Abstract
Protective immunity against pathogens or cancer is mediated by the activation and clonal expansion of antigen-specific naive T cells into effector T cells. To sustain their rapid proliferation and effector functions, naive T cells switch their quiescent metabolism to an anabolic metabolism through increased levels of aerobic glycolysis, but also through mitochondrial metabolism and oxidative phosphorylation, generating energy and signalling molecules1,2,3. However, how that metabolic rewiring drives and defines the differentiation of T cells remains unclear. Here we show that proliferating effector CD8+ T cells reductively carboxylate glutamine through the mitochondrial enzyme isocitrate dehydrogenase 2 (IDH2). Notably, deletion of the gene encoding IDH2 does not impair the proliferation of T cells nor their effector function, but promotes the differentiation of memory CD8+ T cells. Accordingly, inhibiting IDH2 during ex vivo manufacturing of chimeric antigen receptor (CAR) T cells induces features of memory T cells and enhances antitumour activity in melanoma, leukaemia and multiple myeloma. Mechanistically, inhibition of IDH2 activates compensating metabolic pathways that cause a disequilibrium in metabolites regulating histone-modifying enzymes, and this maintains chromatin accessibility at genes that are required for the differentiation of memory T cells. These findings show that reductive carboxylation in CD8+ T cells is dispensable for their effector response and proliferation, but that it mainly produces a pattern of metabolites that epigenetically locks CD8+ T cells into a terminal effector differentiation program. Blocking this metabolic route allows the increased formation of memory T cells, which could be exploited to optimize the therapeutic efficacy of CAR T cells.
Similar content being viewed by others
Main
After antigenic stimulation and expansion, most CD8+ effector T (TE) cells are short-lived effector cells (SLECs), but a small fraction consists of memory precursor effector cells (MPECs) that will survive in an antigen-independent manner and generate a pool of long-lived central memory T (TCM) cells4. The potent cytotoxic nature of CD8+ T cells has led to the development of several cell-based immunotherapies for cancer. These therapies include adoptive cell transfer (ACT), which consists of the ex vivo stimulation, expansion and re-infusion of either naturally occurring tumour-infiltrating lymphocytes (TILs) or genetically engineered autologous T cells, such as CAR T cells. Unfortunately, transferred cells are often terminally differentiated, and thus exhibit a low self-renewing and proliferative potential, resulting in poor therapeutic efficacy. Consistently, the use of T cells with memory features for ACT has been shown to provide more robust and sustained antitumour responses5,6.
The differentiation of TE versus memory T (TM) cells is regulated by cytokines and transcription factors7,8, as well as by post-translational modifications of histones to epigenetically alter chromatin accessibility9,10. In addition, activated CD8+ T cells depend on increased anabolic metabolism to sustain their clonal expansion and effector function. Citrate, produced by the tricarboxylic acid (TCA) cycle, is a major source of cytosolic acetyl-coenzyme A (acetyl-CoA), which is a central metabolite supporting cell growth and function, by serving as a precursor of lipid synthesis and as a donor for acetylation11. Although citrate is mainly produced by the oxidation of pyruvate, fatty acids or amino acids, studies in cancer cells under hypoxia or with mitochondrial defects have shown that reductive carboxylation of glutamine (RC) is an indispensable contributor to the citrate pool12,13. RC can be mediated by two enzymes in mammalian cells: the NADP+-dependent isocitrate dehydrogenase (IDH) 1 in the cytosol and IDH2 in the mitochondria12. Mutations in IDH1 or IDH2 in different cancer types lead to the production of the oncometabolite 2-hydroxyglutarate (2-HG), which induces epigenetic changes that drive oncogenesis14. A third IDH isoform (IDH3) is mitochondrial, NAD+-dependent and only able to catalyse the oxidative reaction15. Although it has been suggested that CD8+ T cells could reductively metabolize glutamine to lipids under hypoxia13, how this affects the function and differentiation of TE cells remains unknown.
TE cells reductively carboxylate glutamine
To investigate oxidative versus reductive glutamine use in highly proliferating TE-like CD8+ T cells, we performed an in vitro kinetic labelling of [U-13C]-glutamine in T cells three days after activation (Fig. 1a and Extended Data Fig. 1a). The accumulation of m + 4 metabolites versus m + 5 citrate and m + 3 malate, fumarate, succinate and aspartate identifies oxidative glutamine metabolism versus RC, respectively (Fig. 1a). In TE cells, oxidative and reductive labelling into citrate quickly equilibrates (Fig. 1b), whereas RC in TM cells (TE cells cultured for four extra days in IL-15; Extended Data Fig. 1a) was barely detectable (Extended Data Fig. 1b). Consistently, TE cells showed significantly higher levels of aspartate, malate and fumarate derived from reductive metabolism than did TM cells (Extended Data Fig. 1c–e), and the ratios of reductive over oxidative metabolites were much higher in TE than in TM cells (Fig. 1c and Extended Data Fig. 1f–h). RC produces acetyl-CoA species for lipid biosynthesis13, and, accordingly, we observed higher levels of glutamine labelling into fatty acids in TE than in TM cells (Extended Data Fig. 1i–k). In general, TE cells consumed more glutamine and were more proliferative than TM cells (Extended Data Fig. 1l,m).
a, Oxidative (green) and reductive (red) glutamine metabolism. Ac-CoA, acetyl-CoA; OAA, oxaloacetate. b, Citrate mass isotopologues in mouse CD8+ TE cells labelled with [U-13C]-glutamine (n = 3 biological replicates). c, Ratio of m + 5 to m + 4 citrate detected by [U-13C]-glutamine labelling in TE or TM cells (n = 4 biological replicates). d, Gene expression of IDH1 and IDH2 in CD8+ T cells from volunteers who were vaccinated against yellow fever. Data are reads per kilobase of transcript per million reads mapped (RPKM) in the indicated cell subsets (n = 3 (TE), n = 5 (TM) and n = 6 (TN) biological replicates). e, Percentage of m + 1 citrate labelling from [1-13C]-glutamine in control scramble (Scr) gRNA-transduced cells or IDH2-deficient cells (n = 3 biological replicates). f, Schematic representation of the experiment. D, day. g, Number of Thy1.1+ cells per microlitre of blood. h, Percentage of CD44+CD62L+ cells among Thy1.1+ cells in the blood 28 days after infection. i, Number of Thy1.1+ cells per milligram of spleen seven days after secondary challenge. j,k, Percentages of IL-2+ (j) and IFNγ+TNF+ (k) cells among Thy1.1+ cells after restimulation seven days after secondary challenge. l,m, LCMV-Armstrong viral titres per kidney (l) and liver (m). PFU, plaque-forming units. In g,h: n = 13 (Idh2 gRNA) and n = 11 (Scr gRNA) biological replicates; pooled data from three independent experiments. In i–m: n = 8 (Idh2 gRNA) and n = 9 (Scr gRNA) biological replicates; pooled data from two independent experiments. Data are mean ± s.e.m. and were analysed by one-way ANOVA using Tukey’s multiple comparison test (d) or unpaired, two-tailed Student’s t-test (c,e,h–m). The P value in c is 0.00005633.
To investigate which IDH isoform mediates RC in T cells, we analysed publicly available RNA sequencing (RNA-seq) data from human yellow-fever-tetramer-positive CD8+ naive T (TN), TE and TM cells from the blood of healthy volunteers who had been vaccinated with the yellow fever vaccine16 (Extended Data Fig. 1n). The expression of all IDH isoforms was induced in TE cells compared to TN and TM cells, which can be explained by the fact that TE cells are highly metabolically active. However, among the IDH isoforms, the expression of IDH2 showed by far the highest fold-change increase (Fig. 1d and Extended Data Fig. 1o). We next characterized IDH gene expression in mouse ovalbumin (OVA)-specific T cell subsets over the course of a transient bacterial infection with Listeria monocytogenes expressing OVA (LM-OVA). SLECs and MPECs were isolated seven days after infection and effector memory T (TEM, CD44+CD62L−) and TCM (CD44+CD62L+) cells were collected on day 28. The expression of IDH1 mRNA was not detectable, and IDH3 expression remained unaltered (Extended Data Fig. 1p), whereas the expression of IDH2 was significantly higher in SLECs than in MPECs, TEM or TCM cells (Extended Data Fig. 1q). We then generated ‘IDH2-deficient’ T cells by CRISPR–Cas9 deletion in P14 cells (Extended Data Fig. 1r). The significant reduction in m + 1 metabolites after [1-13C]-glutamine labelling—the heavy carbon of which can only be incorporated into downstream metabolites through RC—confirmed the role of IDH2 in mediating RC in CD8+ T cells (Fig. 1e and Extended Data Fig. 1s–v).
HIF-1α orchestrates metabolic adaptations after engagement of the T cell receptor (TCR)17, and could thus drive the expression of IDH2 and RC in CD8+ T cells. Indeed, inhibition of HIF-1α with echinomycin after T cell activation reduced the expression of IDH2 mRNA and protein (Extended Data Fig. 2a–c), as well as that of its known targets GLUT1 and PDK1 (Extended Data Fig. 2d,e; see ref. 18), whereas stabilization of HIF-1α by dimethyloxalylglycine (DMOG) further boosted the expression of IDH2 in TE cells, and also in metabolically less active TM cells (Extended Data Fig. 2f–h). In addition, computational analyses uncovered a binding site for the HIF-1α transcription factor on the promoter of mouse and human IDH2 (Extended Data Fig. 2i). However, IDH2 is a bidirectional enzyme, and it was previously shown that reductive IDH2 activity is a function of the α-ketoglutarate (α-KG) and citrate ratio19. In line with this, we observed a markedly higher α-KG/citrate ratio in TE than in TM cells (Extended Data Fig. 2j). Next, we asked how the α-KG/citrate ratio is altered in TE cells. High HIF activity, as well as promoting IDH2 expression, induces PDK1 expression (Extended Data Fig. 2e), which, by suppressing pyruvate dehydrogenase20, limits the entry of carbon into the TCA cycle, thus explaining the decreased citrate levels that promote RC21. Indeed, using sodium dichloroacetate (DCA) to inhibit PDK1 in TE cells decreased the α-KG/citrate ratio (Extended Data Fig. 2k), and consequently inhibited RC (Extended Data Fig. 2l–n). Altogether, our data suggest that IDH2-mediated RC is specifically used by TE cells and is orchestrated through transcriptional and metabolic remodelling by HIF-1α.
Knockout of IDH2 promotes TM differentiation
To determine the role of IDH2-driven metabolism in T cell function and differentiation, we transferred Idh2-gRNA-transduced P14 cells into whole-body Cas9 mice that were infected with lymphocytic choriomeningitis virus (LCMV)-Armstrong concurrently with the activation of the P14 cells (Fig. 1f). Despite the essential role of RC in sustaining cancer-cell proliferation, deletion of IDH2 did not alter the kinetics of the CD8+ T cell response (Fig. 1g). However, the differentiation of IDH2-deficient CD8+ T cells was skewed towards a memory phenotype, with a significantly higher proportion of circulating TCM cells, and with the expression of TCF1 and IL-2 (Fig. 1h and Extended Data Fig. 2o–q). To functionally test the increased differentiation of TM cells, we transferred equal amounts of either Thy1.1+ control or IDH2-deficient CD8+ T cells into naive Rag1-knockout (KO) hosts, followed by infection with LCMV-Armstrong (Fig. 1f). IDH2-deficient T cells showed a greater re-expansion capacity after secondary challenge (Fig. 1i), and maintained higher expression of TCF1 and CD62L seven days after secondary challenge (Extended Data Fig. 2r,s). Furthermore, deletion of IDH2 increased the production of IL-2, IFNγ and TNF (Fig. 1j,k), which correlated with enhanced viral clearance in the kidneys and liver (Fig. 1l,m). We validated these observations in the LM-OVA bacterial-infection model (Extended Data Fig. 3a–m). Altogether, these data reveal that RC is dispensable for clonal expansion and antiviral effector functions, and that genetic deletion of IDH2 instead promotes the differentiation of TM cells.
Inhibition of IDH2 improves CAR T cell function
Successful ACT immunotherapy against cancer depends on the memory-like status of the adoptively transferred cells6. Because genetic deletion of IDH2 favoured the differentiation of TM cells, we evaluated whether the simple and straightforward integration of a small-molecule IDH2 inhibitor during the ex vivo manufacturing of T cells for ACT would be sufficient to enrich TM cells, and thus could be a valuable strategy to improve therapeutic efficacy. We tested two structurally different small-molecule inhibitors of IDH2 (AGI-6780 and the US Food and Drug Administration (FDA)-approved AG-221 (enasidenib; referred to as IDH2i in this manuscript)), both of which were designed to inhibit mutant IDH2, but were also found to inhibit wild-type IDH2 (half-maximum inhibitory concentration (IC50) values of around 2 µM (AGI-6780) and 1.8 µM (AG-221)) (Extended Data Fig. 4a). Activating and culturing T cells with either IDH2 inhibitor suppressed RC (Extended Data Fig. 4b,c) and induced the expression of the memory markers CD62L and TCF1, but did not affect activation markers or cell proliferation (Extended Data Fig. 4d–j). Notably, selective inhibition of IDH1 with AG-120 did not affect the expression of CD62L (Extended Data Fig. 4k,l), and IDH2i did not further boost the differentiation of TM cells in activated splenic T cells from whole-body Idh2-KO mice (Extended Data Fig. 4m), showing that the induction of TM cells by IDH2i is IDH2 specific. In addition, IDH2i-conditioned OVA-specific T cell differentiation was skewed from SLECs to MPECs and TCM cells producing more cytokines, when transferred into mice followed by LM-OVA infection (Extended Data Fig. 4n–s), suggesting that short-term in vitro interference of IDH2 is sufficient to maintain a memory phenotype after in vivo transfer.
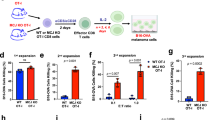
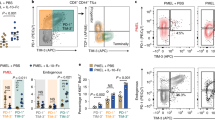
We then transduced mouse CD8+ T cells with a CAR construct targeting the human oncogene HER2 (encoded by ERBB2) (Extended Data Fig. 5a), or with a control blue fluorescent protein (BFP), followed by ACT into mice bearing HER2-overexpressing B16 tumours (Extended Data Fig. 5b,c). Whereas transfer of a sub-therapeutic number of dimethyl sulfoxide (DMSO)-conditioned HER2-CAR T cells did not confer antitumour effects, as compared with the transfer of untreated or BFP-transduced T cells, infusion of IDH2i-conditioned HER2-CAR T cells provided substantial control of tumour growth (Fig. 2a and Extended Data Fig. 5d,e). The tumour-draining lymph nodes of IDH2i-CAR-treated mice contained more HER2-CAR T cells, with increased expression of TCF1 and CD62L (Fig. 2b–d), thus probably constituting a pool of potent TM cells that are able to continuously generate antitumour TE cells, as evidenced by the increased number of HER2-CAR T cells in the tumour (Fig. 2e). IDH2i also caused a shift towards a more progenitor exhausted and less terminally exhausted T cell phenotype (Fig. 2f,g), with increased total numbers of both TIL populations (Extended Data Fig. 5f,g). IDH2i-conditioned HER2-CAR TILs expressed higher levels of TCF1 and lower levels of the exhaustion markers PD-1, TIM3 and LAG3, and produced more cytokines and cytotoxic granules (Fig. 2h and Extended Data Fig. 5h–m). Of note, we observed a similar enhanced antitumour efficacy after ACT of IDH2i-conditioned transgenic OVA-specific CD8+ T cells into Yumm1.7-OVA and B16-OVA tumour-bearing mice (Extended Data Fig. 5n–y and Extended Data Fig. 6a–m), including therapeutic synergy with anti-PD-1 treatment (Extended Data Fig. 6n–p), and by using AGI-6780 for in vitro conditioning of T cells before ACT into B16-OVA tumour-bearing mice (Extended Data Fig. 5z).
a, Tumour growth of B16-HER2 tumour-bearing mice treated with DMSO-conditioned or IDH2i-conditioned BFP-transduced T cells or HER2-CAR T cells. b–d, Number of HER2-CAR+ T cells (b) and their percentage of TCF1+ (c) and CD44+CD62L+ cells (d) in tumour-draining lymph nodes (dLNs) at dissection. e, Number of HER2-CAR+ TILs per milligram of tumour at dissection. f,g, Percentages of PD-1+TCF1+ cells (f) and PD-1+TCF1− cells (g) among HER2-CAR+ TILs. h, Percentage of IFNγ+TNF+IL-2+ HER2-CAR+ TILs. In a–h: n = 12 (IDH2i) and n = 13 (DMSO) biological replicates; pooled data from two independent experiments. i, Mean fluorescence intensity (MFI) of CD62L out of CD62L+ CD19-CAR+ CD8+ T cells nine days after activation, in mice infused with non-transduced (NTD) or anti-CD19 CAR T cells (n = 3 biological replicates; pooled data from two independent experiments). j, Survival curve of NALM6 tumour-bearing mice infused with NTD or anti-CD19 CAR T cells. k, Number of CD19+ NALM6 cells per microlitre of blood at days 14, 21 and 25 after tumour engraftment. In j,k: n = 7 (DMSO or IDH2i CAR), n = 5 (DMSO or IDH2i NTD) and n = 4 (untreated) mice; pooled data from two independent experiments using three human donors. l, MFI of CD62L out of CD62L+ BCMA-CAR+ CD8+ T cells nine days after activation, in mice infused with NTD or BCMA CAR T cells (n = 6 biological replicates; pooled data from two independent experiments). m, Survival curve of NCI-H929 tumour-bearing mice treated by ACT. n, Representative bioluminescence images of NCI-H929 tumour burden. In m,n: n = 19 (DMSO or IDH2i CAR), n = 9 (DMSO or IDH2i NTD) and n = 8 (untreated) mice; pooled data from two independent experiments using two human donors. Data are mean ± s.e.m. and were analysed by unpaired (a–h) or paired (i,l) two-tailed Student’s t-test, log-rank test (j,m) or two-way ANOVA using the original false discovery rate (FDR) test of Benjamini and Hochberg (k).
We next activated and expanded peripheral blood T cells from healthy human donors with IDH2i, which, as in mouse T cells, enhanced CD62L expression, and increased the population of stem cell memory T (TSCM)-like cells (defined as CD45RA+CD62L+CD45RO−CCR7+CD95+CD28+CD27+) (Extended Data Fig. 7a–c). We then generated human brexu-cel-like CD19-28ζ CAR T cells in the presence of IDH2i or DMSO (Extended Data Fig. 7d). IDH2i conditioning did not affect transduction efficiency but strongly increased the expression of CD62L in both untransduced and CAR T cells (Fig. 2i). Notably, transfer of IDH2i-conditioned CAR T cells into NOD-scid-γc−/− (NSG) mice bearing NALM6 B-cell acute lymphoblastic leukaemia (B-ALL) cured almost all mice—in contrast with the transfer of DMSO-treated CAR T cells, which had only a marginal therapeutic effect (Fig. 2j,k).
Finally, we assessed the effect of IDH2 inhibition in two potential ACT applications for multiple myeloma. Autologous bone marrow-infiltrating lymphocytes (MILs) have been proposed as candidates for ACT after autologous transplantation of haematopoietic stem cells in high-risk patients with multiple myeloma22. We thus used IDH2i to activate and expand MILs that were isolated from patients with multiple myeloma, which enhanced the expression of CD62L and resulted in a TSCM-like phenotype (Extended Data Fig. 7e–g). Next, we generated ide-cel-like B cell maturation antigen (BCMA) CAR T cells using an 11D5-3-BBζ CAR (Extended Data Fig. 7h). IDH2i conditioning again led to significant induction of CD62L in CD8+ CAR T cells (Fig. 2l). Most compellingly, infusion of IDH2i-conditioned BCMA CAR T cells into an NCI-H929 myeloma xenograft mouse model markedly improved antitumour activity and survival (Fig. 2m,n and Extended Data Fig. 7i). IDH2 inhibition did not affect the expansion of peripheral blood mononuclear cells (PBMCs), CAR T cells or MILs (Extended Data Fig. 7j–l). Collectively, these data show that transient in vitro conditioning with an IDH2 inhibitor is sufficient to induce the formation of memory-like CD8+ T cells with enhanced antitumour capacities in different mouse models of melanoma tumours and in human xenograft models of FDA-approved CAR T cell therapies for B-ALL and multiple myeloma.
IDH2i shapes chromatin for a memory phenotype
To investigate changes induced by IDH2i in the gene transcriptional landscape, we performed bulk RNA-seq. A total of 476 genes were significantly differentially expressed after inhibition of IDH2. Gene set analysis using Enrichr23 showed that IDH2i upregulated pathways associated with TN cell-surface expression markers and STAT signalling (Fig. 3a). More specifically, gene set enrichment analysis (GSEA) on gene sets from effector versus memory CD8+ T cells confirmed the enrichment of a memory CD8+ T cell gene signature after inhibition of IDH2 (Fig. 3b).
a, OVA-specific CD8+ T cells were activated and cultured for seven days with IDH2i or DMSO, followed by RNA-seq analysis. Shown are pathways significantly enriched among upregulated genes after IDH2 inhibition, identified by Enrichr23 in the Elsevier Pathway Collection (https://www.elsevier.com/solutions/pathway-studio-biological-research) (n = 3 biological replicates). LDL, low-density lipoprotein. b, GSEA of a memory-versus-effector signature from genes upregulated in IDH2i- compared to DMSO-conditioned T cells. NES, normalized enrichment score; running ES, running enrichment score. c, Representative ATAC-seq tracks in OVA-specific CD8+ T cells activated and cultured for seven days with IDH2i or DMSO (n = 3 biological replicates). d, GSEA of a memory-versus-effector signature on more accessible chromatin regions after IDH2 inhibition. e, Differentially accessible chromatin regions in DMSO- versus IDH2i-conditioned cells (FDR P < 0.05), which were correlated with gene-expression data (log2-transformed fold change > 1 and Padj < 0.05). f, Over-representation analysis of the indicated gene signatures from genes exhibiting higher chromatin accessibility and transcription after IDH2 inhibition. g, Top transcription factors identified in more open chromatin regions of IDH2i-conditioned cells using HOMER. h, Top five most significant mouse transcription factors identified by Enrichr in the TRRUST 2019 database (https://www.grnpedia.org/trrust/) in upregulated genes after IDH2 inhibition. Histograms depict negative log of P values (−log10 > 1.5 and P < 0.05). i, Significantly enriched transcription factors in more accessible chromatin regions that correlated with increased gene expression after IDH2 blockade. Transcription factors were found in the TRRUST 2019 database (https://www.grnpedia.org/trrust/) using Enrichr software. Histograms depict negative log of P values (−log10 > 1.5 and P < 0.05).
The expression of genes that drive the differentiation and function of memory CD8+ T cells is dictated by a permissive chromatin state10. We therefore performed an assay for transposase-accessible chromatin with sequencing (ATAC-seq) to compare the epigenetic landscape of IDH2i- versus DMSO-conditioned CD8+ T cells. In general, we observed a total of 8,000 differentially open regions, with a ratio of approximately 50/50%, suggesting that global chromatin accessibility is similar between the two groups (Extended Data Fig. 7m). However, regions that were more accessible after IDH2 inhibition were located at memory genes such as Sell, Tcf7 and Ccr7, thus correlating with increased gene transcription (Fig. 3c and Extended Data Fig. 7n) and enrichment of a memory CD8+ T cell gene signature identified by GSEA (Fig. 3d). Integration of both ATAC-seq and RNA-seq data confirmed that, among several other genes, Sell, Tcf7 and Ccr7 were more accessible in their transcription start site or enhancer regions and were upregulated at the transcriptional level (Fig. 3e). Accordingly, GSEA for this set of genes with increased chromatin accessibility and transcription showed an enrichment for memory CD8+ T cell signatures after IDH2 inhibition (Fig. 3f). Finally, the most enriched transcription factor motifs in more accessible genomic loci after IDH2 inhibition were important transcription factors for memory CD8+ T cell identity and function, such as FOXO1, LEF1 and TCF1 (Fig. 3g). Binding motifs for FOXO1 and STAT1 were also some of the most upregulated genes after IDH2 inhibition (Fig. 3h), and were consistently enriched in those upregulated genes that exhibited more accessible chromatin after integration of ATAC-seq and RNA-seq data (Fig. 3i). Altogether, these results show that increased transcription of memory genes after the inhibition of IDH2 in activated T cells is strongly correlated with profound alterations in chromatin accessibility.
IDH2i induces metabolic compensation
T cell metabolism is tightly linked with phenotypic differentiation. To elucidate the mechanism by which IDH2 interference induces the differentiation of TM cells, we examined the potential functions of IDH2. Reductive IDH1 and oxidative IDH2 can coordinate to generate mitochondrial NADPH to buffer mitochondrial reactive oxygen species (ROS)15, an key signalling molecule for the activation of T cells3. However, basal mitochondrial ROS levels were unchanged, and when exposing the cells to increasing doses of H2O2, oxidative stress was buffered equally in DMSO- versus IDH2i-treated cells (Extended Data Fig. 8a). We then investigated whether there could be a link between the suppression of RC after IDH2 inhibition and the differentiation of TM cells. We made use of T cells that lack mitochondrial transcription factor A (TFAM), which have mitochondrial defects and, in analogy to keratinocytes, might have high rates of RC24. We activated TFAM KO splenocytes and observed an overt lower viability compared to wild-type T cells, which was further reduced by treatment with IDH2i (Extended Data Fig. 8b). Notably, in the viable TFAM KO cells, IDH2 inhibition was able to boost the expression of CD62L expression to the same level as that in wild-type cells. Furthermore, DMSO-treated TFAM KO T cells showed lower expression of CD62L than did DMSO-treated wild-type T cells (Extended Data Fig. 8c). These data suggest that RC, which is likely to be an important metabolic activity in TFAM KO T cells, locks T cells in an effector differentiation state. Nevertheless, the observed differentiation of TM cells could still be an artefact of general disruption of the TCA cycle, because both IDH2 and TFAM are key regulators of mitochondrial metabolism and homeostasis. However, genetic disruption of other major TCA-cycle enzymes, such as α-KG dehydrogenase (OGDH) and succinate dehydrogenase (SDHB), could not recapitulate the induction of TM cell differentiation that was observed after deletion of IDH2 (Extended Data Fig. 8d,e), indicating that IDH2i-orchestrated TM cell differentiation does not result from general disruption of the TCA cycle.
We then investigated how inhibition of IDH2 affects mitochondrial metabolism. Metabolomics analyses revealed overall alterations in the levels of TCA-cycle intermediates after IDH2 inhibition, including higher levels of succinate, fumarate, malate and 2-HG, but equal levels of citrate, acetyl-CoA and α-KG (Fig. 4a,b). The maintenance of cellular citrate and acetyl-CoA pools after the inhibition of RC is notable, and suggests that compensatory metabolic fluxes are activated. We therefore performed an extensive analysis of how inhibition of IDH2 affects the metabolism of three major mitochondrial fuels previously characterized in T cells: glucose, glutamine and fatty acids. [U-13C]-glutamine labelling confirmed a major drop in RC-derived citrate and acetyl-CoA, from 20% and 30%, respectively, to almost 0% after IDH2 inhibition, whereas oxidative m + 4 labelling in metabolites was maintained (Fig. 4c and Extended Data Fig. 8f–i). We then measured [U-13C]-glucose and [U-13C]-palmitate labelling into citrate and acetyl-CoA, and detected a significant increase in the incorporation of palmitate, but not glucose, after IDH2 inhibition, suggesting that fatty acid oxidation (FAO) provides a major compensatory flow to maintain the levels of acetyl-CoA (Fig. 4c and Extended Data Fig. 8j–l). After inhibition of IDH2, T cells indeed become much more oxidative, as measured by Seahorse assay (Fig. 4d and Extended Data Fig. 8m–o). Treatment with etomoxir (an inhibitor of carnitine palmitoyltransferase 1A (CPT1A)) led to a stronger reduction in both maximal respiration and the basal oxygen consumption rate (OCR) in IDH2i-treated T cells (Fig. 4d,e). To support increased fatty acid oxidation and maintain citrate levels after inhibition of RC, an anaplerotic substrate needs to be provided. In rapidly proliferating T cells and cancer cells, glutamine is a major anaplerotic carbon source, driving oxidative phosphorylation1,25,26. Glutamine consumption was increased (Fig. 4f), and the basal OCR was more affected by glutaminase inhibition with BPTES, in IDH2i-treated TE cells (Fig. 4e), suggesting increased glutamine oxidation. In IDH2i-treated cells, either interfering with the oxidative metabolism of glutamine downstream of α-KG through α-KGDH deletion, or inhibiting FAO with etomoxir, significantly suppressed the enhanced differentiation of TM cells (Extended Data Fig. 8p,q). Of note, inhibition of mitochondrial pyruvate oxidation by UK5099 did not differentially reduce the basal OCR, and glucose consumption remained unaltered as well (Fig. 4e and Extended Data Fig. 8r), suggesting no compensatory increase in glucose metabolism after IDH2 inhibition. However, we cannot exclude the possibility that other substrates contribute to anaplerosis and compensation after the inhibition of RC. Branched-chain amino acids can also provide TCA-cycle intermediates as either acetyl-CoA or succinyl-CoA (ref. 27). Indeed, we found that the levels of many amino acids, as well as the expression of the branched-chain amino-acid transporter CD98, were significantly increased in IDH2i-treated T cells (Extended Data Fig. 8s–u). In conclusion, fatty acid and glutamine oxidation compensate for the loss of RC after inhibition of IDH2 and are required for the enhanced differentiation of TM cells. The accompanying higher oxygen consumption rate, which has been shown to correlate with larger pools of TCA metabolites in T cells28 as well as in cancer cells29, could explain the increase in metabolite abundance.
a, Quantification of metabolites in TE cells. The TCA cycle is depicted in blue, with purple circles representing quantified metabolites and grey circles representing metabolites that were not quantified. IDH2 inhibition is represented by a red cross. (n = 6 biological replicates; pooled data from two independent experiments). b, Acetyl-CoA abundance (n = 3 biological replicates). a.u., arbitrary units. c, Percentages of acetyl-CoA m + 2 labelled by [U-13C]-glutamine, [U-13C]-glucose or [U-13C]-palmitate (n = 3 biological replicates). d, OCR by Seahorse (n = 4 biological replicates; pooled data from two independent experiments). e, Reduction in basal OCR after addition of BPTES, UK5099 or etomoxir (n = 4 (UK and BPTES) and n = 5 (Eto) biological replicates; pooled data from two independent experiments). f, Glutamine consumption in TE cells (n = 3 biological replicates). g, Immunoblot quantification of H3K4me3 (n = 5 biological replicates; pooled data from five independent experiments). h, Immunoblot of H3K4me3 in T cells supplemented or not with dimethyl 2-oxoglutarate (an analogue of α-KG). Representative of four independent experiments with four biological replicates. i, CD62L expression in T cells supplemented with α-KG (n = 3 biological replicates; pooled data from three independent experiments). j, Immunoblot of histone marks after supplementation with cell-permeable metabolites. Representative blot from three independent experiments with three biological replicates. k, CD62L expression in T cells with the indicated treatments. Representative histograms from three independent experiments with three biological replicates. l, KDM5 activity after treatment with DMSO or IDH2i, with α-KG supplementation. RFU, relative fluorescence units (n = 3 biological replicates). m,n, Immunoblot of histone marks (m) and CD62L expression (n). KDM5i, KDM5 inhibitor. Representative of two (m) or three (n) independent experiments with two (m) or 3 (n) biological replicates). Data are mean ± s.e.m. and were analysed by unpaired two-tailed Student’s t-test (b,c,e–g), multiple unpaired two-tailed t-tests using the Benjamini and Hochberg method (a), one-way ANOVA using Tukey’s multiple comparison test (i) or two-way ANOVA using the original FDR test of Benjamini and Hochberg (l). For gel source data, see Supplementary Fig. 1.
TCA metabolites dictate epigenetic memory
The epigenetic induction of TM cell differentiation after inhibition of IDH2 suggests changes in the post-translational modifications of histones. We observed that both permissive- and repressive-specific histone modifications were altered after IDH2 inhibition (Extended Data Fig. 9a–c). Among the altered permissive histone marks, the trimethylation of lysine residue 4 on histone H3 (H3K4me3) was previously shown to be involved in the regulation of TM cell differentiation10 (Fig. 4g). By mining previously published H3K4me3 chromatin immunoprecipitation followed by sequencing (ChIP–seq) data, we found that key pro-memory genes with enhanced chromatin accessibility after IDH2 inhibition were associated with H3K4me3 in TN cells, and that this association was maintained in TCM cells, but lost in TE cells30,31 (Extended Data Fig. 9d,e). Of note, higher levels of H3K4me3 were also conserved in human MILs from five patients with multiple myeloma, after culturing the MILs with IDH2i (Extended Data Fig. 9f).
The TCA-derived metabolites succinate, fumarate, 2-HG and malate can compete with the cofactor α-KG for binding to certain histone demethylases, and can inhibit the function of these enzymes32,33. To restore the metabolite balance, we supplemented IDH2i-conditioned T cells with a cell-permeable α-ΚG analogue, dimethyl oxoglutarate, at concentrations that maintained the intracellular levels of α-KG within physiological range (Extended Data Fig. 9g). Increasing intracellular α-KG reverted the IDH2i-mediated increase in H3K4me3, and this was accompanied by a concomitant decrease in CD62L expression (Fig. 4h,i and Extended Data Fig. 9h), suggesting that an imbalance of metabolites after IDH2 inhibition epigenetically drives the expression of TM cell genes. Indeed, an altered metabolite balance alone is sufficient to drive the differentiation of TM cells, as supplementation of CD8+ T cells with cell-permeable fumarate (100 µM) or S-2-HG (250 µM)—but not succinate (0.5, 1 or 2 mM)—increased the levels of trimethylation at H3K4 and the expression of CD62L, which could be reverted by resupplying cells with the α-KG analogue (Fig. 4j,k and Extended Data Fig. 9i–k). Methylation on the suppressive mark H3K27 was not affected (Fig. 4j), which suggests that metabolite imbalance does not lead to a random global increase in histone methylation.
The KDM5 family of histone demethylases are α-KG-dependent and specific for H3K4 (ref. 34). As both the Michaelis constant (Km) value of α-KG and the IC50 values of inhibitory TCA metabolites towards KDM5B activity have been reported before33, we estimated the intracellular concentration of these metabolites, on the basis of the absolute quantifications in Fig. 4a, to calculate a ratio that could indicate the propensity of T cells to exhibit KDM5 inhibition. This ratio was strongly and significantly shifted towards inhibition after IDH2 inhibition (Extended Data Fig. 10a). Indeed, the activity of KDM5 was significantly reduced in IDH2i-treated T cells, which could be restored by increasing intracellular α-KG (Fig. 4l). Furthermore, KDM5 inhibition increased the levels of H3K4me3 and the expression of CD62L in DMSO-conditioned cells (Fig. 4m,n and Extended Data Fig. 10b). Notably, in IDH2-inhibited T cells, inhibition of KDM5 did not further increase the levels of H3K4me3 and CD62L, but it abrogated the suppression of H3K4me3 and CD62L after concomitant supplementation with the α-KG analogue (Fig. 4m,n and Extended Data Fig. 10b), suggesting that the metabolite imbalance induced by IDH2 inhibition favours the differentiation of TM cells by inhibiting the KDM5-dependent demethylation of H3K4.
H3K4me3 was shown to promote histone acetylation by recruiting histone acetyltransferases (HATs)35, and in line with this, we observed strong increases in the acetylation of histone lysine residues (Extended Data Fig. 9a–c), which has been shown to drive pluripotency in TM cells9. Because FAO is involved in the enhanced differentiation of TM cells after IDH2 inhibition, and is compensating for the loss of RC-derived acetyl-CoA, we wondered to what extent FAO contributes to the enhanced histone acetylation. Treatment with etomoxir partially reduced acetylation on H3K27 and H3K14 in IDH2i-treated cells, and this effect was reverted by acetate supplementation (Extended Data Fig. 10c,d). The inhibition of ATP citrate lyase (ACLY), which is essential to form cytosolic acetyl-CoA from mitochondrial-generated citrate to fuel histone acetylation36, partially reverted histone acetylation on H3K27 and H3K14 and CD62L expression in IDH2i-treated T cells (Extended Data Fig. 10e,f). These results indicate that FAO- and citrate-derived acetyl-CoA have a partial role in supporting the phenotype observed after IDH2 inhibition. Preventing histone acetylation by inhibition of the HAT p300 with the inhibitor C646 abrogated the increase in H3K27ac and CD62L expression after IDH2 inhibition (Extended Data Fig. 10g–i). Notably, the improved antitumour effect of IDH2i conditioning was lost when p300 was inhibited simultaneously, indicating that the increased histone acetylation essentially contributes to the induction of memory-like T cells with increased antitumour properties (Extended Data Fig. 10j). Together, these observations reveal the crucial role of RC in epigenetically locking T cells in a terminal effector differentiation state (Extended Data Fig. 10k).
It was previously suggested that, similarly to cancer cells12, clonally expanding T cells rely on RC to sustain their proliferation; however, scientific evidence was lacking37. In this study, we show that IDH2-mediated RC is a unique metabolic characteristic of TE cells, but that it is dispensable for proliferation and effector function. Inactivation of IDH2 epigenetically enabled T cells to express genes that are associated with a memory phenotype. The post-translational modification of histones is a key mechanism that determines gene expression and phenotypic differentiation in CD8+ T cells. TE cells lose the association of the permissive mark H3K4me3 on naive and memory genes after priming by influenza infection, as compared to TN and TM cells. This suggests that H3K4me3 determines the pluripotency potential of CD8+ T cells. We have shown that H3K4 trimethylation is regulated by KDM5 in CD8+ T cells. To allow the activity of KDM5, a specific ratio of metabolites is required, which is established when RC by IDH2 can take place. Therefore, we hypothesize that when naive CD8+ T cells are activated, the induction of RC permits KDM5 activity—which is required for the demethylation of H3K4 at naive and memory genes—and thereby locks the T cells into a terminal effector differentiation program. Inhibition of IDH2-mediated RC alters the levels of metabolites and inhibits KDM5, thus maintaining high levels of H3K4me3 at naive and memory genes. This does not diminish effector function—as evidenced by potent antiviral activity—but instead maintains pluripotency, which favours the differentiation of TM cells after antigen clearance.
Finally, we identify IDH2 inhibition as a promising solution to boost the differentiation of memory cells during the in vitro expansion of T cell products for ACT, which could result in markedly improved antitumour efficacy. Numerous genetic approaches have been reported to induce the formation of TM cells or to enhance the persistence and efficacy of CAR T cells after ACT38,39. The method that we propose —the addition of a small-molecule IDH2 inhibitor to maintain pluripotency during the ex vivo manufacturing of CAR T cells—could complement these next-generation CAR T cell constructs.
Methods
Mice and cell lines
C57BL/6 (B6) (CD45.2) mice were purchased from Charles River (France). Cd4-Cre (B6.Cg-Tg(Cd4-cre)1Cwi/BfluJ), whole-body Cas9 (B6J.129(Cg)- Gt(ROSA)26Sortm1.1(CAG-cas9*,-EGFP)Fezh/J) and conditional Cas9 or Rosa26-LSL-Cas9 knock-in (B6;129-Gt(ROSA)26Sortm1(CAG-cas9*,-EGFP)Fezh/J) mice were bought from the Jackson Laboratory and bred in-house. OT1 TCR transgenic mice, expressing a TCR specific for the chicken egg OVA SIINFEKL epitope in the context of H2Kb, and CRISPR–Cas9 knock-in OT1 TCR transgenic mice were obtained by crossing Rosa26-LSL-Cas9 knock-in mice with Cd4-Cre mice on an OT1 background. Female NOD-scid-γc−/− (NSG) mice (6 to 8 weeks old) were purchased from the Jackson Laboratory and were bred and maintained at the animal facility of the University of Lausanne. Mice were kept in the SPF animal facility of the University of Lausanne. Males and females between 6 and 10 weeks old were used for experiments and were age and sex matched for ACT experiments. Sample size was chosen based on previous experience. Researchers were not blinded to the different treatment groups. Mice were kept in the SPF animal facility of the University of Lausanne in individually ventilated cages, at 19–23 °C with 45–65% humidity and a 12-h dark–light cycle. Experimentation was performed in respect of protocols approved by the veterinary authorities of the Canton de Vaud (VD2688.2, VD3763b, VD3390x1 and VD3309x1e).
Yumm1.7, B16-F10 melanoma cells, Phoenix-Eco and 293T cells were obtained from the American Type Culture Collection and were not further authenticated. Yumm1.7 and B16 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (P/S; Gibco, 15070-063), and 1% l-glutamine (Q; Gibco, 25030-081). Phoenix-Eco cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM), containing 10% FBS and 1% P/S. The 293T cells were maintained in DMEM (Gibco, 21885025) supplemented with 10% FBS. PCR testing was systematically done to confirm that cell lines were mycoplasma-free. NCI-H929 cells were purchased from the German Cell Culture Collection (DSMZ) and cultured in RPMI 1640 with 20% FBS, 1% sodium pyruvate (Gibco, 11360070) and 50 μM β2-mercaptoethanol (Gibco, 31350010). CD4Cre+/wtTfamfl/fl mouse splenocytes were gifted by the laboratory of M. Mittelbrunn and IDH2 whole-body-knockout mouse splenocytes by the laboratory of S.-S. Im.
Culture of mouse T cells
Mouse spleens were processed on a 70-µm cell strainer, followed by centrifugation to obtain a single-cell suspension. Red blood cell lysis was done using Qiagen Red Blood Cell Lysis Buffer. Splenocytes were seeded at 106 cells per ml in RPMI (Gibco, 61870-01) supplemented with 10% FBS (Gibco, 10270-106), 10 mM HEPES (Gibco, 15630-080), 1 mM sodium pyruvate (Gibco, 11360-039), 1% P/S (Gibco, 15070-063), 1× non-essential amino acids (Gibco, 11140-035), 1% l-glutamine (Gibco, 25030-081) and 50 µM β-mercaptoethanol. Recombinant human (rh) IL-2 100 IU ml−1 (in-house) and OVA peptide (SIINFEKL) 1 µg ml−1, an IDH2 inhibitor (IDH2i: AG-221 (ref. 40), Cayman Chemical, 21277; AGI-6780 (ref. 41), Sigma-Aldrich, SML0895; AG-120, Cayman Chemical, 19894-5; all at 5 µM) or the control solvent DMSO (Sigma-Aldrich, 20-139) were also added to the cells.
For phenotypic analyses, immunoblotting and ACT, cells were washed at day 3 and cultured for another four days with rhIL-2 (100 IU ml−1), hIL-7 (10 ng ml−1, Peprotech 200-07), IDH2i or DMSO.
Culture of human T cells
Culture of PBMCs
Buffy coats from de-identified healthy human volunteer blood donors were obtained from the Center of Interregional Blood Transfusion SRK Bern.
PBMCs were isolated by density gradient centrifugation (Lymphoprep). Cells were seeded at 106 cells per ml in round-bottomed 96-well plates in RPMI 1640 (Gibco, 61870-010) supplemented with 8% human AB serum (Blutspendedienst SRK Bern, 92041), kanamycin 1× (Gibco, 11360-039), 1% l-glutamine (Gibco, 25030-024), 1% sodium pyruvate (Gibco, 11360-039), 0.1% 2-mercaptoethanol (5 × 10−2 stock, Sigma-Aldrich, M-7522), 1% non-essential amino acids (Gibco, 11140-035), 150 IU ml−1 rhIL-2 (Peprotech, 200-02) and AG-221 or DMSO. PBMCs were activated with Dynabeads Human T-Activator CD3/CD28 (1:1 cell:bead ratio, Thermo Fisher Scientific, 11161D). Beads were removed five days after activation and cell phenotype was analysed by flow cytometry nine days after activation.
Culture of MILs
Bone marrow aspirates were obtained from patients with multiple myeloma at the Lausanne University Hospital under a research protocol approved by the cantonal ethical commission for human research (CER-VD), and after obtaining informed consent. Malignant plasma cells were separated from whole bone marrow by performing a CD138 positive selection with beads (EasySep, 17877). The positive fraction containing CD138+ cells and the negative fraction containing MILs and other cells were frozen using 40% RPMI 1640 (Gibco, 61870-010), 50% FBS (Gibco, 10270-106; lot: 2166446) and 10% DMSO (Sigma-Aldrich, 41639) and stored in liquid nitrogen until use. The negative fraction was used in these experiments. Cells of the negative fraction were seeded at 106 cells per ml in round-bottomed 96-well plates in RPMI 1640 (Gibco, 61870-010) supplemented with 8% AB serum, kanamycin 1× (Gibco, 11360-039), 1% l-glutamine (Gibco, 25030-024), 1% sodium pyruvate (Gibco, 11360-039), 0.1% 2-mercaptoethanol (5 × 10−2 stock, Sigma-Aldrich, M-7522), 1% non-essential amino acids (Gibco, 11140-035), 150 IU ml−1 rhIL-2 (Peprotech, 200-02) and AG-221 or DMSO, and were activated with Dynabeads Human T-Activator CD3/CD28 (1:1 cell:bead ratio, Thermo Fisher Scientific, 11161D). Beads were removed five days after activation and cell phenotype was analysed by flow cytometry nine days after activation.
Genetic deletion by CRISPR–Cas9
Design of small guide RNA (gRNA) for mouse IDH2 (gRNA 1: GGGCCACCCAGAAGTACAGTG; gRNA 2: GAGAGCCCTAACGGAACGATC), SDHB (gRNA: TCGAATGCAGACGTACGAGG), ACO2 (gRNA: GGTATCCTCACGGTGAAAGG), and α-KGDH (gRNA: GGACTTGTGCTGCTAAGTTA) was done using a publicly available gRNA design tool to improve on-target activity and reduce off-target activity42. Cloning of gRNAs in a self-inactivating version of the MSCV retroviral vector was done under the control of the pU6 promoter. Co-infection of Phoenix-Eco cells with gRNA retroviral plasmids and pCL-ECO allowed for the generation of retroviral particles, which were concentrated by ultracentrifugation. Two different gRNAs were pooled, aliquoted and stored at −80 °C until use.
Mouse CD8+ T cells were isolated from conditional Cas9.Cd4-Cre P14 or OT1 mice splenocytes using the EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies) following the manufacturer’s protocol. Purified CD8+ T cells were plated at 0.5 × 106 cells per ml in RPMI 1640 supplemented as described above, containing rhIL-2 at 50 IU ml−1 and IDH2i or DMSO. Cells were activated using Activator CD3/CD28 Dynabeads (Gibco, 2 beads:1 cell ratio). Retroviral transduction was performed for 24 h in wells previously coated with recombinant human fibronectin (Takara Clontech) for 12 h at 4 °C and blocked with 2% BSA in phosphate-buffered saline (PBS) for 30 min at room temperature. Concentrated retroviruses were added to the fibronectin-coated wells and centrifuged for 90 min at 2,000g at 32 °C. Twenty-four-hour-activated CD8+ T cells were placed in the fibronectin-coated wells containing retroviruses, and centrifuged for 10 min at 400g at 32 °C. Three days after transduction, the medium was changed for fresh medium containing rhIL-2 (10 IU ml), rhIL-7 (10 ng ml−1), rhIL-15 (10 ng ml−1).
Forty-eight hours after transduction, cells were expanded in completed RPMI containing 50 IU ml−1 rhIL-2. Five days later, activation beads were removed and Thy1.1+ transduced cells were purified (STEMCELLl Technologies).
Preparation of mouse CAR T cells
Cloning of HER2-CAR and control BFP in the MSGV retroviral transfer vector was done43 under the control of the 5′ LTR promoter44. The template plasmid pIG6-4D5, which contains the scFv fragment derived from the human-specific anti-HER2 mouse antibody 4D5, was used for the HER2-CAR (ref. 45; a gift from A. Pluckthun). Fusion of the single-chain antibody fragment to the CD8α hinge and transmembrane domains was followed by fusion to the mouse intracellular 4-1BB and CD3ζ signalling endodomains.
Calcium phosphate transfection of Phoenix-Eco cells with HER2-CAR or BFP plasmid and pCL-Eco-packaging plasmid was done and supernatant containing the produced virus was collected at 48 h and 72 h after transfection and ultracentrifuged.
Mouse CD8+ T cells were isolated from the spleen of C56BL/6 mice, using the the EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies) following the manufacturer’s protocol. Purified CD8+ T cells were plated at 0.5 × 106 cells per ml in RPMI 1640 supplemented as described above, containing rhIL-2 at 50 IU ml−1 (Peprotech, 200-02), and IDH2i or DMSO. Cells were activated using Activator CD3/CD28 Dynabeads (Gibco, 2 beads:1 cell ratio). Retroviral transduction was performed as described above in the ‘Genetic deletion by CRISPR–Cas9’ section.
Preparation of human CAR T cells
Anti-CD19 CAR T cells
The hCD19-28z CAR was constructed by ligating the hCD19 scFv (FMC63) into the CAR backbone sequences of a third-generation viral vector pTRPE-28z. Third-generation lentiviral vectors were produced as previously described46. In brief, 95% confluent HEK293T cells were transfected with the pTRPE, psPAX2 encoding gag-pol and pMD2.G encoding the VSV-G envelope, using Lipofectamine 2000 (Invitrogen). Viral supernatant was collected 24 h and 48 h after transfection, filtered, concentrated by ultracentrifugation and frozen. For the generation of CAR T cells, human T cells were enriched from peripheral blood by RosetteSep (STEMCELL Technologies). T cells were activated with human anti-CD3/CD28 Dynabeads (Gibco, 11131D) and cultured in RPMI supplemented with 10% FBS, 1 mM HEPES, 1% P/S and 1% non-essential amino acids (all Gibco). Twenty-four hours later, T cells were transduced with the lentiviral vector encoding anti-human CD19scFv fused to CAR backbones containing human CD28 and CD3ζ (CD247) signalling domains and expanded ex vivo for 12 days. Transduced T cells were maintained at a concentration of 0.75 × 106 cells per ml throughout the culture period by cell enumeration every two to three days. T cells were exposed to 5 μM AG-221 or DMSO over the entire culture period.
Anti-BCMA CAR T cells
The m11D5-3BBζ CAR construct was previously generated47. In brief, the 11D5-3 scFv was linked to the human CD8α hinge and transmembrane domains followed by the human 4-1BB and CD3ζ endodomains, and the CAR construct was ligated into the SFG retroviral vector backbone with a ΔCD271 selectable marker. Retroviral supernatant was produced by transiently co-transfecting 50% confluent 293T cells with (1) RDF plasmid encoding the RD114 envelope or a plasmid encoding the VSV-G envelope; (2) the Peg-Pam plasmid encoding MoMLV gag-pol or a plasmid encoding gag-pol; and (3) the SFG retroviral plasmid of interest or WGXIP retroviral plasmid, using GeneJuice transfection reagent (Merck, 70967-3). Retroviral supernatant was collected at 48 h and 72 h after transfection, filtered, snap-frozen and stored at −80 °C until use, or used fresh. For the generation of CAR T cells, PBMCs were isolated from buffy coats by density gradient centrifugation (Lymphoprep, STEMCELL Technologies, 07851) and were activated with human anti-CD3/CD28 Dynabeads (Gibco, 11131D). T cells were transduced with the retroviral vector and expanded ex vivo for 10 days in the presence of rhIL-2 at 150 IU ml−1 and either AG-221 5 µM or DMSO.
Mouse xenograft models
B-ALL xenograft model
Male NSG mice (8–10 weeks old) were inoculated with 106 Nalm6 cells in the tail vein. Fifteen days after NALM6 infusion, human CAR T cells were thawed and washed, and 2 × 106 CAR+ cells were adoptively transferred into the tail vein. The body weight was frequently measured and the health of mice was monitored. When the physical and behavioural health of mice declined below the levels established by the Swiss cantonal authorities or when mouse body weight dropped by more than 15%, mice were euthanized. The numbers of NALM6 cells in the blood were measured by anti-human CD19 flow cytometry analysis.
Multiple myeloma xenograft model
To establish an in vivo stress test using the NCI-H929 myeloma xenograft model, the dose of BCMA CAR T cells was titrated down to a dose at which standard CAR T cells were only minimally effective. Female NSG mice (6–8 weeks old) were injected with 3 × 106 NCI-H929-GFP-FFluc myeloma cells intravenously in the tail vein at day 0 (ref. 47). Tumour burden was monitored by bioluminescence imaging (BLI) (photons per second per cm2 per steradian) using the Xenogen in vivo imaging system (IVIS) (Caliper Life Sciences) using Living Image software (v.4.7.3, 64 bit). Mice were randomized on the basis of BLI intensity. One dose of 0.5 × 106 T cells was administered intravenously at day 10 after tumour engraftment, and tumour growth was monitored by BLI. The survival of mice was determined according to humane end-points as defined in the score sheet of the approved animal protocol.
LCMV infection model
Whole-body Cas9 mice were infected with 200,000 PFU of LCMV-Armstrong by intraperitoneal injections. The same day, P14 Cas9.Cd4-Cre CD8+ T cells were isolated and activated for transduction as described above. Twenty-four hours later, activated T cells were transduced with Idh2 gRNA as explained above and injected into infected recipients six hours after transduction. Tail-vein blood sampling was performed at different time points. Thirty-five days after infection, transduced CD8+ T cells were sorted by fluorescence-activated cell sorting (FACS) on the basis of their Thy1.1 expression, and transferred into Rag1-knockout recipients, which were infected with LCMV-Armstrong (200,000 PFU, intraperitoneal) the following day.
Plaque assay
Vero cells were grown in DMEM containing 10% FBS and 1% P/S, to a loose confluence, resuspended in medium, counted and diluted to 8 × 105 cells per ml. Mouse kidneys were collected and directly frozen at −80 °C. Kidneys were then smashed in MEM-5 and solution was used for serial dilutions, which were then plated in 24-well plates. Vero cells were added to the serial dilutions of kidney preparations and the mixture was incubated for 3 h at 37 °C. Wells were gently coated with 2× DMEM methylcellulose (1:1 ratio; FLUKA 64620) and incubated for 60–72 h at 37 °C.
Staining was performed as follows. The supernatant was aspired and cells were fixed with 4% PFA for 30 min at room temperature. Cells were washed and incubated with Triton X-100 (FLUKA 93418) for 20 min at room temperature. After the removal of Triton, cells were incubated with 5% FBS in PBS for 20 min at room temperature. Cells were washed and incubated with 2% FBS in PBS containing VLA-4 antibody (BioXcell) for 60 min at room temperature. Cells were washed twice with PBS and incubated with secondary antibody Peroxidase AffiniPure Goat Anti-Rat IgG (Jackson ImmunoResearch Laboratories) in 2% FBS in PBS for 60 min at room temperature. Cells were washed twice in PBS and incubated with freshly prepared ortho-phenylenediamine (Sigma-Aldrich, P3888) and 30% H2O2 perhydrol (Sigma-Aldrich, 216763) for 15 min, until a brown colour appeared. Cells were washed twice with PBS, plaques were counted and PFU calculated using the formula: PFU per ml = number of counts × dilution steps.
Listeria infection model
For IDH2 pharmacological inhibition, splenocytes from OT1 mice were activated and cultured in vitro for seven days with DMSO or AG-221 (IDH2i) as described above, collected and purified on a Ficoll gradient, allowing dead and live T cells to be separated. For genetic deletion of IDH2, OT1 Cas9.Cd4-Cre CD8+ T cells were transduced with Idh2 gRNA as described above, and transduced T cells were isolated after eight days on the basis of their Thy1.1 expression (STEMCELL Technologies). A total of 100,0000 DMSO- or IDH2i-conditioned T cells were injected into the tail vein of C57BL/6 mice, and 10,000 Thy1.1-positive Scr- or Idh2 gRNA-transduced T cells were injected into whole-body Cas9 mice.
Recombinant L. monocytogenes deficient for actA and expressing the OVA peptide SIINFEKL were grown and titrated. Bacterial concentration was calculated using spectrophotometer-measured optical density, and 2,000 colony-forming units (CFU) were injected in the tail of each mouse 4 h after T cell transfer. Tail-vein blood sampling was performed at different time points.
Tumour models
Engraftment of either 106 OVA-expressing Yumm1.7, 105 OVA-expressing B16-F10 or 4 × 105 HER2-expressing B16-F10 tumour cells was performed in recipient C57BL/6 mice. Mice were lymphodepleted six days after tumour engraftment, either by whole-body irradiation with 5 Gy (RS2000, Rad Source, for B16-OVA and Yumm1.7-OVA) or by intraperitoneal injection of 100 mg kg−1 cyclophosphamide (Sigma-Aldrich, C7397, for B16-HER2). Randomization was performed according to tumour volume and 105 IDH2i- or DMSO-conditioned T cells (for Yumm1.7-OVA and B16-OVA) and 5 × 106 BFP or HER2-CAR T cells were adoptively transferred intravenously. B16-OVA and Yumm1.7-OVA tumour-bearing mice were administered a vaccine consisting of CpG-ODN oligonucleotide (Microsynth, 45355) and OVA peptide (Sigma-Aldrich) at the time of T cell transfer. Tumours were measured with a caliper every two days and volumes were calculated using the formula: V = π × (d2 × D)/6, in which d is the minor tumour axis and D is the major tumour axis. Mice bearing tumours larger than 1 g were euthanized. Tumours were dissected and single-cell suspensions were obtained using the Mouse Tumour Dissociation Kit (Miltenyi, 130–096-730) following the manufacturer’s protocol. Draining lymph nodes and spleens were smashed on a 70-µm cell strainer. Antibody staining was done on single-cell suspensions for flow cytometry analysis.
Flow cytometry
Fluorochrome-conjugated antibodies were all from BioLegend or Thermo Fisher Scientific and non-conjugated antibodies were from Cell Signaling. Staining for flow cytometry was done in PBS with 2% FBS and 2 mM EDTA, at 4 °C in the dark. The LIVE/Dead Fixable Blue Cell Stain Kit (Thermo Fisher Scientific) was used to mark dead cells. The FOXP3 staining kit (Thermo Fisher Scientific) was used for intracellular staining, following the manufacturer’s instructions. HER2-CAR T cells were stained with recombinant human Fc-tagged Her2/ErbB2 protein (Sino Biological) and anti-human-Fc Alexa Fluor 488 conjugate (Thermo Fisher Scientific).
Acquisition was done on LSR II, Fortessa and Cytek Aurora flow cytometers with FACSDiva Software.
The flow cytometry gating strategy is provided in Supplementary Fig. 2.
Seahorse assay
The extracellular flux assay was performed with a Seahorse XF96 Extracellular Flux Analyzer, following the manufacturer’s protocol. In brief, T lymphocytes were plated in a cell-TAK (Corning, 354241) coated Seahorse plate with a density of 200,000 cells per well. Cells were treated successively with oligomycin (2 μM), FCCP (2 µM), rotenone (0,5 µM), antimycin A (0,5 µM), etomoxir (50 µM), BPTES (5 µM) and UK5099 (10 µM). The OCR was measured by the analyzer.
Colorimetric and fluorometric assays
The histone multiplex assay was done using the EpiQuik Histone H3 Modification Multiplex Assay Kit (BioCat, P-3100-96-EP), and the KDM5 activity assay was done using the KDM5/JARID Activity Quantification Assay Kit Fluorometric (Abcam, ab113464), according to the manufacturer’s protocol, using 100 ng of extracted histones (for the EpiQuik Histone H3 Modification Multiplex Assay) and 10 µg of nuclear extract (for the KDM5 activity assay) as starting material. The α-KG assay was done using the colorimetric α-KG Assay Kit (Abcam, ab83431) and the 2-HG assay was done using the D 2 Hydroxyglutarate Assay Kit (Abcam, ab211070) according to the manufacturer’s protocol, using 10 million cells as starting material. Glutamine and glucose were detected in the cell culture supernatant using the Glutamine Assay Kit (Sigma-Aldrich, mak438) and the Glucose Assay Kit (Sigma-Aldrich, mak263), respectively, according to the manufacturer’s protocol. OT1 cells were activated in the presence of DMSO or IDH2i as described above. The medium was replaced at day 2 and the supernatant was collected at day 3 after activation to measure glucose and glutamine consumption over 24 h. Glutamine consumption was also measured in TE and TM cells at day 3 and day 7 after activation, respectively. The culture medium was replaced at day 2 (TE cells) and day 6 (TM cells) and the supernatant was collected at day 3 (TE cells) and day 7 (TM cells) after activation. Glutamine and glucose consumption were then normalized with cellular protein content.
RNA extraction and qPCR
For analysing upstream regulation of IDH gene expression, mouse CD8+ T cells were isolated from the spleen from OT1 mice using the EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies) following the manufacturer’s protocol. Purified CD8+ T cells were plated at 106 cells per ml in RPMI 1640 supplemented as described above, containing rhIL-2 at 100 IU ml−1, and cells were activated using Activator CD3/CD28 Dynabeads (Gibco, 2 beads:1 cell ratio). Twenty-four hours after activation, cells were treated with an mTOR inhibitor (Torin 2, Sigma-Aldrich, SML1224, 500 nM), a HIF-1α inhibitor (Echinomycin, Abcam, ab144247-1, 100 nM) or a MYC inhibitor (10058-F4, c-Myc-Max dimerization inhibitor, Abcam, ab145065, 100 µM). Cells were lysed at day 3 after activation.
For the analysis of CD8+ T cell subsets over the course of a bacterial infection, mouse CD45.1+ CD8+ T cells were isolated from the spleen of OT1 mice using the EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies) and adoptively transferred into recipient C57BL/6 (B6) CD45.2+ mice, which were subsequently infected with LM-OVA (2,000 CFU). CD45.1+ CD8+ T cells were then sorted by FACS for RNA extraction at different time points after infection.
RNA was extracted using the Qiagen RNA isolation kit (Qiagen). cDNA was converted from mRNA using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). Quantitative PCR (qPCR) was done with a LightCycler 480 Instrument II (Roche Life Science) using KAPA SYBR FAST qPCR Kit Master Mix (KAPA Biosystems) according to the manufacturer’s instructions. Relative expression was normalized by the expression of β2-microglobulin (β2M forward: AGA CTGATACATACGCCTGCAG; reverse: GCAGGTTCAAATGAATCTTCAG; PDK1 forward: 5′-GGGCCAGGTGGACTTCTATG; reverse: 5′-TGGATATACCAACTTTGCACC; GLUT1 forward: TGGCAGGCTGTGCTGTGCTCATGAC; reverse: AAGCCAGCCACAGCAATAGCAGCA; IDH1 forward: AGTCCAGAGTGAAGAGGGTT; reverse: ACTTGACGCCCACGTTGTAT; IDH2 forward: GTACAACACCGACGAGTCCAT; reverse: AGCCTCAGCCTCAATTGTCT; IDH3A forward: AGGGAAGTTGCGGAGAACTG; reverse: GGGCTGTTCCATGAACCGAT).
Western blotting
Whole-cell lysis was done in RIPA lysis buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate and 0.1% SDS) to which 500 µM of fresh protease inhibitors (4-(2-aAminoethyl)benzenesulfonyl fluoride hydrochloride; Sigma-Aldrich) and phosphatase inhibitors (Phosstop; Sigma-Aldrich) were added. For histone analyses, nuclei were isolated and washed in Triton extraction buffer (PBS containing 0.5% Triton X-100) containing 5 mM sodium butyrate and 500 µM protease inhibitor, and histones were acid-extracted with 0.2 M HCl, followed by precipitation with trichloroacetic acid. Histones were then resuspended in 50 mM Tris-HCl.
Whole-cell-lysate proteins were quantified with a BCA protein assay kit (Thermo Fisher Scientific) and histone extracts were quantified with Bradford reagent (Sigma-Aldrich). Equal amounts of proteins were denatured for 5 min at 95 °C in SDS loading dye containing 5% β-mercaptoethanol, followed by separation on 12.5% polyacrylamide gradient gels and transferring onto 0.2 µM nitrocellulose membranes (Bio-Rad). After transfer, blocking of unspecific binding sites was done in 5% milk and incubation with primary antibodies (Cell Signaling, GeneTex, Santa Cruz Biotechnology) was done overnight at 4 °C. The next day, membranes were incubated with HRP-conjugated secondary anti-rabbit and anti-mouse antibodies (Santa Cruz Biotechnology). Chemiluminescence imaging was performed with ECL and Femto reagents (Super Signal West, Thermo Fisher Scientific).
Uncropped blots are available in Supplementary Fig. 1.
13C-glutamine, 13C-glucose and 13C-palmitate labelling and liquid chromatography–high-resolution mass spectrometry metabolomics
To detect glutamine, glucose and fatty acid metabolism in TE versus TM cells and after pharmacological inhibition of IDH2, splenocytes from OT1 were activated as described above. For IDH2 inhibition experiments, cells were either activated and cultured in 21% O2 and 5% CO2, or placed in a hypoxic hood set at 2% O2 and 5% CO2. At day 3 after activation, cells were washed with PBS and cultured for 2 h with [U-13C]-glutamine (for the IDH2i experiment; in either 21% O2 or 2% O2 conditions), with [1-13C]-glutamine (for the TE versus TM in vitro differentiation experiment), with [U-13C]-glucose or with [U-13C]-palmitate. To detect RC after genetic deletion of IDH2, OT1 OT1 Cas9.Cd4-Cre CD8+ T cells were activated, transduced and cultured as described above. At day 7 after activation, transduced cells were isolated on the basis of their Thy1.1 expression and were rested for 24 h. Next, cells were restimulated for 70 h with Activator CD3/CD28 Dynabeads (Gibco, 2 beads:1 cell ratio) and then labelled for 2 h with [1-13C]-glutamine. Cells were all labelled in glucose- and glutamine-free RPMI (Biological Industries) containing 10% dialysed FBS (Thermo Fisher Scientific), 1% HEPES, 1% P/S, 50 µM β-mercaptoethanol, 1× non-essential amino acids, 11 mM glucose (Sigma-Aldrich, G7021) or 11 mM [U-13C]-glucose, 4 mM [U-13C]-glutamine or 4 mM [1-13C]-glutamine (Cambridge Isotope Laboratories) or 4 mM l-glutamine (Gibco, 25030-081), and DMSO or IDH2i when applicable. For the palmitate labelling experiment, cells were labelled for 6 h in glucose- and glutamine-free RPMI (Biological Industries) containing 10% dialysed FBS (Thermo Fisher Scientific), 1% HEPES, 1% P/S, 11 mM glucose (Sigma-Aldrich, G7021), 4 mM l-glutamine (Gibco 25030-081), DMSO or AG-221 and [U-13C]-palmitate dissolved in ethanol at 0.2% of the final volume. Cells were then washed once with PBS, and pellets were snap-frozen in liquid nitrogen and stored at −80 °C. Metabolites were extracted by resuspending frozen pellets in 80% methanol containing 2 µM d27 myristic acid. Samples were centrifuged at 20,000g for 15 min at 4 °C, supernatant containing metabolites was analysed as described below and protein pellets were resuspended in NaOH, heated at 95 °C for 20 min, cooled down, spun for 10 min at 5,000 rpm and used for BCA analyses.
Absolute quantification of 2-hydroxyglutaric acid, citric acid, fumaric acid, malic acid and succinic acid was performed using a strategy described previously48. In brief, 50 μl of each sample was transferred to a new tube, while a pool consisting of aliquots from all samples was composed in a separate tube. The samples and a standard mix of the target compounds with known concentrations were then derivatized with 12C dimethylaminophenacyl bromide (DmPA), and the pool sample with 13C DmPA. Each derivatized sample and the standard mix was mixed with an equal amount of derivatized pool sample and transferred to MS vials. The absolute concentrations of the 12C derivatized target compounds were calculated on the basis of the abundances of the 13C derivatized target compounds from the pool sample spiked in each sample and standard. Samples were analysed using a platform consisting of a Vanquish UHPLC (Thermo Fisher Scientific) equipped with a C-18 column (Aquity UPCL BEH 1.7 µm 2.1 × 150 mm) coupled to a Q Exactive Orbitrap Focus mass spectrometer (Thermo Fisher Scientific) operating in positive-ion mode. The samples were analysed in sequential order and a derivatized standard was analysed before and after the samples. A step gradient was carried out using solvent A (formic acid 0.1% in mQ) and solvent B (acetonitrile in 0.1% formic acid). Samples (10 μl) were loaded at 0.2% of solvent B and from 1 to 25 min a ramp to 99.8% solvent B was carried out. From minute 25 to 27.5 the gradient was kept constant (99.8% B). The gradient returned to initial conditions (99.8% A) and was kept constant for 4.5 min. The flow rate was held constant at 250 µl per min. The mass spectrometer operated in full-scan (range 120–1200) and positive mode using a spray voltage of 3.2 kV, capillary temperature of 320 °C, sheath gas at 15.0 and auxiliary gas at 2.0. The AGC target was set at 1e6 using a resolution of 70,000.
A different platform was used for the absolute quantification of α-KG, by running an external calibration using an α-KG standard series (0, 0.5, 1, 2, 5 and 25 µM), and for the relative quantification of all other metabolites. Ten microlitres of each sample supernatant was loaded into a Dionex UltiMate 3000 LC System (Thermo Fisher Scientific) equipped with a C-18 column (Acquity UPLC -HSS T3 1. 8 µm; 2.1 × 150 mm, Waters) coupled to a Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific) operating in negative-ion mode. A step gradient was carried out using solvent A (10 mM TBA and 15 mM acetic acid) and solvent B (100% methanol). The gradient started with 5% of solvent B and 95% solvent A and remained at 5% B until 2 min after injection. A linear gradient to 37% B was used until 7 min and increased to 41% until 14 min. Between 14 and 26 min the gradient increased to 95% of B and remained at 95% B for 4 min. At 30 min the gradient returned to 5% B. The chromatography was stopped at 40 min. The flow was kept constant at 0.25 ml per min at the column was placed at 40 °C throughout the analysis. The mass spectrometer operated in full-scan mode (m/z range: (70.0000–1050.0000]) using a spray voltage of 4.80 kV, capillary temperature of 300 °C, sheath gas at 40.0 and auxiliary gas at 10.0. The AGC target was set at 3e6 using a resolution of 140,000, with a maximum IT fill time of 512 ms.
Data from both platforms was collected using Xcalibur software, and analysed using El-Maven and Polly (Elucidata).
For the estimation of the intracellular concentration of TCA metabolites, we have first estimated the volume of an activated T cell. We measured the average diameter (11 μm) of the T cells using the CountessII (Invitrogen). We then calculated the volume assuming that T cells are spherical: 696.9 μm3, which we used to derive the volume of 1 cell: 0.6969 pl. Because the absolute quantification of the TCA metabolites was normalized per μg of protein, we calculated that 1 million activated T cells represent on average 75 μg of protein. This allowed us to estimate the intracellular concentration of the TCA metabolites.
Pathway enrichment analysis
Pathway analysis was performed with the online publicly available software Enrichr23. Genes assigned to sets of differentially accessible regions were compared to gene sets from the TRRUST 2019 database (https://www.grnpedia.org/trrust/) (for ATAC-seq data as well as combined ATAC-seq and RNA-seq data) and genes that were upregulated after IDH2 inhibition were compared to gene sets from the Elsevier Pathway Collection.
Expression level of IDH genes in yellow-fever-specific CD8 T cells
Normalized raw counts data from RNA-seq of human yellow-fever-specific T cells were retrieved from GSE100745, imported into R (v.3.5.3) and converted to log2(normalized counts + 1) (ref. 16). To determine the fold change of genes among TN, TE and TM cells, we fitted a linear model to each gene using the lmFit function of the limma49 package (v.3.38.3), followed by moderated t-statistic computation with the eBayes function. We also converted gene-expression levels to RPKM by obtaining the length of human transcripts using biomaRt (v.2.38.0)50. The RPKM was calculated at the gene level by using the length of the longest transcript and the rpkm function of the edgeR (v.3.24.3) package51.
HIF-1α- and MYC-binding motifs in the promoter of IDH2
The promoter region of IDH2 for both the human genome and the mouse genome was obtained from the Eukaryotic Promoter Database (EPDnew)52. For the analysis of the human genome, the location of HIF-1α- and MYC-binding sites compiled from ChIP–seq data (‘meta clusters’) was obtained from the Gene Transcription Regulation Database (GTRD)53. We verified whether or not HIF-1α- and MYC-binding sites overlapped with the IDH2 promoter using the Integrative Genomics Viewer (IGV v.2.8.0; Homo sapiens hg38).
For the analysis of the mouse genome, we obtained the motif of the HIF-1α and MYC transcription factors from the Jaspar2022 database (https://jaspar.genereg.net/). The best match for these motifs was searched within a region extending ±2 kb from the promoter region of Idh2 using the FIMO tool of the online MEME Suite (v 5.3.2; https://meme-suite.org/meme/meme_5.3.2/). The location of the HIF-1α and MYC motifs within the extended Idh2 promoter region was visualized with IGV (Mus musculus mm10).
RNA-seq analysis
Splenocytes from OT1 mice were activated and cultured as described above, in the presence of either AG-221 5 µM or DMSO. Seven days after activation, cells were gently washed with PBS and RNA was collected and extracted using the Qiagen RNA isolation kit (Qiagen), according to the manufacturer’s instructions. RNA quality was assessed on a Fragment Analyzer (Agilent Technologies). All RNAs had an RQN between 9.4 and 10. RNA-seq libraries were prepared from 500 ng of total RNA with the Illumina TruSeq Stranded mRNA reagents (Illumina) using a unique dual indexing strategy, and following the official protocol automated on a Sciclone liquid handling robot (Perkin Elmer). Libraries were quantified by a fluorometric method (QubIT, Life Technologies) and their quality was assessed on a Fragment Analyzer (Agilent Technologies).
Cluster generation was performed with 2 nM of an equimolar pool from the resulting libraries using the Illumina HiSeq 3000/4000 SR Cluster Kit reagents, followed by sequencing on the Illumina HiSeq 4000 using HiSeq 3000/4000 SBS Kit reagents for 150 cycles (single read). Sequencing data were demultiplexed using the bcl2fastq2 Conversion Software (v.2.20, Illumina).
Sequencing reads were pre-processed and aligned to the mouse genome using the RNA-seq method of the bcbio-nextgen pipeline (v.20.11). The reads were first trimmed for quality and adapter using Atropos (v.1.1.28)54. Trimmed reads were aligned to the Mus musculus genome (mm10) using hisat2 (v.2.2.1)55. Numbers of reads were summarized at the gene level per sample using htseq-count function of HTSeq (v.0.9.1)56. Raw counts were imported into R (v.3.5.3). Genes were filtered to only retain the ones expressed at one count per million in at least one sample. The raw counts were then pre-processed using the voomWithQualityWeights function of the limma package (v.3.38.3), using the mouse ID of each sample as a covariate in the design matrix. Next, a linear model was fitted for every gene with the lmFit function, and moderated t-statistics were computed using the eBayes function for the Idh2i versus the DMSO contrast. Genes with P < 0.05 after Benjamini–Hochberg adjustment were considered as significantly differentially expressed between IDH2i- and DMSO-conditioned cells.
GSEA57 for genes linked to the memory phenotype was performed using the clusterProfiler package (v.4.0.5) for R (v.4.1.0)58. A set of memory-related gene sets was obtained from the Molecular Signature Database (MSigDB), and subjected to GSEA using the GSEA function, with parameters eps = 1e-60, pvalueCutoff = 1, seed = T and a seed = 1234.
ATAC-seq analysis
Splenocytes from OT1 mice were activated and cultured in the presence of AG-221 or DMSO for seven days, following the above protocol, and a previously described ATAC-seq protocol was followed59. In brief, at day 7 after activation, 5 × 104 T cells were washed with ice-cold 1 × PBS and resuspended in 50 μl of ice-cold lysis buffer (10 mM Tris-Cl (pH 7.4), 10 mM NaCl, 3 mM MgCl2 and 0.1% (v/v) NP-40). Cells were centrifuged, and pellets containing nuclei were resuspended in 50 µl of transposase reaction mix (25 μl 2× TDbuffer, 2.5 μl Tn5 transposase (Illumina) and 22.5 μl of nuclease-free water), and incubated for 30 min at 37 °C. The Qiagen MinElute PCR Purification kit was used to clean tagmented DNA and library preparation was done with custom Nextera PCR primers previously described60 and NEBNext High-Fidelity 2X PCR Master Mix (M0541), with the program: 5 min 72 °C, 30 s 98 °C; 10 cycles: 10 s 98 °C, 30 s 63 °C, 1 min 72 °C. Agencourt AMPure XP magnetic beads (A63880, Beckman) were used to clean libraries, which were then quantified using a Fragment Analyzer and sequenced on an Illumina HiSeq 4000 with paired-end 75 nucleotides at the Gene Expression Core Facility at the Ecole Polytechnique Fédérale de Lausanne. Filtration of low-quality reads and trimming were done with AdapterRemoval (v.2.1.7)61.
Sequencing reads were pre-processed and aligned to the mouse genome using the bcbio-nextgen pipeline (v.19.03). Reads were first trimmed for quality and adapter using Atropos (v.1.1.21), and aligned to the Mus musculus genome (mm10) using bwa (v.0.7.17-r1188)62. Reads were shifted to correct for the transposon event. Peak calling was performed using MACS2 (v.2.1.1.20160309), using the --broad option, generating one broad peak list for IDH2i-conditioned samples, and one for the DMSO-conditioned samples. Differential chromatin accessibility analyses were next performed within R (v.3.5.3) using the DiffBind (v.2.10.0) package63. Counts per genomic region were computed using the dba.count function, with the parameter Score=DBA_SCORE_TMM_READS_FULL. Differential accessibility analysis was performed with the edgeR implementation within the dba.analyze function. All peaks were annotated to nearest genes using the annotatePeakInBatch function of the ChIPpeakAnno package (v.3.16.1)64 and the mouse transcription start site annotation (GRCm38.p1), with parameters output = “overlapping”, bindingType = “fullRange”, PeakLocForDistance = “middle” and bindingRegion = c(-15000,15000). A peak that was located further upstream of Tcf7 but fell within a known enhancer region was manually annotated to Tcf7 (ref. 65). Enrichment of transcription-factor-binding motifs within the chromatin regions that were more accessible after IDH2 inhibition was performed using Homer (v.4.11.1). For GSEA of memory-related gene signatures, only one peak per gene was used by retaining the one that had the highest absolute log2-transformed fold-change value. The log2-transformed fold-change values were provided to the GSEA function of the clusterProfiler package with parameters eps = 1e-50, pvalueCutoff = 1, seed = T and a seed = 1234.
Finally, for genes that were both significantly differentially expressed at the mRNA level and had associated more accessible chromatin regions (that is, 52 genes), we performed an over-representation analysis of memory-related gene signatures using the enricher function of the clusterProfiler package.
Raw sequencing and pre-processed RNA-seq (GSE192395) and ATAC-seq (GSE192394) data were deposited in the NCBI’s Gene Expression Omnibus (GEO) with the SuperSeries accession number GSE192396.
Quantification and statistical analysis
FlowJo v.10 was used to analyse flow cytometry data. ImageJ software was used for the quantification of western blots. Prism v.9 software (GraphPad) was used for statistical analyses. Results are represented as mean ± s.e.m. Information on each statistical test used is provided in the figure legends, with the sample size and number of independent repeats. Two group comparisons were tested by unpaired two-tailed Student’s t-tests. Comparisons of more than two groups were done with one-way ANOVA and Tukey’s multiple comparison correction. Grouped data comparisons were calculated using two-way ANOVA with the original FDR method of Benjamini and Hochberg. Sample sizes were based on previous experience. Graft rejection or death led to the exclusion of some mice from analyses. Samples were excluded from flow cytometric analyses when the number of events recorded was lower than 20 in the population of interest, preventing accurate analyses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. RNA-seq data are available in the NCBI’s GEO database under the accession code GSE192395. ATAC-seq data are available in the NCBI’s GEO database under the accession code GSE192394. Source data are provided with this paper.
References
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51, 856–870 (2019).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Joshi, N. S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Klebanoff, C. A. et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA 102, 9571–9576 (2005).
Chen, G. M. et al. Integrative bulk and single-cell profiling of premanufacture T-cell populations reveals factors mediating long-term persistence of CAR T-cell therapy. Cancer Discov. 11, 2186–2199 (2021).
Schluns, K. S. et al. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168, 4827–4831 (2002).
Dominguez, C. X. et al. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J. Exp. Med. 212, 2041–2056 (2015).
Gray, S. M. et al. Polycomb repressive complex 2-mediated chromatin repression guides effector CD8+ T cell terminal differentiation and loss of multipotency. Immunity 46, 596–608 (2017).
Araki, Y. et al. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity 30, 912–925 (2009).
Pietrocola, F. et al. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012).
Reitman, Z. J. & Yan, H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J. Natl Cancer Inst. 102, 932–941 (2010).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Akondy, R. S. et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature 552, 362–367 (2017).
Pollizzi, K. N. & Powell, J. D. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 14, 435–446 (2014).
Finlay, D. K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Fendt, S. M. et al. Reductive glutamine metabolism is a function of the a-ketoglutarate to citrate ratio in cells. Nat. Commun. 4, 2236 (2013).
Menk, A. V. et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 (2018).
Rajagopalan, K. N. et al. Metabolic plasticity maintains proliferation in pyruvate dehydrogenase deficient cells. Cancer Metab. 3, 7 (2015).
Noonan, K. A. et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci. Transl. Med. 7, 288ra278 (2015).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Hamanaka, R. B. et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 6, ra8 (2013).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Fan, J. et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712 (2013).
Neinast, M. D. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 29, 417–429 (2019).
Vardhana, S. A. et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 21, 1022–1033 (2020).
Griss, T. et al. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 16, e1002309 (2015).
Tyrakis, P. A. et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 540, 236–241 (2016).
Russ, B. E. et al. Distinct epigenetic signatures delineate transcriptional programs during virus-specific CD8+ T cell differentiation. Immunity 41, 853–865 (2014).
Xiao, M. et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012).
Tarhonskaya, H. et al. Studies on the interaction of the histone demethylase KDM5B with tricarboxylic acid cycle intermediates. J. Mol. Biol. 429, 2895–2906 (2017).
Klose, R. J., Kallin, E. M. & Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 7, 715–727 (2006).
Zhang, T., Cooper, S. & Brockdorff, N. The interplay of histone modifications—writers that read. EMBO Rep. 16, 1467–1481 (2015).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Ye, L. et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 34, 595–614 (2022).
Corria-Osorio, J. et al. Orthogonal cytokine engineering enables novel synthetic effector states escaping canonical exhaustion in tumor-rejecting CD8+ T cells. Nat. Immunol. 24, 869–883 (2023).
Yen, K. et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 7, 478–493 (2017).
Wang, F. et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science 340, 622–626 (2013).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Coren, L. V., Jain, S., Trivett, M. T., Ohlen, C. & Ott, D. E. Production of retroviral constructs for effective transfer and expression of T-cell receptor genes using Golden Gate cloning. Biotechniques 58, 135–139 (2015).
Tschumi, B. O. et al. CART cells are prone to Fas- and DR5-mediated cell death. J. Immunother. Cancer 6, 71 (2018).
Worn, A. & Pluckthun, A. An intrinsically stable antibody scFv fragment can tolerate the loss of both disulfide bonds and fold correctly. FEBS Lett. 427, 357–361 (1998).
Tatari, N. et al. Preclinical testing of CAR T cells in a patient-derived xenograft model of glioblastoma. STAR Protoc. 1, 100174 (2020).
Camviel, N. et al. Both APRIL and antibody-fragment-based CAR T cells for myeloma induce BCMA downmodulation by trogocytosis and internalization. J. Immunother. Cancer 10, e005091 (2022).
Guo, K. & Li, L. Differential 12C-/13C-isotope dansylation labeling and fast liquid chromatography/mass spectrometry for absolute and relative quantification of the metabolome. Anal. Chem. 81, 3919–3932 (2009).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Dreos, R., Ambrosini, G., Groux, R., Cavin Perier, R. & Bucher, P. The eukaryotic promoter database in its 30th year: focus on non-vertebrate organisms. Nucleic Acids Res. 45, D51–D55 (2017).
Kolmykov, S. et al. GTRD: an integrated view of transcription regulation. Nucleic Acids Res. 49, D104–D111 (2021).
Didion, J. P., Martin, M. & Collins, F. S. Atropos: specific, sensitive, and speedy trimming of sequencing reads. PeerJ 5, e3720 (2017).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 (2015).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Schubert, M., Lindgreen, S. & Orlando, L. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Res. Notes 9, 88 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Zhu, L. J. et al. ChIPpeakAnno: a Bioconductor package to annotate ChIP–seq and ChIP–chip data. BMC Bioinformatics 11, 237 (2010).
He, B. et al. CD8+ T cells utilize highly dynamic enhancer repertoires and regulatory circuitry in response to infections. Immunity 45, 1341–1354 (2016).
Acknowledgements
We thank C. Vuillefroy de Silly for technical assistance; G. Gyülvészi for scientific discussion; and M. Mittelbrunn and S.-S. Im for providing TFAM and IDH2 KO splenocytes. Metabolomic analysis was performed at the VIB Metabolomics Core at the University of Leuven; RNA library preparation and RNA-seq were performed at the Lausanne Genomic Technologies Facility, University of Lausanne; and ATAC-seq was performed at the Gene Expression Core Facility at the Ecole Polytechnique Fédérale de Lausanne. D.M. is supported by the ISREC Foundation, PHRT Foundation, Ligue Genevoise Contre le Cancer, Fondation Dr Henri Dubois-Ferrière Dinu Lipatti and the Swiss Innovation Agency (Innosuisse). M.W., P.-C.H. and P.R. were supported in part by a grant from Roche. P.R. was supported in part by the SNSF grant 310030_182735 and Oncosuisse KFS-4404-02-2018. P.-C.H. is funded in part by a European Research Council Starting Grant (802773-MitoGuide), a European Molecular Biology Organization (EMBO) Young Investigator award, SNSF project grants (31003A_182470), the Cancer Research Institute (Lloyd J. Old STAR award) and a Melanoma Research Alliance Established Investigator award. N.M.-P. was funded by the EMBO and the European Federation of Immunological Societies (EFIS). C.A. receives funding from Swiss Cancer Research KFS-4542-08-2018-R, Stiftung für Krebsbekämpfung, Fondation Leenaards, Helmut Horten Stiftung and Fondation Muschamp. J.A.R. was a recipient of a Swiss Government Excellence Scholarship.
Author information
Authors and Affiliations
Contributions
A.J. conceived, designed and performed most experiments, analysed data and wrote the manuscript. T.W. performed bioinformatics analyses. N.M.-P., A.L., N.C. and C.V.G. performed experiments on human T cells. J.A.R. contributed to BCMA CAR T cell experiments and analysis. A.B. provided assistance with Seahorse experiments. J.-J.P., F.F. and K.-C.K. helped with cloning. B.G. supervised metabolomics experiments. C.A., D.M. and F.M. designed and supervised human CAR T cell experiments and provided scientific input. C.A. edited the manuscript. P.R. and P.-C.H. provided scientific input, supervised the project and edited the manuscript. M.W. conceived the study, designed experiments, analysed data, supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
P.R. and M.W. are inventors on a patent application filed by the University of Lausanne related to memory induction by IHD2 inhibition and its application in cellular therapies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Stephen Gottschalk and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 RC is a unique metabolic feature of rapidly proliferating TE cells.
a, Experimental set-up of expanding effector (TE, blue) and resting memory (TM, red) T cell differentiation. OVA-specific CD8+ T cells from OT1 mice were activated with SIINFEKL (OVA) peptide and cultured with IL-2 for 3 days and with IL-15 for 4 additional days. TE cells were collected at day 3 and TM cells were collected at day 7 post-activation. Before collection, cells were cultured with [U-13C]-glutamine for 2 h and labelling patterns were analysed by mass spectrometry. b, Bar graphs representing the percentages of citrate isotopologues m + 0, m + 1, m + 2, m + 3, m + 4, m + 5 and m + 6 detected by [U-13C]-glutamine labelling in TE or TM cells. (n = 4 biological replicates per group). c–e, Percentages of aspartate (c), malate (d) and fumarate (e) isotopologues m + 0, m + 1, m + 2, m + 3 and m + 4 detected by [U-13C]-glutamine labelling in TE or TM cells. f–h, Ratios of m + 3 over m + 4 isotopologues of aspartate (f), malate (g) and fumarate (h) detected by [U-13C]-glutamine labelling in TE and TM cells. (n = 4 biological replicates per group). i–k, Percentage of isotopologues of palmitate (i), myristate (j) and stearate (k) labelling from [U-13C]-glutamine in TE or TM cells. (n = 3 biological replicates per group (TM cells) and n = 2 biological replicates per group (TE cells)). l, Glutamine consumption in mouse CD8+ TE and TM cells over 24 h at day 3 and day 7 post-activation respectively. (n = 3 biological replicates per group). m, Percentages of KI67+ out of CD8+ T cells in TE and TM cells. (n = 6 biological replicates per group, pooled data from 2 independent experiments). n, Experimental set-up of yellow fever vaccine administration to healthy volunteers, from which yellow fever tetramer+ CD8+ T cells were isolated from the blood at indicated time points. o, Gene expression in CD8+ T cells from healthy human volunteers vaccinated with yellow fever vaccine. Shown here are IDH3A, B and G reads per kilobase of transcript per million reads mapped in indicated cell subsets isolated from peripheral blood. (n = 3 (TE), n = 5 (TM) and n = 6 (TN) biological replicates). p, mRNA expression of Idh3a in indicated T cell subsets isolated from mouse spleens at day 7 or day 28 post-LM-OVA infection. Data are represented as fold change as compared to SLECs, normalized to β2-microglobulin expression. (n = 3 biological replicates). q, mRNA expression of Idh2 in indicated T cell subsets isolated from spleens of mice at day 7 or day 28 post-LM-OVA infection. Data are represented as fold change as compared to SLECs, normalized to β2-microglobulin expression. (n = 4 biological replicates). r, Representative immunoblot for IDH2 in control Scr or IDH2 deleted cells. s–v, Percentage of m + 1 cis-aconitate (s), fumarate (t), malate (u) and succinate (v) labelling from [1-13C]-glutamine in Scr or IDH2 deleted CD8+ T cells. (n = 3 biological replicates per group). Data represent mean ± s.e.m. and were analysed by unpaired, two-tailed Student’s t-test (c–h,l,m,s–v), multiple unpaired, two-tailed t-test (b,i–k) or one-way ANOVA using Tukey’s multiple comparison test (o–q). Only relevant statistical comparisons are shown. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 HIF1 drives IDH2 expression and RC in CD8+ T cells.
a, mRNA expression of Idh2 in mouse CD8+ TE cells upon treatment with DMSO and HIF-1α inhibitor (HIF-1αi). Data are represented as fold change as compared to DMSO-treated, normalized to β2-microglobulin expression. (n = 3 biological replicates per group). b,c, Representative immunoblot (b) and quantification (c) of IDH2 in mouse CD8+ T cells upon treatment with DMSO and HIF-1αi. A dashed line indicates where the immunoblot membrane was cropped (b) (n = 3 biological replicates per group). d,e, mRNA expression of Glut1 (d) and Pdk1 (e) in mouse TE cells treated with HIF-1αi. Data are represented as fold change as compared to DMSO-treated, normalized to β2-microglobulin expression. (n = 3 biological replicates per group). f–h, Representative immunoblot (f) and quantification of HIF-1α (g) and IDH2 (h) in TE or TM cells upon treatment with control or DMOG. (n = 3 biological replicates per group). i, Representation of human and mouse Idh2 transcripts, promoters and extended promoters with HIF-1α- recognized sequences highlighted in red. j, Ratio of α-KG over citrate in TE or TM cells at day 3 and day 7 post-activation respectively. (n = 4 biological replicates). k, Ratio of α-KG over citrate in mouse CD8+ TE cells treated with either 5 mM sodium dichloroacetate (DCA) or an equimolar sodium chloride control solution (-). (n = 3 biological replicates per group). l,m, Percentages of citrate m + 5 (l) and m + 4 (m) detected by 4 h [U-13C]-glutamine labelling in mouse CD8+ TE cells treated with either 5 mM sodium dichloroacetate (DCA) or an equimolar sodium chloride control solution (-). (n = 3 biological replicates per group). n, Ratio of m + 5 over m + 4 isotopologues of citrate detected by [U-13C]-glutamine labelling in mouse CD8+ TE cells treated with either 5 mM sodium dichloroacetate (DCA) or an equimolar sodium chloride control solution (-). (n = 3 biological replicates per group). o, Percentage of IL-2+ cells out of transferred Thy1.1+ CD8+ T cells upon ex vivo restimulation at day 28 post-primary infection. p,q, Percentage of TCF1+ cells out of transferred Thy1.1+ CD8+ T cells in the blood at day 8 (p) and day 28 (q) post-primary infection. r, Percentage of TCF1+ cells out of transferred Thy1.1+ CD8+ T cells in the spleen at day 7 post-secondary challenge. s, Percentage of CD44+CD62L+ cells out of transferred Thy1.1+ CD8+ T cells in the spleen at day 7 post-secondary challenge. (o–q, n = 11 (Scr gRNA) and n = 13 (Idh2 gRNA) biological replicates per group, pooled data from 3 independent experiments. r,s, n = 9 (Scr gRNA) and n = 8 (Idh2 gRNA) biological replicates per group, pooled data from 2 independent experiments.). Data represent mean ± s.e.m. and were analysed by unpaired, two-tailed Student’s t-test (a,c–e,j–s) or two-way ANOVA using the original FDR test of Benjamini and Hochberg (h,i). Only relevant statistical comparisons are shown. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 3 Genetic deletion of IDH2 promotes the differentiation of memory CD8+ T cells.
a, Experimental set-up of OT1CD4CreCas9 CD8+ T cell retroviral transduction with Idh2 gRNA or control scramble (Scr) gRNA, followed by ACT into mice that are subsequently infected with LM-OVA. 34 days post-primary infection, Thy1.1-positive cells were isolated and transferred in new hosts followed by LM-OVA infection ( = secondary infection). b, Percentage of Thy1.1+ CD8+ out of live cells over the course of the primary infection. c–e, Percentage of TCF1+ (c), IFNγ+ TNF+ IL-2+ (d) and CD44+CD62L+ (e) cells out of transferred Thy1.1+ CD8+ T cells in the blood at day 28 post-primary infection. (b–e, n = 4 biological replicates per group). f, Representative flow cytometry graphs showing the percentage of TCF1+ cells out of transferred Thy1.1+ CD8+ T cells in the spleen at day 28 post-primary infection. g, Graphs representing the percentage of CD44+CD62L+ cells out of transferred Thy1.1+ CD8+ T cells in the spleen at day 34 post-primary infection. h,i, Percentage of CD44+ CD62L+ (h) and TCF1+ (i) cells out of transferred Thy1.1+ CD8+ T cells in the spleen, lymph nodes (LN) and bone marrow (BM) at day 34 post-primary infection. (h,i, n = 5 biological replicates per group). j–l, Percentage of Thy1.1+ cells out of CD8+ T cells in the spleen (j), lymph nodes (k) and bone marrow (l) at day 34 post-secondary infection with LM-OVA. m, Percentage of IFNγ+TNF+ cells out of transferred Thy1.1+ CD8+ T cells in the spleen at day 34 post-secondary infection with LM-OVA. (j–m, n = 4 (Idh2 gRNA) and n = 6 (Scr gRNA) biological replicates per group). Data represent mean ± s.e.m. and were analysed by unpaired, two-tailed Student’s t-test.
Extended Data Fig. 4 Pharmacological inhibition of IDH2 boosts the formation of memory-like CD8+ T cells.
a, TCA-cycle schematic with indication of two different IDH2 inhibitors. b,c, Bar graphs representing the percentage of m + 1 citrate labelling from [1-13C]-glutamine in DMSO, AGI-6780-conditioned CD8+ T cells (b) or AG-221-conditioned cells (c). (n = 3 biological replicates per group). d, Experimental set-up of in vitro T cell culture. OVA-specific CD8+ T cells from the spleen of OT1 mice were activated with OVA and IL-2 and cultured in the presence of DMSO or IDH2i for 7 days, with IL-7 supplementation from day 3. e,f, Representative flow cytometry histograms indicating the relative expression of CD62L (e) and of the indicated cell-surface proteins (f) on the surface of CD8+ T cells at day 7 post-activation upon inhibition of IDH2 using AG-221 (IDH2i) or control (DMSO) treatment. g, Cell count at 24 h, 48 h, 72 h and 7 days post-activation of DMSO- or IDH2i-conditioned CD8+ T cells. (n = 4 biological replicates, data pooled from 2 independent experiments). h, Percentages of TCF1+ CD8+ T cells at day 7 post-activation upon DMSO or IDH2i conditioning. (n = 2 biological replicates). i,j, Representative histogram indicating the relative expression of CD62L (i) and bar graph showing the percentage of CD62L+ CD8+ T cells (j) treated with AGI-6780 at day 7 post-activation. (n = 5 biological replicates, data pooled from 4 independent experiments). k,l, Representative histogram indicating the relative expression of CD62L (k) and bar graph showing the percentage of CD62L+ CD8+ T cells (l) treated with an IDH1 inhibitor (AG-120) at day 7 post-activation. (n = 2 biological replicates, data pooled from 2 independent experiments). m, Percentages of CD62L+ CD8+ T cells 7 days post-activation of splenocytes from IDH2 wild-type or knockout mice cultured with or without IDH2i (n = 2 biological replicates, data pooled from 2 independent experiments). n, Experimental set-up of DMSO- or IDH2i-conditioned T cells transferred into mice that are subsequently infected with LM-OVA. o,p, Graphs representing the percentage of SLECs (KLRG1+CD127−) (o) and MPECs (KLRG1−CD127+) (p) out of transferred CD8+ T cells in the spleen at day 14 post-infection. q, Percentage of TCM (CD44+CD62L+) cells out of transferred CD8+ T cells in the spleen at day 28 post-infection. r, Percentage of IFNγ+TNF+ cells out of transferred CD8+ T cells upon ex vivo restimulation at day 28 post-infection. s, Percentage of IFNγ+TNF+ cells out of transferred CD8+ T cells upon ex vivo restimulation over one year post-infection. (n–q, n = 3(IDH2i) and n = 5 (DMSO) biological replicates per group). Data represents mean ± s.e.m. and were analysed by unpaired, two-tailed Student’s t-test (b,c,g,h,j,l,o–s) or two-way ANOVA using the original FDR test of Benjamini and Hochberg (m). Only relevant statistical comparisons are shown.
Extended Data Fig. 5 Pharmacological inhibition of IDH2 boosts the antitumour function of adoptively transferred cells in B16 melanoma tumor models.
a, Representative dot plots of anti-HER2-CAR expression on BFP- or anti-HER2-CAR- transduced CD8+ T cells and representative histogram of CD62L protein expression on the cell surface of HER2-CAR CD8+ T cells treated with DMSO or IDH2i, analysed by flow cytometry at day 7 post-activation. b, Representative histogram of HER2 protein expression and isotype control on the cell surface of B16-HER2 tumour cells compared to B16 tumour cells, analysed by flow cytometry. c, Experimental set-up of B16-HER2 tumour experiment. Polyclonal CD8+ T cells were transduced with blue fluorescent protein (BFP)-expressing vector or anti-HER2-CAR, expanded during 7 days in the presence of DMSO or IDH2i and subsequently transferred into B16-HER2 tumour-bearing mice. d,e, Weight (d) and photo (e) of B16-HER2 tumours from mice transferred with either DMSO or IDH2i-conditioned anti-HER2-CAR T cells. f,g, Number of transferred PD-1+TCF1+ cells (f) and PD-1+TCF1− cells (g) DMSO- or IDH2i-conditioned CD8+ HER2-CAR TILs, analysed by flow cytometry 21 days post-tumour engraftment. h–k, Percentages of TCF1+ (h), PD-1+ (i), TIM3+ (j) and LAG3+ (k) cells out of transferred HER2-CAR TILs. l, Percentages of PD-1+TIM3+LAG3+ cells out of transferred HER2-CAR TILs. m, Percentage of granzyme B+ HER2-CAR+ CD8+ TILs upon ex vivo restimulation with PMA-ionomycin. (d–m, n = 13 (DMSO HER2-CAR), n = 12 (IDH2i HER2-CAR) and n = 8 (control, DMSO BFP and IDH2i BFP) biological replicates per group, pooled data from 2 independent experiments). n,o, Tumour growth curve (n) and weight (o) of B16-OVA tumour-bearing mice transferred with T cells conditioned with DMSO or IDH2i (AG-221). p, Percentages of CD44+CD62L+ cells out of transferred CD8+ T cells in the spleen 23 days post-tumour engraftment. q, Percentages of TCF1+ cells out of transferred CD8+ T cells in the spleen 23 days post-B16-OVA tumour engraftment. r, Number of transferred DMSO- or IDH2i-conditioned CD8+ TILs per milligram of tumour, analysed by flow cytometry 23 days post-B16-OVA tumour engraftment. s,t, Percentages of PD-1+TCF1+ cells (s) and PD-1+TCF1− cells (t) out of transferred CD8+ TILs. u–y, Percentage of IFNγ and TNF (u), granzyme B (v), and CD107a (y) production and representative FACS plots of IFNγ and TNF (w) and granzyme B (x) production by transferred TILs upon ex vivo restimulation with OVA peptide. (n–y, n = 7 biological replicates per group). z, Tumour growth curve of B16-OVA tumour-bearing mice transferred with OT1 cells conditioned with DMSO, AG-221 or AGI-6780. (n = 7 biological replicates per group). Data represent mean ± s.e.m. and were analysed by unpaired, two-tailed Student’s t-test (f–v,y,z) and one-way ANOVA using Tukey’s multiple comparison test (d). Only relevant statistical comparisons are shown.
Extended Data Fig. 6 Pharmacological inhibition of IDH2 boosts the antitumour function of adoptively transferred cells in a Yumm1.7 melanoma model.
a, Experimental set-up of in vivo Yumm1.7-OVA tumour model. b–d, Tumour growth curve (b), weight (c) and representative photo (d) of B16-OVA tumour-bearing mice either left untreated (unt.) or transferred with T cells conditioned with DMSO or IDH2i (AG-221). e, Number of transferred DMSO- or IDH2i-conditioned CD8+ T cells per tumour-draining lymph node, analysed by flow cytometry 25 days post-Yumm1.7-OVA tumour engraftment. f, Percentages of TCF1+ cells out of transferred CD8+ T cells in tumour-draining lymph node 25 days post-Yumm1.7-OVA tumour engraftment. g, Percentages of CD44+CD62L+ cells out of transferred CD8+ T cells in the spleen 25 days post-tumour engraftment. h, Number of transferred DMSO- or IDH2i-conditioned CD8+ TILs per milligram of tumour, analysed by flow cytometry 25 days post-Yumm1.7-OVA tumour engraftment. i,j, Number of transferred PD-1+ TCF1+ cells (i) and PD-1+TCF1− cells (j) DMSO- or IDH2i-conditioned CD8+ TILs, analysed by flow cytometry 25 days post-tumour engraftment. k,l, Percentages of PD-1+TCF1+ cells (k) and PD-1+TCF1− cells (l) out of transferred CD8+ TILs. m, Bar plots representing the percentage of IFNγ and TNF production by transferred TILs upon ex vivo restimulation with OVA peptide. (b–m, n = 8 (unt.), n = 11 (DMSO) and n = 10 (IDH2i) biological replicates per group, pooled data from 2 independent experiments). n, Survival curve of mice treated with OVA-specific CD8+ T cells conditioned with DMSO or IDH2i, with α-PD-1 or IgG control combination treatment. (n = 8 biological replicates per group). o, Number of transferred DMSO- or IDH2i-conditioned TILs with α-PD-1 or IgG control combination treatment per milligram of tumour, analysed by flow cytometry 25 days post-Yumm1.7-OVA tumour engraftment. p, Percentage of IFNγ+TNF+IL-2+ CD8+ TILs upon ex vivo restimulation with OVA peptide. (o–q, n = 5 (DMSO+IgG and IDH2i+IgG), n = 6 (IDH2i + α-PD-1) and n = 8 (DMSO + α-PD-1) biological replicates per group). Data represent mean ± s.e.m. and were analysed by unpaired, two-tailed Student’s t-test (b,e–m), one-way ANOVA using Tukey’s multiple comparison test (c), two-way ANOVA corrected for multiple testing by the original FDR of Benjamini and Hochberg (o,p) or log-rank test (n). Only relevant statistical comparisons are shown.
Extended Data Fig. 7 Inhibition of IDH2 induces memory features in human T cells.
a,b, MFI of CD62L (a) and representative histogram (b) at day 9 post-activation on the surface of human CD8+ T cells isolated from healthy volunteer PBMCs, measured by flow cytometry. PBMCs from healthy donors were activated with anti-CD3/CD28 beads for 5 days and cultured 4 additional days prior phenotypic analyses. The cells were cultured in the presence of DMSO or IDH2i during the entire experiment (a–c). (n = 7 donors, pooled data from 2 independent experiments). c, Percentages of human CD8+ TSCM-like cells, characterized as CD45RA+, CD62L+, CD45RO−, CCR7+, CD95+, CD28+, CD27+. (n = 7 donors, pooled data from 2 independent experiments). d, Experimental set-up of NAML6 tumour experiment. Human T cells were transduced with anti-CD19 CAR T cells, expanded during 12 days in the presence of DMSO or IDH2i and subsequently transferred into tumour-bearing NSG mice. e–g, Human MILs from patients with multiple myeloma were activated with anti-CD3/CD28 beads for 5 days and cultured 4 additional days prior phenotypic analyses. The cells were cultured in the presence of DMSO or IDH2i during the entire experiment. Shown here is MFI of CD62L (e) and representative histogram (f), and percentage of TSCM (g) at day 9 post-activation in human CD8+ MILs. (n = 6 donors, pooled data from 2 independent experiments). h, Experimental set-up of NCI-H929 tumour experiment. Human T cells were transduced with anti-BCMA CAR T cells, expanded during 10 days in the presence of DMSO or IDH2i and subsequently transferred into tumour-bearing NSG mice. i, BLI quantification of total flux (p/s) (representative of 2 independent experiments). j–l, Fold-change expansion at day 9 post-activation of human PBMCs (j), human MILs (k) and human CAR T cells (l) cultured with DMSO or IDH2i. (n = 3 (j), n = 6 (k) and n = 2 donors (l), pooled data from 2 independent experiments (k). m, Representation (row z-scores) of differentially accessible regions in mouse CD8+ T cells after DMSO or IDH2i conditioning (ATAC-seq). n, Bar plots representing log2 fold change in mRNA expression of Sell, Tcf7 and Ccr7 upon IDH2i-compared to DMSO-conditioned CD8+ T cells (RNA-seq). Data represent mean ± s.e.m. and were analysed by paired two-tailed Student’s t-test (a,c,e,g,j–m).
Extended Data Fig. 8 Inhibition of IDH2 induces metabolic compensation.
a, MitoSOX MFI in DMSO- or IDH2i-treated cells upon addition of H2O2 at indicated concentrations, measured by flow cytometry. (n = 5 biological replicates, pooled data from 2 independent experiments). b, Number of live TFAM WT and TFAM KO T cells per millilitre upon DMSO or IDH2i treatment. (n = 3 biological replicates, pooled data from 2 independent experiments). c, Percentage of CD62L+ out of CD8+ TFAM WT and KO T cells upon DMSO or IDH2i treatment. (n = 3 biological replicates, pooled data from 2 independent experiments). d, Percentage of CD62L+ out of Thy1.1+ CD8+ T cells at day 7 post-activation upon deletion of indicated genes. (n = 6 (Scr, Ogdh and Sdhb) and n = 5 (Idh2) biological replicates per group, pooled data from 3 independent experiments). e, Immunoblots for OGDH and SDHB in control Scr and OGDH- or SDHB-deleted cells. (Representative of 2 independent experiments). f, Percentages of citrate m + 5 detected by [U-13C]-glutamine labelling in DMSO or IDH2i-treated T cells. (n = 3 biological replicates per group). g, Percentages of m + 3 succinate, fumarate and malate detected by 2 h [U-13C]-glutamine labelling in DMSO or IDH2i-treated T cells. (n = 3 biological replicates per group). h, Percentages of m + 4 succinate, fumarate and malate detected by 2 h [U-13C]-glutamine labelling in DMSO or IDH2i-treated T cells. (n = 3 biological replicates per group). i, Percentages of m + 5 α-KG detected by 2 h [U-13C]-glutamine labelling in DMSO or IDH2i-treated T cells. (n = 3 biological replicates per group). j, Percentages of m + 2 acetyl-CoA, detected by [U-13C]-glucose labelling for indicated time in DMSO or IDH2i-treated T cells. (n = 3 biological replicates per group). k, Percentages of m + 2 citrate, succinate, fumarate and malate detected by 2 h [U-13C]-glucose labelling in DMSO or IDH2i-treated T cells. (n = 3 biological replicates per group). l, Percentages of citrate m + 2 detected by [U-13C16]-palmitate labelling in mouse CD8+ T cells treated with DMSO or the IDH2 inhibitor (IDH2i). (n = 3 biological replicates per group). m–o, Quantification of basal OCR (m), ATP-linked OCR (n) and spare respiratory capacity (SRC; o) from data presented in Fig. 4d. (n = 4 biological replicates, pooled data from 2 independent experiments). p, Percentage of CD62L+ T cells in control Scr or ΟGDH-deleted CD8+ T cells, treated with IDH2i. (n = 4 biological replicates, pooled data from 2 independent experiments). q, Percentage of CD62L+ T cells in DMSO or IDH2i-conditioned CD8+ T cells, treated with etomoxir (FAOi). (n = 5 biological replicates, pooled data from 2 independent experiments). r, Glucose consumption in DMSO- or IDH2i-treated mouse CD8+ T cells over 24 h, at day 3 post-activation. (n = 3 biological replicates per group). s, Intracellular abundances of indicated amino acids (arbitrary units, a.u) measured by mass spectrometry upon DMSO or IDH2i treatment. (n = 9 biological replicates, pooled data from 3 independent experiments). t,u, CD98 MFI in DMSO- or AG-221- (t) or AGI-6780- (u) treated CD8+ T cells, measured by flow cytometry at day 3 post-activation. (n = 9 (AG-221) and n = 3 (AGI-6780) biological replicates, pooled data from 4 (AG-221) and 2 (AGI-6780) independent experiments). Data represent mean ± s.e.m. and were analysed by unpaired two-tailed Student’s t-test (f,i,l–o,r,t,u), multiple unpaired, two-tailed t-tests using Benjamini and Hochberg method (g,h,j,k,s), one-way ANOVA using Tukey’s multiple comparison test (d) or two-way ANOVA using the original FDR test of Benjamini and Hochberg (a–c,p,q). Only relevant statistical comparisons are shown. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 9 Inhibition of IDH2 leaves an epigenetic imprint.
a, Bar graphs representing permissive and repressive histone marks in DMSO or IDH2i-conditioned CD8+ T cells. b, Immunoblot and quantification of indicated histone marks in DMSO or IDH2i-conditioned CD8+ T cells. (n = 2 (H3K36me2), pooled from 2 independent experiments, n = 3 (H3K79me3, H3K14ac) and n = 5 (H3K27ac) biological replicates, pooled data from 3 independent experiments). c, Immunoblot and quantification of total acetylated lysines in DMSO or IDH2i-conditioned CD8+ T cells. (n = 3 biological replicates, data representing 2 independent experiments). d, Barcode plots representing gene set enrichment analysis of a memory signature on H3K4me3-enriched regions in OT1 cells. NES, normalized enrichment score; Running ES, running enrichment score. e, H3K4me3 ChIP–seq tracks at Sell, Ccr7 and Tcf7 loci from mouse naive (TN, green), effector (TE, blue) and memory (TM, pink) OT1 cells and from human naive (TN, green), effector memory (TEM, blue) and central memory (TCM, pink) CD8+ T cells from the peripheral blood of healthy adults. f, Immunoblot and quantification of H3K4me3 histone mark in DMSO- or IDH2i-conditioned MILs. (n = 5 biological replicates, pooled data from 2 independent experiments). g, Intracellular abundance of α-KG in DMSO or IDH2i-conditioned CD8+ T cells, supplemented with indicated doses of cell-permeable α-KG (n = 2 biological replicates). h, Immunoblot quantification of H3K4me3 in DMSO or IDH2i-conditioned CD8+ T cells, supplemented with α-KG. (n = 4 biological replicates, pooled data from 4 independent experiments). i, Immunoblot quantification of H3K4me3 in T cells treated with DMSO, fumarate or 2-HG. j, Percentage of CD62L+ T cells upon DMSO, fumarate or S-2-HG treatment and α-KG supplementation. (i,j, 3 biological replicates, pooled data from 3 independent experiments). k, Representative histogram indicating the relative expression of CD62L on the surface of CD8+ T cells treated with 0.5, 1 or 2 mM succinate (dimethyl succinate, Sigma-Aldrich) at day 7 post-activation. Data represent mean ± s.e.m. and were analysed by unpaired (c,i,j) or paired (d) two-tailed Student’s t-test or two-way ANOVA using the original FDR test of Benjamini and Hochberg (e–h,k). Only relevant statistical comparisons are shown. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 10 Compensatory metabolism upon IDH2 inhibition influences histone modification and memory T cell differentiation.
a, KDM5 activating potential represented by the ratio of fold increase in Km value for α-KG versus the sum of fold increased IC50 concentrations of inhibitory metabolites (2-HG, succinate, fumarate and malate), calculated as: (concentration α-KG/Km α-KG)/((concentration 2-HG/IC50 2-HG) + (concentration succinate/IC50 succinate) + (concentration fumarate/IC50 fumarate) + (concentration malate/IC50 malate)). Ratio based on cellular metabolite concentrations calculated from absolute metabolite quantifications. (n = 6 biological replicates, pooled data from 2 independent experiments). b, Immunoblot quantification of H3K4me3 in DMSO- and IDH2i-treated CD8+ T cells, supplemented with α-KG and/or KDM5 inhibitor. (n = 2 biological replicates, pooled data from 2 independent experiments). c,d, Immunoblot (c) and quantifications (d) of H3K27ac and H3K14ac in DMSO or IDH2i-conditioned CD8+ T cells, treated with etomoxir (FAOi) and supplemented with acetate. (n = 3 biological replicates). e, Immunoblot quantification of H3K27ac and H3K14ac in DMSO or IDH2i-conditioned CD8+ T cells, treated with ACLYi. (n = 3 biological replicates). f, Percentage of CD62L+ T cells in DMSO or IDH2i-conditioned CD8+ T cells, treated with ACLYi. (n = 5 biological replicates, pooled data from 2 independent experiments). g,h, Immunoblot (g) and quantification (h) of H3K27ac in DMSO, IDH2i-, DMSO+HATi-and IDH2i+HATi-conditioned CD8+ T cells. (n = 3 biological replicates, pooled data from 3 independent experiments). i, CD62L expression in DMSO, IDH2i-, DMSO+HATi- and IDH2i+HATi-conditioned cells. (HATi = histone acetyltranferase p300/CBP inhibitor, 8 μM C646). (Representative blot and histogram from 3 independent experiments with 3 biological replicates). j, Tumour growth curve of B16-OVA tumours from mice treated with either DMSO, IDH2i-, DMSO+HATi- or IDH2i+HATi-conditioned OVA-specific CD8+ T cells. (n = 13 (IDH2i), n = 14 (DMSO+HATi, IDH2i+HATi) and n = 15 (DMSO) biological replicates per group, pooled data from 2 independent experiments). k, Pluripotent naive CD8+ T cells are characterized by low metabolic activity and permissive H3K4me3 deposition at pro-memory genes, including Sell, Ccr7 and Tcf7. After activation, TE cells increase glutamine metabolism along both the oxidative and reductive pathways (represented by two red arrows on the left panel of the figure). RC generates citrate and acetyl-CoA, which is a key metabolite supporting cell growth and function. This specific TE cell metabolism creates a unique metabolite composition (represented by balanced levels of α-KG on one side and succinate, fumarate, malate and 2-HG on the other side) enabling the activity of the α-KG-dependent histone demethylase KDM5. KDM5-mediated demethylation of H3K4me3 in activated T cells induces a repressive chromatin state at pro-memory genes, which prevents the maintenance of pluripotency and facilitates terminal effector differentiation. Blocking RC through inhibition of IDH2 does not hinder T cell proliferation and function, as compensatory fatty acid oxidation can refuel acetyl-CoA pools, with the support of glutamine anaplerosis, which is now redirected entirely in the oxidative branch of the TCA cycle (represented by the larger red arrow on the right panel of the figure), altogether enhancing cellular OCR. This leads to a perturbation of the metabolite balance by increasing the levels of the TCA intermediates succinate, fumarate, malate and 2-HG (represented by a shift in the balance upon IDH2 inhibition). The accumulation of these inhibitory metabolites impairs KDM5 demethylase activity, and elevated H3K4me3 is likely to foster a permissive chromatin state at pro-memory genes, allowing for the maintenance of pluripotency and memory differentiation. Data represent mean ± s.e.m. and were analysed by unpaired two-tailed Students t-test (a,j) or two-way ANOVA using the original FDR test of Benjamini and Hochberg (b,d–f,h). Only relevant statistical comparisons are shown. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Figure 1
This file contains uncropped immunoblot images with size marker indications.
Supplementary Figure 2
This file contains figures exemplifying the flow cytometry gating strategies for mouse and human cells.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaccard, A., Wyss, T., Maldonado-Pérez, N. et al. Reductive carboxylation epigenetically instructs T cell differentiation. Nature 621, 849–856 (2023). https://doi.org/10.1038/s41586-023-06546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06546-y
- Springer Nature Limited
This article is cited by
-
A new era of cancer immunotherapy: combining revolutionary technologies for enhanced CAR-M therapy
Molecular Cancer (2024)
-
Fueling CARs: metabolic strategies to enhance CAR T-cell therapy
Experimental Hematology & Oncology (2024)
-
CD28 co-stimulation: novel insights and applications in cancer immunotherapy
Nature Reviews Immunology (2024)
-
Targeting ROS in cancer: rationale and strategies
Nature Reviews Drug Discovery (2024)
-
T cell dysfunction and therapeutic intervention in cancer
Nature Immunology (2024)