania and Australia). Int. J. Coal Geol. 191, 80–94 (2018).
Grey, K. A modified palynological preparation technique for the extraction of large Neoproterozoic acanthomorph acritarchs and other acid-insoluble microfossils. Western Australia Geological Survey, Record 1999/10 (1999).
Acknowledgements
We thank the Royal Museum for Central Africa (Tervuren, Belgium) and D. Baudet for access to the Kanshi SB13 drill core; S. Spinks and M. Kunzmann (CSIRO Mineral Resources, Australia) for samples from the GSD7 drill core at the Darwin core facility (Australia); and the Geological Survey of Canada’s Geomapping for Energy and Minerals programme, G. Halverson (McGill University, Canada), R. Rainbird (GSC, Canada), E. Turner (Laurentian University, Canada), T. Gibson (McGill University, Canada) and C. Loron (ULiege, Belgium and University of Edinburgh, UK) for sampling the Shaler Supergroup in the Northwest Territories of Arctic Canada. We thank M. Giraldo at the Early Life Traces & Evolution–Astrobiology laboratory and C. López-Iglesias and H. Duimel at the Microscopy CORE Lab (University of Maastricht) for technical support. FRS-FNRS-FWO EOS ET-Home (grant no. 30442502), ERC Stg ELiTE FP7/308074, an Agouron Institute geobiology grant and BELSPO BRAIN project B2/212/PI/PORTAL supported this project.
Author information
Authors and Affiliations
Contributions
C.F.D., Y.J.L. and E.J.J. conceived the study and interpreted the data. A.L. performed acid demineralization and prepared microfossil slides. C.F.D. and E.J.J. performed TEM sample preparation and observations. C.F.D. and E.J.J. prepared samples for Raman spectroscopy. C.F.D., Y.J.L. and E.J.J. performed Raman analyses. C.F.D., Y.J.L. and E.J.J. wrote the paper. E.J.J. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Helmut Kirchhoff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 TEM pictures of a specimen of Navifusa majensis, from the Grassy Bay Formation (Shaler Supergroup, Canada).
Pictures a, c and d show the width and limits of each layer interpreted as stacked thylakoidal membranes that were measured. Measurements are compiled in Extended Data Table 1 below. Picture b is a zoom of the section through the microfossil rounded end in a (black box). The parietal arrangement is clearly visible (dotted black lines), as well as the variable thicknesses of stacked thylakoidal membranes, due to merging of several thylakoids during burial, compression and diagenesis, dotted yellow lines show possible limits of several layers in the ticker one. These TEM pictures are from the same specimen illustrated in Fig. 2c,d; 3a,b.
Extended Data Fig. 2 TEM pictures of the second specimen of Navifusa majensis from the Grassy Bay Formation (Shaler Supergroup, Canada).
Pictures a and b show some positions where thickness of layers were measured and clearly illustrate the limits of each layer interpreted as stacked thylakoidal membranes. Measures are summarized in the Extended Data Table 1 below. Picture c shows knife marks (dotted lines) creating artefacts on ultrathin sections. These knife marks are clearly distinguishable from limits of stacked thylakoidal layers. Picture c also shows that on a same ultrathin section, the limits between layers may be less clear, due to merging during burial and compression. n = 2 N. majensis for Grassy Bay Formation. “n” represents the number of specimens observed by TEM.
Extended Data Fig. 3 TEM picture of a specimen of Navifusa majensis from the McDermott Formation (Tawallah Group, Australia).
This picture shows the positions where the thylakoidal membranes are measured. Measurements are compiled in table S4 below. n = 2 N. majensis for McDermott Formation. “n” represents the number of specimens observed by TEM.
Extended Data Fig. 4 Schematic drawings showing how compressed microfossils were cut transversally for TEM observations.
a represents a whole flattened specimen of Navifusa majensis, with transversal section shown (black line). b represents a transversal TEM ultrathin section through the microfossil. The length of the ultrathin section in b corresponds to the width of the microfossil (red line in a and b), while the thickness of the ultrathin section corresponds to the thickness of the compressed microfossil (T in a and b). L: length of the whole microfossil; W: width; T: thickness.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demoulin, C.F., Lara, Y.J., Lambion, A. et al. Oldest thylakoids in fossil cells directly evidence oxygenic photosynthesis. Nature 625, 529–534 (2024). https://doi.org/10.1038/s41586-023-06896-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06896-7
- Springer Nature Limited