Abstract
All-carbon materials based on sp2-hybridized atoms, such as fullerenes1, carbon nanotubes2 and graphene3, have been much explored due to their remarkable physicochemical properties and potential for applications. Another unusual all-carbon allotrope family are the cyclo[n]carbons (Cn) consisting of two-coordinated sp-hybridized atoms. They have been studied in the gas phase since the twentieth century4,5,6, but their high reactivity has meant that condensed-phase synthesis and real-space characterization have been challenging, leaving their exact molecular structure open to debate7,8,9,10,11. Only in 2019 was an isolated C18 generated on a surface and its polyynic structure revealed by bond-resolved atomic force microscopy12,13, followed by a recent report14 on C16. The C18 work trigged theoretical studies clarifying the structure of cyclo[n]carbons up to C100 (refs. 15,16,17,18,19,20), although the synthesis and characterization of smaller Cn allotropes remains difficult. Here we modify the earlier on-surface synthesis approach to produce cyclo[10]carbon (C10) and cyclo[14]carbon (C14) via tip-induced dehalogenation and retro-Bergman ring opening of fully chlorinated naphthalene (C10Cl8) and anthracene (C14Cl10) molecules, respectively. We use atomic force microscopy imaging and theoretical calculations to show that, in contrast to C18 and C16, C10 and C14 have a cumulenic and cumulene-like structure, respectively. Our results demonstrate an alternative strategy to generate cyclocarbons on the surface, providing an avenue for characterizing annular carbon allotropes for structure and stability.
Similar content being viewed by others
Main
Early theory21,22,23,24 predicted C10 to be the watershed between cyclic (for n ≥ 10) and linear (for n < 10) Cn and the largest cumulenic aromatic cyclocarbon, whereas C14 is understood to be the point at which the cumulenic structure seen with C10 transitions, via Peierls distortion, to the polyynic structure seen with C18 (refs. 10,16,17). Investigating the structure of C10 and C14 is thus of particular interest.
Advanced scanning tunnelling microscopy (STM) and atomic force microscopy (AFM), especially when carried out with a CO-terminated tip, enable both bond-resolved characterization of molecular structures25,26 and triggering of chemical reactions by manipulating individual atoms27,28. Importantly, in high-resolution AFM images of single molecules, polyynic moieties can be unambiguously distinguished from cumulenic moieties (that is, characteristic bright features of triple bonds and uniform line features of consecutive double bonds, respectively)28,29.
The essential first step is the precise synthesis of C10 and C14 in the condensed phase, for which we follow earlier on-surface synthesis approaches in which molecules are stabilized on the surface, which is kept at extremely low temperatures. Inspired by the Bergman reaction30 and previous methods generating carbon cluster ions31,32, we used the reaction schemes shown in Fig. 1. As illustrated in Fig. 1a, Bergman and retro-Bergman reactions in solution involve a cyclization and a ring-opening reaction; the retro-Bergman ring-opening reaction has also been shown to be feasible on a surface (Fig. 1b)33,34. We used a fully halogenated naphthalene (octachloronaphthalene, C10Cl8)31 and anthracene (decachloroanthracene, C14Cl10) as molecular precursors, with the aim of generating C10 and C14 on the surface through tip-induced dehalogenation and retro-Bergman reactions (Fig. 1c,d).
a, Bergman cyclization and its reverse process (retro-Bergman) involving a cyclization and a ring-opening reaction. b, The on-surface retro-Bergman ring-opening reaction. c,d, Reaction schemes for the formation of cyclo[10]carbon (c) and cyclo[14]carbon (d) by dehalogenation and retro-Bergman reactions. The double bonds indicated by blue and black in d represent two different bond lengths within cyclo[14]carbon.
The cyclo[10]carbon product can, in principle, adopt one of the four possible structures shown in Fig. 2. The two polyynic structures with D5h and C5h symmetries exhibit non-zero bond length alternations (BLA ≠ 0) (Fig. 2a,b), whereas the two cumulenic structures with D10h and D5h symmetries have zero bond length alternations (BLA = 0) (Fig. 2c,d). In contrast to the polyynic structure of C18 (refs. 10,16,17), calculations at different levels of theory (for example, density functional theory (DFT)11,16,17 and coupled cluster methods10,24) have predicted a ground state for C10 with the D5h cumulene geometry shown in Fig. 2d, and with BLA = 0 and bond angle alternation (BAA) ≠ 0 (see also detailed calculations in Extended Data Figs. 1 and 2).
For the experiments, all molecules were placed on a bilayer NaCl/Au(111) surface at 4.7 K. C10Cl8 was first introduced on the cold sample held at approximately 6 K and then imaged with STM (Extended Data Fig. 3a,b), revealing that single C10Cl8 molecules display an oval shape without internal features. In AFM images (Fig. 3a, (ii) and (iii)), the Cl atoms in C10Cl8 exhibit different contrasts in brightness. The brightness differences imply differences in adsorption heights caused by steric hindrance of Cl atoms in the highly strained molecule34, confirmed by AFM simulations (Fig. 3a, (iv) and (v)). In the Laplace-filtered AFM image (Fig. 3a, (vi)), more prominent features associated with the positions of Cl atoms in C10Cl8 are seen.
a, Precursor images. b–d, The most frequently observed reaction intermediates: C10Cl6 (b); C10Cl5 (c); C10Cl2 (d). e, Cyclo[10]carbon. Images shown are: (i) molecular structures; (ii) and (iii) AFM images; (iv) and (v) AFM simulations (sim.); (vi) Laplace-filtered (Lap.) AFM images. The double bonds indicated by blue and black in d (i) represent two different bond lengths within C10Cl2. AFM images were recorded with a CO-terminated tip at different tip offsets, Δz, with respect to STM set points (a (ii)–(iii), b (ii)–(iii): I = 1 pA, V = 0.3 V; c (ii)–(iii), d (ii)–(iii): I = 2 pA, V = 0.3 V; e (ii)–(iii): I = 0.5 pA, V = 0.3 V) above the NaCl surface. AFM simulations are based on gas-phase DFT-calculated geometries. The scale bar in a (ii) applies to all experimental, simulated and Laplace-filtered AFM images.
To remove Cl atoms from the molecule, the tip was initially positioned on a single molecule, and retracted by about 4 Å from the STM set point (typically I = 1 pA, V = 0.3 V) and the sample bias then gradually increased from 0.3 V to 4 V. This process typically resulted in the loss of two or three Cl atoms, yielding C10Cl6 or C10Cl5 intermediates that were structurally characterized by AFM imaging and the structures were confirmed by AFM simulations (Fig. 3b,c, (ii)–(vi), and Extended Data Fig. 4a–c). Note that the retro-Bergman ring-opening reaction has not occurred yet in the C10Cl5 molecule, which has two of its α-Cl atoms removed from the left carbon ring. Further voltage sweeping resulted in successive further dehalogenation of the C10Cl6 and C10Cl5 intermediates and usually yielded C10Cl1 or C10Cl2 intermediates consisting of a single carbon ring (Fig. 3d, (ii)–(vi), and Extended Data Fig. 4d–f), and thus suggesting occurrence of the retro-Bergman ring-opening reaction. The tip-induced dehalogenation could be related to anionic charge states of molecules or an applied electric field34,35. In addition, inelastic electron tunnelling may also help to trigger dehalogenative reactions12.
Further voltage sweeping induced complete dehalogenation of the intermediates and generated the final product, as shown in the STM image (Extended Data Fig. 3c) and AFM images (Fig. 3e, (ii)–(iii)). The images clearly show that the final product contains a carbon ring, which could be unambiguously assigned to a single C10. In addition, the C10 molecule represents a uniform feature in AFM images resembling the cumulenic structures reported before29 and differing from the characteristic bright features seen with polyynic C18 and C16 (refs. 12,13,14). This naturally indicates a cumulenic structure for C10, in excellent agreement with theoretical calculations (BLA = 0) (Extended Data Fig. 1)10,11,16,17,24. Further analysis of the AFM images (Fig. 3e (ii),(iii),(vi)) reveals that the C10 structure deviates from the structure of a perfect circle. Optimizing the C10 structure placed on different NaCl surface sites (Extended Data Fig. 5a–d) does not noticeably change the five-fold symmetry, indicating that this pentagon-like shape appearing in the AFM images (even if only faintly resolved) is probably related to the theoretically predicted intrinsic D5h symmetry of C10 (shown in Fig. 2d). Note that AFM images acquired at a smaller oscillation amplitude (50 pm) exhibited similar features both in ‘AFM far’ and ‘AFM close’ images (Extended Data Fig. 6). Also note that C10 shows remarkable stability under our manipulation conditions (V ≤ 4 V), retaining its cyclic structure.
Calculations based on modern theory10,16,17 for cyclo[14]carbon indicate that the ground state is a Peierls-transition intermediate with a structure between that of the cumulenic C10 and the polyynic C18. Figure 4a shows the six possible C14 structures considered, with our calculations indicating a BAA of 25.3° (Extended Data Fig. 7) for the ground state, and a small BLA of 0.05 Å, which lies between the values for C10 (BLA = 0; Extended Data Fig. 1) and C18 (BLA = 0.12 Å; Extended Data Fig. 8)17. Such a Peierls-transition structure corresponds to a C7h intermediate (Fig. 4a, (iv)), with the optimized structures for C14 adsorbed at Cl-top and Na-top sites on the NaCl surface shown in Extended Data Fig. 5e–h.
a, Six possible structures of cyclo[14]carbon. The cumulenic forms with D14h (i) and D7h (ii) symmetries, the intermediate structures with D7h (iii) and C7h (iv) symmetries, and the polyynic forms with D7h (v) and C7h (vi) symmetries are shown. The bond length alternation is defined as BLA = d1 − d2 and the bond angle alternation is defined as BAA = θ1 − θ2. b–d, AFM images (b (i)–(iii)), AFM simulations (c (i)–(iii)) and Laplace-filtered AFM images (d (i)–(iii)) of cyclo[14]carbon at different tip heights. e, AFM simulations of C14 with varying BLAs. f–h, Intermediates C14Cl6 (f (i)–(iv)), C14Cl4 (g (i)–(iv)) and C14Cl1 (h (i)–(iv)). The double bonds indicated by blue and black in a (iii),(iv), h (i) and e represent two different bond lengths within the structures, respectively. AFM images were recorded with a CO-terminated tip at different tip offsets, Δz, with respect to STM set point (b (i)–(iii), f (ii), g (ii) and h (ii): I = 1 pA, V = 0.3 V) above the NaCl surface. AFM simulations are based on gas-phase DFT-calculated geometries. The scale bar in b (i) applies to all experimental, simulated AFM and Laplace-filtered images of cyclo[14]carbon. The scale bar in f (ii) applies to all experimental, simulated AFM and Laplace-filtered images of intermediates. The scale bar in e applies to all simulated AFM images with varying BLAs.
Analogous to the on-surface synthesis strategy used with C10, we used the molecular precursor C14Cl10 for generating C14 (Fig. 1d). C14Cl10 precursor molecules were introduced onto a bilayer NaCl surface held at approximately 6 K. Due to steric hindrance experienced by the Cl atoms in the highly strained molecule, C14Cl10 exhibits a non-planar configuration in both STM and AFM images (Extended Data Fig. 9a–c). As with C10, atom manipulation induces complete dehalogenation of the precursor and is accompanied by two-step retro-Bergman ring opening to give the C14 product on the surface with a yield of approximately 24% (see STM and AFM images and simulations in Extended Data Fig. 9e and Fig. 4b–d). AFM imaging at different heights (Fig. 4b, (i)–(iii)) shows that the final product has a carbon ring that is unambiguously assigned to a single C14. In all AFM-derived images, C14 exhibits a cumulenic feature resembling that of C10, despite a BLA of 0.05 Å calculated for the former. We therefore assign a cumulene-like structure for C14, which is also obviously different from the polyynic C18 and C16 with characteristic bright features. We note that a BLA of only 0.05 Å cannot be distinguished experimentally by AFM imaging, so more detailed structural characterization of C14 was performed by AFM image simulations, as shown in Fig. 4e (more details are shown in Extended Data Fig. 10). We simulated AFM images of C14 with varying BLAs, ranging from cumulenic to intermediate and polyynic structures (that is, BLA = 0, 0.03 Å, 0.05 Å, 0.07 Å, 0.09 Å, 0.11 Å, 0.13 Å, 0.15 Å), to explore the limits of AFM imaging for differentiating between structures with different BLAs. The simulations suggest that, in the case of 0 < BLA < 0.09 Å, which covers the Peierls-transition region, structures can be assigned as cumulene-like, although they are difficult to differentiate. When BLA ≥ 0.09 Å, structures can be identified as polyynic, and larger BLAs give rise to more pronounced bright features over the triple bonds.
Cyclo[14]carbon formation and the accompanying skeletal rearrangements were further probed by investigating the intermediates involved, using AFM imaging and simulations (Fig. 4f–h). Dehalogenation of the intermediate C14Cl6 (Fig. 4f) results in the formation of C14Cl4, which consists of a ten-membered ring with characteristic bright features above the triple bonds resolved (Fig. 4g); this suggests that the first-step retro-Bergman reaction has occurred. Further dehalogenation leads to the formation of C14Cl1, imaged as a larger carbon ring that lacks characteristic bright features and with one Cl atom attached; this suggests that the second-step retro-Bergman reaction has occurred (Fig. 4h). Compared to the ten-membered ring of the preceding C14Cl4 intermediate, the experimental observations and calculations both indicate that the BLA decreases for most C–C bonds within the C14Cl1 ring (Extended Data Figs. 11 and 12 (for other intermediates and side reaction products)).
To conclude, our successful generation of the aromatic cyclo[10]carbon and cyclo[14]carbon by atom manipulation on the bilayer NaCl/Au(111) surface at 4.7 K has enabled us to confirm a cumulenic structure for C10 by bond-resolved AFM imaging, as predicted by theory. More interestingly, the experimental AFM images of the Peierls-transition intermediate C14 also show cumulenic characteristics. Although the AFM imaging resolution is not sufficient to detect the small bond length alternation of 0.05 Å calculated for this molecule, it does identify C14 as an intermediate structure between the cumulenic C10 and the polyynic C18. We anticipate that the complement of our and previous on-surface synthesis strategies will enable the generation of other cyclo[n]carbons that might exhibit interesting properties.
Methods
Experimental details for STM and AFM measurements
STM and AFM measurements were carried out in a commercial (Createc) low-temperature system operated at 4.7 K with base pressure better than 1 × 10−10 mbar. Single crystalline Au(111) surface was cleaned by several sputtering and annealing cycles. The NaCl films were obtained by thermally evaporating NaCl crystals onto a clean Au(111) surface at room temperature, resulting in islands of two and three monolayer thickness. Octachloronaphthalene (C10Cl8, purchased from Aladdin, greater than 99%) and decachloroanthracene (C14Cl10, synthesized using procedures in ref. 36) molecules were deposited on a cold NaCl/Au(111) surface by thermal sublimation from a molecular evaporator. CO molecules for tip modification25 were dosed onto the cold sample via a leak valve. We used a qPlus sensor37 with a resonance frequency f0 = 29.49 kHz, quality factor Q ≈ 45,000 and a spring constant k ≈ 1,800 N m−1 operated in frequency-modulation mode38. The bias voltage V was applied to the sample with respect to the tip. AFM images were acquired in constant-height mode at V = 0 V and an oscillation amplitude of A = 1 Å (unless otherwise noted). The tip-height offsets, Δz, for constant-height AFM images are defined as the offset in tip-sample distance relative to the STM set point at the NaCl surface. The positive (negative) values of Δz correspond to the tip-sample distance increased (decreased) with respect to a STM set point.
DFT calculations and AFM simulations
DFT calculations were carried out in the gas phase using the Gaussian 16 program package39. The ωB97XD exchange-correlation functional40 in conjunction with 6-311++G(d,p)41 basis sets was used for all C10 and C14 related calculations in the gas phase17. The electrostatic potentials, localized orbital locator (LOL) and interaction region indicator (IRI) of molecules were calculated at the ωB97XD/6-311++G(d,p) level combined with Multiwfn v.3.8 code42 and Visual Molecular Dynamics (VMD v.1.9)43.
The AFM simulations were conducted using the PP-AFM code provided by Hapala et al.44. The detailed parameters are listed below. The lateral spring constant for the CO tip was 0.2 N m−1, and a quadrupole-like charge distribution at the tip apex was used to simulate the CO tip with q = −0.05e (e is the elementary charge and refers to |e|, and q is the magnitude of quadrupole charge at the tip apex). The amplitude was set as 1 Å (unless otherwise noted). The difference in probe height between ‘sim. far’ and ‘sim. close’ corresponded to the respective difference between ‘AFM far’ and ‘AFM close’.
The Vienna ab initio simulation package45,46 was used to perform DFT calculations on the NaCl surface. For describing the interaction between electrons and ions, the projector-augmented wave method47,48 was used, and the Perdew–Burke–Ernzerhof generalized gradient approximation exchange-correlation functional was used49. The van der Waals corrections to the Perdew–Burke–Ernzerhof density functional were also included using the DFT-D3 method of Grimme50. The kinetic energy cutoff was set to 400 eV. We used a bilayer NaCl(001) slab separated by a vacuum thicker than 20 Å and the bottom layer of the NaCl was fixed. The atomic structures were relaxed until the atomic forces were less than 0.03 eV Å−1.
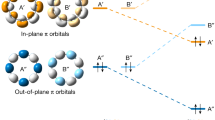
Theoretical calculations were used to gain more insight into the electronic structures of C10. As an sp-hybridized carbon allotrope, C10 generally possesses two perpendicular π-conjugated electron systems (in-plane and out-of-plane π molecular orbitals (πin and πout MOs)), thus we performed the LOL function to reveal the delocalization of π electrons in the cumulenic C10 ring on the basis of π MOs. As illustrated in the LOL-πin and LOL-πout isosurface maps (Extended Data Fig. 2a,c), the favourable global delocalization channels of the electrons in πin and πout MOs are clearly depicted. More importantly, the isosurfaces around all C–C bonds are volumetrically similar, indicating that the electrons over all C–C bonds are equally delocalized, which is significantly different from the C18 with alternating bond lengths20,51. The colour-filled LOL-π map draws the same conclusion from a complementary perspective (Extended Data Fig. 2b,d). The IRI analysis was also performed to reveal the interactions within the C10. As shown in the IRI isosurface (Extended Data Fig. 2e), both covalent bond and van der Waals interaction regions are nicely revealed by the blue and green isosurfaces, respectively, according to the standard colouring method (Extended Data Fig. 2g)52. Notably, the covalent interactions (blue isosurfaces) in all C–C bonds are homogeneous, indicating no bond length alternation, which is consistent with the IRI colour-filled map (Extended Data Fig. 2f) and LOL results. Thus, both the LOL and IRI analyses exhibited that all the C–C bonds in cyclo[10]carbon are identical, further indicating that the C10 ring is cumulenic, in accordance with the conclusion drawn by bond order analysis (Extended Data Fig. 1).
The standard colouring method and chemical explanation of sign(λ2)ρ on the IRI isosurfaces are shown in Extended Data Fig. 2g. The IRI function is defined as follows:
where ρ is the electron density, r is the coordinate vector and \(a\) is an adjustable parameter, \(a\) = 1.1 is adopted for the standard definition of IRI52. The isosurfaces of IRI can exhibit various kinds of interaction regions by properly choosing isovalue. The sign(λ2) denotes the sign of the second largest eigenvalue of Hessian of ρ, which has a certain ability to distinguish attractive and repulsive interactions42,53. The region showing relatively high ρ and thus large magnitude of sign(λ2)ρ implies a relatively strong interaction, whereas the area with low ρ and thus small sign(λ2)ρ does not participate in a noticeable interaction, or the interaction can be at most attributed to the very weak interatomic van der Waals interaction52.
Data availability
All data supporting the findings of this study are available within the paper and its online Extended Data files.
References
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Iijima, S. & Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605 (1993).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Parent, D. C. & McElvany, S. W. Investigations of small carbon cluster-ion structures by reactions with hydrogen cyanide. J. Am. Chem. Soc. 111, 2393–2401 (1989).
Van Orden, A. & Saykally, R. J. Small carbon clusters: spectroscopy, structure, and energetics. Chem. Rev. 98, 2313–2357 (1998).
Grutter, M. et al. Electronic absorption spectra of linear C6, C8 and cyclic C10, C12 in neon matrices. J. Chem. Phys. 111, 7397–7401 (1999).
Pitzer, K. S. & Clementi, E. Large molecules in carbon vapor. J. Am. Chem. Soc. 81, 4477–4485 (1959).
Parasuk, V., Almlof, J. & Feyereisen, M. W. The [18] all-carbon molecule: cumulene or polyacetylene? J. Am. Chem. Soc. 113, 1049–1050 (1991).
Torelli, T. & Mitas, L. Electron correlation in C4N+2 carbon rings: aromatic versus dimerized structures. Phys. Rev. Lett. 85, 1702–1705 (2000).
Arulmozhiraja, S. & Ohno, T. CCSD calculations on C14, C18, and C22 carbon clusters. J. Chem. Phys. 128, 114301 (2008).
Remya, K. & Suresh, C. H. Carbon rings: a DFT study on geometry, aromaticity, intermolecular carbon–carbon interactions and stability. RSC Adv. 6, 44261–44271 (2016).
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 365, 1299–1301 (2019).
Scriven, L. M. et al. Synthesis of cyclo[18]carbon via debromination of C18Br6. J. Am. Chem. Soc. 142, 12921–12924 (2020).
Gao, Y. et al. On-surface synthesis of a doubly anti-aromatic carbon allotrope. Nature https://doi.org/10.1038/s41586-023-06566-8 (2023).
Baryshnikov, G. V., Valiev, R. R., Kuklin, A. V., Sundholm, D. & Agren, H. Cyclo[18]carbon: insight into electronic structure, aromaticity, and surface coupling. J. Phys. Chem. Lett. 10, 6701–6705 (2019).
Baryshnikov, G. V. et al. Aromaticity of even-number cyclo[n]carbons (n = 6–100). J. Phys. Chem. A 124, 10849–10855 (2020).
Charistos, N. D. & Muñoz-Castro, A. Induced magnetic field in sp-hybridized carbon rings: analysis of double aromaticity and antiaromaticity in cyclo[2N]carbon allotropes. Phys. Chem. Chem. Phys. 22, 9240–9249 (2020).
Baryshnikov, G. V. et al. Odd-number cyclo[n]carbons sustaining alternating aromaticity. J. Phys. Chem. A 126, 2445–2452 (2022).
Brémond, E., Pérez-Jiménez, A. J., Adamo, C. & Sancho-García, J. C. Stability of the polyynic form of C18, C22, C26, and C30 nanorings: a challenge tackled by range-separated double-hybrid density functionals. Phys. Chem. Chem. Phys. 24, 4515–4525 (2022).
Li, M. et al. Potential molecular semiconductor devices: cyclo-Cn (n = 10 and 14) with higher stabilities and aromaticities than acknowledged cyclo-C18. Phys. Chem. Chem. Phys. 22, 4823–4831 (2020).
Hoffmann, R. Extended Hückel theory―V: cumulenes, polyenes, polyacetylenes and Cn. Tetrahedron 22, 521–538 (1966).
Liang, C. & Schaefer, H. F. III Carbon clusters: the structure of C10 studied with configuration interaction methods. J. Chem. Phys. 93, 8844–8849 (1990).
Hutter, J., Lüthi, H. P. & Diederich, F. Structures and vibrational frequencies of the carbon molecules C2–C18 calculated by density functional theory. J. Am. Chem. Soc. 116, 750–756 (1994).
Watts, J. D. & Bartlett, R. J. The nature of monocyclic C10. A theoretical investigation using coupled-cluster methods. Chem. Phys. Lett. 190, 19–24 (1992).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Pavliček, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 7, 623–628 (2015).
Pavliček, N. et al. Polyyne formation via skeletal rearrangement induced by atomic manipulation. Nat. Chem. 10, 853–858 (2018).
Sun, Q. et al. On-surface formation of cumulene by dehalogenative homocoupling of alkenyl gem-dibromides. Angew. Chem. Int. Ed. Engl. 56, 12165–12169 (2017).
Jones, R. R. & Bergman, R. G. p-Benzyne. Generation as an intermediate in a thermal isomerization reaction and trapping evidence for the 1,4-benzenediyl structure. J. Am. Chem. Soc. 94, 660–661 (1972).
Lifshitz, C., Peres, T., Kababia, S. & Agranat, I. C10+· and C7+· carbon cluster ions from overcrowded octachloropentafulvalene and octachloronaphthalene. Int. J. Mass Spectrom. Ion Processes 82, 193–204 (1988).
Lifshitz, C., Peres, T. & Agranat, I. Properties of carbon cluster ions, Cn+·, formed by dissociative ionization. Int. J. Mass Spectrom. Ion Processes 93, 149–163 (1989).
Schuler, B. et al. Reversible Bergman cyclization by atomic manipulation. Nat. Chem. 8, 220–224 (2016).
Albrecht, F. et al. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science 377, 298–301 (2022).
Suresh, R. et al. Cyclo[18]carbon formation from C18Br6 and C18(CO)6 precursors. J. Phys. Chem. Lett. 13, 10318–10325 (2022).
Dobrowolski, M. A., Cyranski, M. K. & Wrobel, Z. Cyclic π-electron delocalization in non-planar linear acenes. Phys. Chem. Chem. Phys. 18, 11813–11820 (2016).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Albrecht, T. R., Grütter, P., Horne, D. & Rugar, D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 69, 668–673 (1991).
Frisch, M. J. et al. Gaussian 16 Rev. C.01 (Gaussian, 2016).
Chai, J. D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Hehre, W. J. Ab initio molecular orbital theory. Acc. Chem. Res. 9, 399–406 (1976).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Liu, Z., Lu, T. & Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: bonding character, electron delocalization, and aromaticity. Carbon 165, 468–475 (2020).
Lu, T. & Chen, Q. Interaction region indicator: a simple real space function clearly revealing both chemical bonds and weak interactions. Chem. Methods 1, 231–239 (2021).
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010).
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22125203).
Author information
Authors and Affiliations
Contributions
W.X. conceived the research. L.S., W.G. and F.K. performed the STM/AFM experiments and carried out the DFT calculations. W.Z. synthesized the C14Cl10 molecules. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Glib Baryshnikov and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The bond lengths, Mayer bond orders and bond angles in a cyclo[10]carbon.
Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level. The bond lengths and bond order in C10 are all nearly the same, indicating the structure of consecutive carbon-carbon double bonds, i.e., cumulenic structure (BLA = 0).
Extended Data Fig. 2 Real-space function analysis of cyclo[10]carbon.
(a to d) Localized orbital locator calculated based on in-plane π MOs (LOL-πin) and out-plane π MOs (LOL-πout). (a) and (c) correspond to isosurface maps of LOL-π = 0.4. (b) shows LOL-πin in the ring plane, and (d) shows LOL-πout above 1 Bohr of the ring plane. (e) Interaction region indicator (IRI) isosurface and (f) color-filled map of C10 showing the homogeneous covalent interactions in carbon-carbon bonds. (g) Standard coloring method and chemical explanation of sign(λ2)ρ on interaction region indicator (IRI) isosurfaces.
Extended Data Fig. 3 STM images of the C10Cl8 precursor and the product cyclo[10]carbon.
(a) C10Cl8 molecules separately adsorbed on bilayer NaCl/Au(111) surface. A single CO molecule appeared as a small depression. (b) Close-up STM image of single C10Cl8 molecules on NaCl. (c) Close-up STM image of C10. Scanning condition: (a) I = 1 pA, V = 0.3 V. (b) I = 2 pA, V = 0.3 V. (c) I = 0.5 pA, V = 0.3 V.
Extended Data Fig. 4 Other intermediates observed during manipulation.
(a-c) C10Cl6, (d-f) C10Cl1. The Laplace-filtered AFM images are also shown. Reference set point of Δz: I = 0.5 pA, V = 0.3 V for (b), I = 1 pA, V = 0.3 V for (e). The double bonds indicated by blue and black in (d)represent two different bond lengths within the structure, respectively.
Extended Data Fig. 5 DFT relaxed C10 and C14 structures on NaCl surface.
(a, b) C10 on Cl-top site. (c, d) C10 on Na-top site. (e, f) C14 on Cl-top site. (g, h) C14 on Na-top site.
Extended Data Fig. 6 AFM images of C10 acquired at the oscillation amplitude A = 50 pm.
We tested the effect of different amplitude on the AFM imaging of C10. The AFM image (a, b) and AFM simulations (c, d) were carried out at the oscillation amplitude A = 50 pm. The Laplace-filtered AFM images of (a) and (b) are also shown in (e) and (f). Reference set point of Δz: I = 0.5 pA, V = 0.3 V. The scale bar in (a) applies to all experimental, simulated and Laplace-filtered AFM images.
Extended Data Fig. 7 The bond lengths, Mayer bond orders and bond angles in a cyclo[14]carbon.
Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level, revealing a small bond length alternation (BLA = 0.05 Å) and bond angle alternation (BAA = 25.3°) within C14. The double bonds indicated by blue and black in C14 represent two different bond lengths within the structure, respectively.
Extended Data Fig. 8 The bond lengths, Mayer bond orders and bond angles in a cyclo[18]carbon.
Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level, revealing a bond length alternation (BLA = 0.12 Å) within C18.
Extended Data Fig. 9 STM images of the C14Cl10 precursor and the product cyclo[14]carbon.
(a) C14Cl10 and CO molecules separately adsorbed on a bilayer NaCl/Au(111) surface. (b, c) STM and AFM images of an individual C14Cl10 molecule. (d) Spectra of frequency shift (Δf) as a function of tip height (Δz). The red and blue spectra were taken at the topmost Cl atom of the C14Cl10 molecule, and the Cl atom at the first layer of NaCl surface, respectively. Inset: schematics of the CO-tip approaching processes. The distance between two topmost Cl atoms of C14Cl10 molecule extracted from the AFM image and experimental absolute height extracted from the spectra reasonably agree with the theoretical values. (e) Close-up STM image of C14. Scanning condition: I = 1 pA, V = 0.3 V for (a); I = 0.5 pA, V = 0.3 V for (b); I = 1 pA, V = 0.3 V for (e). Reference set point of Δz for (c): I = 0.5 pA, V = 0.3 V.
Extended Data Fig. 10 AFM simulations of C14 with varying BLAs.
(a-h) A series of AFM simulations of C14 with varying BLAs from cumulenic to intermediate to polyynic structures at decreasing tip-sample distances from left to right (i.e., BLA = 0 Å, 0.03 Å, 0.05 Å, 0.07 Å, 0.09 Å, 0.11 Å, 0.13 Å, 0.15 Å) followed by the method developed in ref. 13. The simulated AFM images within blue and red boxes are assigned to cumulene-like and polyynic structures, respectively. All atomic coordinates keep the BAA as 25.3°. The double bonds indicated by blue and black in (b), (c) and (d) represent two different bond lengths within the structures, respectively. The scale bar in (a) applies to all simulated AFM images.
Extended Data Fig. 11 The bond lengths in the C14Cl4 and C14Cl1 intermediates.
The bond lengths of a ten-membered carbon ring in the C14Cl4 intermediate (a) and a fourteen-membered carbon ring in the C14Cl1 intermediate (b) are listed. Calculations were conducted at the ωB97XD/6-311 + + G(d,p) level. The double bonds indicated by blue and black in C14Cl1 represent two different bond lengths within the structure, respectively.
Extended Data Fig. 12 Other intermediates and side reaction products observed during manipulations.
(a-c) C14Cl8, (d-f) C14Cl3, (g-i) C14Cl5, (j-l) C14Cl6. The Laplace-filtered AFM images are also shown. Reference set point of Δz: I = 0.5 pA, V = 0.3 V for (b) and (e), I = 1 pA, V = 0.3 V for (h), I = 0.2 pA, V = 0.3 V for (k). The scale bar in (b) applies to all experimental and Laplace-filtered AFM images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, L., Zheng, W., Gao, W. et al. On-surface synthesis of aromatic cyclo[10]carbon and cyclo[14]carbon. Nature 623, 972–976 (2023). https://doi.org/10.1038/s41586-023-06741-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06741-x
- Springer Nature Limited
This article is cited by
-
On-surface synthesis and characterization of anti-aromatic cyclo[12]carbon and cyclo[20]carbon
Nature Communications (2024)
-
A new dual-functional strategy to desensitize and sense the explosive and toxic 1,3,5-trinitro-1,3,5-triazinane by cyclo[n]carbons (n = 10,14,18)
Journal of Molecular Modeling (2024)