Abstract
Temperature affects the rate of all biochemical processes in ectotherms1,2 and is therefore critical for determining their current and future distribution under global climate change3,4,5. Here we show that the rate of biological processes maintaining growth, homeostasis and ageing in the permissive temperature range increases by 7% per degree Celsius (median activation energy Ea = 0.48 eV from 1,351 rates across 314 species). By contrast, the processes underlying heat failure rate within the stressful temperature range are extremely temperature sensitive, such that heat failure increases by more than 100% per degree Celsius across a broad range of taxa (median Ea = 6.13 eV from 123 rates across 112 species). The extreme thermal sensitivity of heat failure rates implies that the projected increase in the frequency and intensity of heatwaves can exacerbate heat mortality for many ectothermic species with severe and disproportionate consequences. Combining the extreme thermal sensitivities with projected increases in maximum temperatures globally6, we predict that moderate warming scenarios can increase heat failure rates by 774% (terrestrial) and 180% (aquatic) by 2100. This finding suggests that we are likely to underestimate the potential impact of even a modest global warming scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Main
Temperature has a profound influence on processes at all levels of biological organization, ranging from the simple catalytic rates of enzymes to the complex biological interactions that underlie metabolism, growth and reproduction of ectothermic animals1,2. The interactions between multiple temperature-sensitive biological rates ultimately shape thermal performance and determine the thermal limits for life and death in ectotherms1,7,8. Accordingly, thermal tolerance limits are robust predictors of the geographical distribution of ectothermic animals3,9,10, and climate change beyond tolerance limits can explain their current redistributions4,11.
Thermal sensitivity of life and death
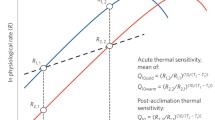
Temperature effects on biological rates are often described using Q10 (the factorial change in biological rate resulting from a 10 °C increase) but are more appropriately expressed by the Arrhenius activation energy Ea (ref. 2). When rates are measured within permissive temperatures, defined as temperatures that allow for long-term survival, Ea typically ranges from 0.5 to 0.8 eV (equivalent to Q10 ≈ 2–3) corresponding to a 7–12% rate increase per degree Celcius12,13,14. The consequences of global warming on the rate of energy metabolism in ectotherms are already implemented in contemporary analyses of ecosystems and agriculture14,15,16. However, temperature also affects biological rate functions at stressful temperatures, defined here as the temperature range causing acute heat injury and mortality. The temperature sensitivity of these processes is much more potent in ectothermic animals17,18,19 but has received little attention in the context of global warming.
The disparate temperature sensitivities in the permissive and stressful temperature range can be exemplified through a combined analysis of temperature effects on the population growth capacity20 and lifespan18 of adult fruit flies (Drosophila subobscura; Fig. 1a). Within the permissive temperature range for this species (3–28 °C), warming increases the rates of biological processes in a manner that initially enhances fitness, that is, the product of egg laying rate, developmental viability and developmental speed20. However, as temperature increases further, the balance between catabolic and anabolic rates shifts and net fitness decreases1,7,8,21 even if it remains positive. This declining fitness occurs even though many biological rates—such as feeding rate, heart rate, metabolic rate and ageing/mortality rate—continue to increase with the same thermal sensitivity throughout the permissive range22. Accordingly, when lifespan is analysed across the permissive temperature range, the increased rates of biological activities coincide with an acceleration of senescence and ageing23,24. In this example, the thermal sensitivity, Q10 = 2.5 for ageing/mortality rate (1/lifespan) (Fig. 1a), corresponds to an Arrhenius activation energy Ea of 0.66 eV (Fig. 1b). Similar moderate thermal sensitivities of ageing/mortality rate (1/lifespan) at permissive temperatures have been documented in a variety of ectothermic species (Ea = 0.56 ± 1 eV (mean ± s.d.) across 97 field and laboratory populations24).
a, Lifespan of adult fruit flies (D. subobscura) depicted on a log10 scale to indicate the exponential relationship between temperature and lifespan (right y axis; data are from ref. 18). The critical temperature (Tc) indicates the transition at which the temperature effect on lifespan (slope) diverts from that of biological processes in the permissive temperature range to become extremely high in the stressful temperature range. A thermal performance curve for reproductive fitness in D. subobscura in the permissive temperature range illustrates that this is the range of temperatures that permits completion of the life cycle (dashed green curve on the left y axis; data are from ref. 20). b, Temperature-specific death rates calculated as 1/lifespan from a were analysed in an Arrhenius plot. Activation energies Ea are indicated for the permissive (Ea = 0.66 eV) and stressful (Ea = 7.49 eV) temperature ranges separated at a breakpoint temperature Tc (28.2 °C) found using Davies’ test for a change in slope (P < 0.001).
There is a substantial shift in the influence of temperature on lifespan above a critical temperature Tc, defined as the temperature or narrow temperature zone that separates the permissive and stressful temperature range (Fig. 1). Although Tc is rarely parametrized experimentally (see the discussion in ref. 22), it represents a temperature at which biological processes dictating the ‘rate of death’ become dominant over those determining the ‘rate of life’. Heat failure rate above Tc is also calculated as 1/lifespan, and the Arrhenius breakpoint1,2 at Tc indicates that the heat failure rate is dictated by different biological processes that are extremely sensitive to temperature (Q10 = 8,726 (Fig. 1a) and Ea = 7.49 eV (Fig. 1b)). For D. subobscura, heat death occurs after 6 h at 33 °C, while 4 °C further warming reduces its lifespan to less than 10 min (Fig. 1a). Similar extreme thermal sensitivities of heat failure have been described in thermal death time curves for many other ectotherms19,25,26.
Analysis of activation energies
The fundamentally different thermal sensitivities for processes associated with life (permissive range) and death (stressful range) are not unique for D. subobscura (Fig. 1). Data compiled on 1,351 rates across different temperatures from 314 species show that the Ea of biological processes within the permissive temperature range (median Ea = 0.48 eV; interquartile range (IQR) = 0.28–0.71 eV; Fig. 2a,b) are indeed consistent with textbook values of Ea ≈ 0.5–0.8 eV (Q10 ≈ 2–3) for most ectothermic animals12,13,14. As previously discussed13,14, these thermal sensitivities mirror most biological processes, including enzyme catalytic rates and integrated biological functions, such as feeding rate and metabolic rate (Fig. 2a,b and Extended Data Table 1). However, note that the integrated effect of many underlying biological rates causes a decline in ‘fitness’ in the warmer part of the permissive temperature range. As a consequence, the population growth rate (fitness) is associated with Ea < 0 or Q10 < 1 at the warmest permissive temperatures (Box 1) even though many underlying biological rates continue to increase after fitness has peaked at the optimal temperature (Topt)22.
Data are organized in five ectotherm groups (fishes, crustaceans, molluscs, amphibians and insects) for which the most published data exist. a, Six representative examples of temperature sensitivity of biological processes measured within the permissive (non-stressful) temperature range (colour refers to the animal group and symbols to the trait; details are provided in Extended Data Table 1). b, Data from 1,351 literature estimates of Ea measured in the permissive temperature range from 314 species grouped by biological process. Coloured points represent averages in cases in which n ≥ 8 for that animal group. Data from ectotherms not belonging to the five groups are also included in the box plots. The dashed line indicates the global median (Ea = 0.48 eV, corresponding to Q10 = 1.9). The box plots summarize each categorized biological process; the centre line shows the median, the box limits represent the first and third quartiles, the whiskers extend to 1.5 × IQR and the grey points show outliers. c, Representative examples of heat failure rates and their activation energy (Ea) measured in the stressful temperature range (the same or closely related species as in a). d, Activation energies of heat failure rate organized by ectothermic group with all 123 Ea values shown (squares, from 112 unique species). The full line indicates the global median (Ea = 6.13 eV, corresponding to Q10 > 1,500). For reference to b, the grey area denotes the Ea range −1.3−4 eV, and the dashed line indicates the median Ea for processes in the permissive range.
In contrast to the modest temperature sensitivity of biological rates in the permissive temperature range, the rate of heat failure is extraordinarily temperature sensitive in the stressful temperature range (Fig. 2c,d). We compiled data on the thermal sensitivity of heat failure for 112 species (123 datasets in total) with the criteria that time to heat failure was measured at three or more constant test temperatures. Heat failure rates (min−1) were calculated as 1/time to heat failure (min) and the activation energy was subsequently calculated using an Arrhenius analysis (Fig. 2c). Heat failure rate has extreme thermal sensitivity across all of the ectotherms examined (Fig. 2d) with a median Ea = 6.13 eV (IQR = 4.42–8.82 eV) corresponding to a median Q10 > 1,500 and more than a doubling of heat failure rate per 1 °C of warming (median increase = 110%, IQR = 71–190%). The median duration of the heat failure experiments was 125 min (IQR = 31.5–422 min), with 122 out of 123 median durations less than 2.5 days, emphasizing that our estimates of heat failure rate are relevant for the acute heat exposures experienced during daily fluctuations and heatwaves25. All five ectothermic groups (fishes, crustaceans, molluscs, amphibians and insects) have a median Ea > 4.63 eV, but vertebrates are particularly sensitive to warming (median Ea = 10.06 eV and 10.30 eV for fishes and amphibians, respectively). This analysis also shows that Ea is high for both terrestrial (Ea = 5.53 eV; IQR = 4.13–6.42 eV) and aquatic species (Ea = 6.69 eV; IQR = 4.61–10.38 eV). Given the extraordinarily high thermal sensitivities in all taxonomic groups, we suggest that the extreme thermal sensitivity of heat failure rate is a general characteristic of all ectothermic animals.
The physiological causes of heat death in ectotherms are still poorly understood, but have been associated with protein denaturation, oxygen limitation, loss of cellular excitability and membrane dysfunction2,7,8,12,21,27. It is also unclear why the rates of these processes accelerate so substantially at extreme temperatures above Tc. Nevertheless, it is likely the same physiological dysfunctions that underlie chronic (hours) and acute (minutes) heat stress as exposure to different temperatures above Tc is additive in both fish19 and insects25. Furthermore, the absence of Arrhenius breakpoints2 above Tc suggests that heat failure is caused by a common heat stress syndrome that accelerates in intensity with an extreme thermal sensitivity. Importantly, many underlying biological rates typically begin to decrease within the stressful temperature range. Thus, metabolic rate, movement rate and heart rate, which typically increase throughout the permissive range22, will eventually decline as temperatures become acutely stressful. The thermal sensitivity of this rate decline in the stressful temperature range is typically higher than the thermal sensitivity of the rate increase occurring in the permissive temperature range13,22,28,29. However, it remains difficult to pinpoint whether the extreme increase in death rate at stressful temperatures substantially limits heart rate, metabolic rate and movement rate or vice versa, as the causalities of the physiological heat stress syndrome are currently poorly understood2,8,22,27.
Implications of global warming
In their active season, ectothermic animals are mostly confined to habitats with permissive temperatures that enable reproduction and population growth1,2,7. Even so, ectotherms may experience stressful temperatures (exceeding Tc) during heatwaves or diurnal/seasonal temperature extremes. Tolerance to extremes is therefore an important determinant of species distributions3,9, and thermal tolerance limits (CTmin and CTmax) often correlate stronger with distribution than the thermal optimum for population growth (Topt), a performance measure within the permissive temperature range20,30.
The severity of stressful temperatures depends on both the intensity (that is, the actual temperature) and the duration of the exposure17,19,22,25,26. The considerable thermal sensitivity of ectothermic heat failure rates more than doubles heat stress with every degree Celsius of warming. Accordingly, even modest increases in maximal exposure temperature—for example, as a result of moderate global warming—can substantially exacerbate the severity of heat injury. The potential magnitude of this problem was assessed by associating the median Ea for terrestrial and aquatic ectotherms with projected increases in maximum temperature for three IPCC warming scenarios (Fig. 3a and Extended Data Table 2). This analysis represents a worst-case scenario based on the assumption that species under current climate conditions experience temperatures equal to or above Tc on the warmest days within their distribution range. Terrestrial environments are projected to warm considerably more than aquatic environments6 (Fig. 3 and Extended Data Fig. 1), but median thermal sensitivity is higher for aquatic ectotherms implying that both aquatic and terrestrial ecosystems will experience substantial increases in heat failure rate (median percentage increase, 180% and 774%, respectively, under the SSP2–4.5 scenario6; Fig. 3a). Furthermore, the more homogenous thermal conditions in aquatic habitats leave considerably fewer options for behavioural mitigation to avoid stressful temperature exposure31. These increases in heat failure rate are much more substantial than the projected 6% and 32% increases in permissive biological rates estimated for aquatic and terrestrial ectotherms, respectively, in association with increases in mean temperature (Fig. 3a and Extended Data Fig. 2).
a, Percentage increases in biological rates associated with future climate change in terrestrial and aquatic environments (in 2081–2100 and 2090–2100, respectively). The temperature change ΔT for three warming scenarios6 corresponds to changes in the mean and maximum temperature for the permissive and stressful range, respectively. SSP1–2.6 is within the limits of the Paris Agreement, whereas SSP2–4.5 and SSP5–8.5 represent intermediate and severe emission scenarios, respectively. Percentage increases in rates (median and IQR) are based on the baseline temperature, ΔT and environment-specific Ea for the permissive and stressful temperature range (Methods; see Extended Data Figs. 2 and 4 for global maps). b, Analysis evaluating the risk of exposure to temperatures above the critical temperature Tc (estimated as the temperature resulting in heat failure in 24 h) for two example species, G. nigricans and P. megacephala, in current and future (SSP2–4.5) climates. Occurrence locations are coloured according to the comparison between Tc and maximal environmental temperature (Tenv max). Grey, Tc > Tenv max in both current and future climates; maroon, Tc < Tenv max in the current climate; red, Tc < Tenv max in future climates. The global distribution of P. megacephala is shown in Extended Data Fig. 3. c, Increases in heat failure rate resulting from SSP2–4.5-projected increase in maximal temperature above Tc using global occurrences and thermal sensitivities for G. nigricans and P. megacephala (Methods). Colours are as described in b. For occurrences in red, the increase in maximal temperature is the difference between future maximum temperature and Tc. For occurrences in maroon (which already experiences temperatures of >Tc), the additional increase in temperature between current and future maximum temperature was used.
To demonstrate that the risk of exposure to temperatures above Tc in current and future climate varies within the species distribution, Fig. 3b presents an analysis of two species (Girella nigricans and Pheidole megacephala). These species-level examples were generated by contrasting current and future (SSP2–4.5 scenario) estimates of maximal environmental temperature against a conservative approximation of Tc (here calculated as the temperature that causes heat failure in 24 h). Although some populations already experience temperatures above Tc in their current distribution, climate warming will result in more populations experiencing temperatures exceeding Tc (Fig. 3b and Extended Data Fig. 3). As evident from Fig. 3c, the consequences of future warming will depend on the current climate and the projected warming but, for some populations, projected warming will exacerbate the heat failure rate relative to current conditions by up to 2,100% and 690% for G. nigricans and P. megacephala, respectively (Fig. 3c). To put this into context, a 1,000% (tenfold) increase in heat failure rate entails that an ectotherm accumulating 15% of its lethal thermal injury on a very hot day under current climate conditions, will instead experience 150% of its lethal dose over the same duration under the future warming scenario. As a corollary, a 1,000% increase in failure rate implies that an ectotherm currently surviving for 5 h during a hot day will instead succumb to heat death within 30 min under the future warming scenario.
The general risk analysis for ectotherms in Fig. 3a suggests that both terrestrial and aquatic species may experience substantial increases in the intensity of injurious heat stress. Although terrestrial ectotherms can often escape short-term heat exposures by seeking permissive microhabitats (<Tc)3,31,32,33,34,35, warming may reduce the availability of such microhabitats. In both terrestrial and aquatic environments, there is considerable spatial variation in regional climate warming with projected increases in maximum temperature greater than 8 °C in some regions even in the SSP2–4.5 scenario6 (Extended Data Fig. 1). As a consequence, the potential increase in heat failure rate for species living close to their Tc can be even more extreme locally, particularly across temperate terrestrial environments in the Northern Hemisphere and in aquatic environments across the Arctic (Extended Data Figs. 4 and 5).
Using air and sea surface maximum temperatures may further underestimate the exposure to stressful temperature as it does not account for temperatures experienced in particular warm microclimates, nor does it consider that solar radiation and convective heat transfer3,31,33 can increase the operative temperature considerably above air temperature. By contrast, the risk estimate presented here does not directly account for mitigation through behavioural selection of permissive microhabitats32,33,34,35 or for acclimation/adaptive responses that could alter thermal tolerance2,7. Species-specific implications of future heatwaves should therefore consider the local risk of exposure to extreme events beyond Tc (Fig. 3b). Nevertheless, most ecosystems will probably include species that are at risk of exposure to temperatures beyond Tc (ref. 3).
The risk analysis presented here is mainly relevant for species that experience temperatures above Tc in their current or future environment (Fig. 3b), and the notable implications primarily pertain to the periods during which environmental temperature is highest. The effect of global warming on processes of life and death should therefore ideally integrate positive and negative warming effects within both the permissive and stressful temperatures (Box 1). Even so, our analysis highlights that heat stress is likely to escalate substantially with even a modest degree of global warming (Fig. 3). The effects of warming on heat failure rates are several magnitudes greater than the temperature effects previously considered when analysing warming of permissive biological processes. As a consequence, both aquatic and terrestrial ectotherms risk considerable increases in heat stress with global warming and this increase will be accentuated markedly on the regional scale and with each degree of further global warming.
Methods
Data collection for the meta-analysis
To estimate the thermal sensitivity of permissive biological rates, we collected data for a meta-analysis of processes covering enzyme activity, heart rate, locomotion, feeding and metabolic rate for a wide range of ectothermic animal species. The dataset includes 1,351 entries of biological rates measured at two temperatures and represents 314 species examined in 304 original publications. Data were mostly sourced from two large collections of published data compiled by Dell et al. (see Supporting Information in ref. 13) (here we used only Ea of the ascending rates derived from trait performance curves) and by Seebacher et al. (see Supplementary Information in ref. 14), and overlapping entries were removed. A few (n = 4) additional entries were included as they were used as examples in Fig. 2a.
To estimate the thermal sensitivity of heat failure rates in the highly stressful temperature range, we compiled data on time to heat failure with associated test temperatures. This dataset includes 123 thermal sensitivities for 112 species. Data were compiled from 69 individual studies and an additional 54 studies sourced from references reported by Rezende et al. (see Supporting Information in ref. 26), and were included only if heat failure times were available for at least three temperatures.
Calculation of E a
The Arrhenius activation energy Ea was calculated to quantify the thermal sensitivity of rates related to either permissive or stressful biological processes. The Ea values of ascending rates (in the permissive temperature range) originating from Supporting Information in ref. 13 were available from the publication, whereas Ea values for all other rates were calculated using a linear regression in an Arrhenius analysis. The Arrhenius analysis was performed by regressing thenatural logarithm to the rate against the reciprocal temperature (1/temperature (K−1)). The regression slope was then used to calculate the activation energy Ea
Where R is the gas constant (8.31 J K−1 mol−1), NA is the Avogadro constant (6.022 × 1023 mol−1), and C is a conversion factor to report Ea in eV (1.602 × 10−19 J eV−1).
To estimate activation energy Ea for heat failure rates in the stressful temperature range, we calculated heat failure rates (min−1) by converting the collected heat failure times (min) as
Accordingly, heat failure rate represents the incremental heat stress that accumulates per minute at a specific constant temperature and, once the increments sum to 1, heat failure occurs (that is, the number of increments (time) to sum to 1 equals the heat failure time). For example, if heat failure time is 100 min at 38 °C, then the corresponding heat failure rate at 38 °C is 1/100 min = 0.01 min−1 and, therefore, accumulating these increments of heat stress over a 100 min exposure to 38 °C results in summation to 1 = heat failure.
The median heat failure times used to calculate Ea vary between studies (median = 125 min, IQR = 31.5–422 min) but a linear regression of log10[median duration] against Ea did not reveal any significant correlation (F1,121 = 0.36, P = 0.55, R2 < 0.01), and we therefore conclude that high Ea is not an artefact of test duration.
Converting E a to estimates of Q 10
In mainstream literature, thermal sensitivities are often presented using the thermal sensitivity quotient Q10 (that is, the factorial change in rate associated with a 10 °C temperature change). To discuss thermal sensitivities using the more commonplace Q10, we converted activation energy Ea using
Where Ea is the activation energy (eV), kB is the Boltzmann constant (8.617 × 10−5 eV K−1) and T is the temperature (K). This conversion is sensitive to temperature and here we used the temperature T = 18.3 °C (291.5 K) for conversion to permissive Q10 and T = 36.3 °C (309.5 K) for stressful Q10. These temperatures were chosen as they represent the mean temperature used to measure the rates in the permissive and stressful temperature range, respectively.
Modelling projected temperature change
To model the impact of increased intensity of heatwaves, we associated the predicted rise in future temperature with the thermal sensitivity Ea in terrestrial and aquatic environments. To make this change spatially and temporally explicit, we used projected global changes in mean and maximum temperature for three different emission scenarios (see below) towards the end of the twenty-first century compared with present-day conditions (Extended Data Fig. 1).
For terrestrial areas, we used the WorldClim v.2.1 climate database (https://worldclim.org)49, based on monthly averages, using the bioclimatic variables ‘mean annual temperature’ (BIO1) and ‘maximum temperature of the warmest month’ (BIO5). In WorldClim, present conditions are produced with monthly averages for the latest climate period 1970–2000. Future layers of mean and maximum temperature (BIO1 and BIO5, respectively) were produced by averaging data from eight general circulation models (GCMs) (Extended Data Table 2) for the period 2081–2100. We used projected changes for three future Shared Socioeconomic Pathways (SSP) scenarios6: (1) the optimistic SSP1–2.6, a peak-and-decline scenario ending with low greenhouse gas concentration levels by the end of the twenty-first century; (2) the SSP2–4.5 ‘middle of the road’ scenario where trends do not shift markedly from historical patterns; and (3) the pessimistic and perhaps unrealistic SSP5–8.5, where fossil-fuelled development increases emissions over time leading to high greenhouse gas concentrations (for discussions on the use and misuse of emission scenarios, see refs. 50,51,52).
For aquatic areas, we used the Bio-ORACLE v.2.0 database (https://bio-oracle.org/)53,54, based on monthly averages, using the variables average and maximum sea surface water temperature (SST). In Bio-ORACLE, present conditions are produced with monthly averages for the period 2000–2014. Future layers of mean and maximum SST were produced by averaging data from three atmosphere–ocean coupled GCMs (AO-GCMs) (Extended Data Table 2) for the period 2090–2100. The SSPs are not yet available for aquatic environments, so we used the corresponding Representative Concentration Pathway (RCP) scenarios (RCP2.6, RCP4.5, and RCP8.5, respectively) that precede the SSP scenarios (hereafter, we refer to all scenarios by the corresponding SSP). In terms of temperature change by the end of the twenty-first century, the SSPs and RCPs yield practically identical predictions52. All spatial data were used at a 5 arcmin resolution in a Behrmann equal area cylindrical projection (approximately 9.2 km) with the WGS84 datum.
Exposure to temperatures above T c
In two example species (G. nigricans and P. megacephala), we estimated exposure to environmental temperatures above the critical temperature Tc separating the permissive and stressful temperature range. In this analysis we first established a proxy of Tc representing the temperature above which heat stress accumulates. Specifically, Tc (K) is estimated as the temperature causing heat failure after 24 h, using the slope and intercept from the linear regression in the Arrhenius analysis
Where R′ is the rate calculated to result in heat failure after 24 h (that is, R′ = 1/1,440 min, compare with equation (2)). This approximation of Tc is conservative as the linearity of heat failure rates often extends beyond 24 h (for example, Fig. 1a), suggesting that we may underestimate the risk of exposure to temperatures above Tc. However, the potent nature of heat failure versus temperature discourages excessive extrapolation of such data (see the discussions in refs. 17,25).
For the species-level risk assessment, we then obtained occurrence records from the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/; downloaded 20 March 2022). After removal of faulty records, we found 647 and 2,063 occurrences for G. nigricans and P. megacephala, respectively, from which we extracted the maximum temperature in the current climate and from the SSP2–4.5 future warming scenario (BIO5 (terrestrial) and maximum SST (aquatic) for P. megacephala and G. nigricans, respectively). Temperature data were aggregated within 46 × 46 km and 92 × 92 km cells for G. nigricans and P. megacephala, respectively, to avoid sampling bias, resulting in 93 and 403 cells for G. nigricans and P. megacephala, respectively. The maximum environmental temperatures at these locations were evaluated against the species-specific estimates of Tc to determine which of the occurrence locations experience temperatures ≥Tc now and under future warming. The increase in maximal environmental temperature above Tc was associated with the resulting increase in heat failure rates using species-specific Ea estimates (8.72 eV and 3.67 eV for G. nigricans and P. megacephala, respectively), and Tc (31.5 °C and 34.4 °C for G. nigricans and P. megacephala, respectively). For the parts of the species-distribution ranges in which populations experience temperatures above Tc only after future climate warming, the increase in maximal temperature was calculated as the difference between the future maximum temperature and Tc. For the populations in which maximal temperature already exceeds Tc, the increase in temperature was calculated from the increase between current and projected future maximum temperatures.
Associating temperature change with E a
The projections on future percentage increases in biological rates in the permissive temperature range were based on the mean annual temperature, whereas projections for increases in heat failure rates were based on the maximum temperatures (Extended Data Fig. 1a,b, respectively). The projected change in local temperature (ΔT) for the three future scenarios (SSP1–2.6, SSP2–4.5 and SSP5–8.5) was determined as follows:
Where Tfuture is the mean annual or maximum temperature for the specific future climate scenario, and Tpresent is the current mean annual or maximum temperature, and both were calculated separately for the terrestrial and aquatic environment. The current mean annual temperature was described by BIO1 or SSTmean for terrestrial and aquatic environments, respectively, and the current maximum temperature was described by BIO5 or SSTmax for terrestrial and aquatic environments, respectively (see the ‘Modelling projected temperature change’ section; Extended Data Fig. 1c–h).
Subsequently, the projected change in temperature ΔT (mean and maximum for terrestrial and aquatic environments separately) was associated with the activation energy Ea (median and first–third quartile) for the specific group to calculate the increase in rate, for example, Ea for heat failure rate in terrestrial ectotherms was associated with ΔT based on the maximum temperature in the terrestrial environment. The projected percentage increase in rates (in the permissive and stressful range) was calculated as follows:
Where Ea is the activation energy (eV), kB is the Boltzmann constant (8.617 × 10−5 eV K−1), ΔT is the projected change in temperature (K) between the current and future climate scenario, and T2 and T1 are the future and current temperature [K], respectively. The following values of Ea were used for rates in the permissive temperature range: Ea = 0.56839 eV (terrestrial) and Ea = 0.44329 eV (aquatic); and for heat failure rates: Ea = 5.52589 eV (terrestrial) and Ea = 6.68649 eV (aquatic). These values are also presented in Fig. 3a, and the projected percentage increases in rates resulting from all three future scenarios are shown in Extended Data Fig. 2 (biological rates in the permissive temperature range) and in Extended Data Fig. 4 (heat failure rates in the stressful temperature range).
Equation (6) was also used to calculate the percentage increase in rates from a 1 °C temperature increase, using the median Ea for the permissive biological rates (Ea = 0.48 eV) or heat failure rates (Ea = 6.13 eV) disregarding the specific environment and using the temperatures listed in the ‘Converting Ea to estimates of Q10’ section.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available online55.
References
Angilletta, M. J. Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford Univ. Press, 2009).
Cossins, A. R. & Bowler, K. Temperature Biology of Animals (Chapman and Hall, 1987).
Sunday, J. M. et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615 (2014).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (2005).
Kellermann, V. et al. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16228–16233 (2012).
IPCC. Climate Change 2021: The Physical Science Basis (eds Masson-Delmotte, V. et al.) (Cambridge Univ. Press, 2021).
Hofmann, G. E. & Todgham, A. E. Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu. Rev. Physiol. 72, 127–145 (2010).
Schulte, P. M. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866 (2015).
Sunday, J. et al. Thermal tolerance patterns across latitude and elevation. Philos. Trans. R. Soc. B 374, 20190036 (2019).
Parratt, S. R. et al. Temperatures that sterilize males better match global species distributions than lethal temperatures. Nat. Clim. Change 11, 481–484 (2021).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690 (2012).
Schmidt-Nielsen, K. Animal physiology: Adaptation and Environment 5th edn (Cambridge Univ. Press, 1997).
Dell, A. I., Pawar, S. & Savage, V. M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. USA 108, 10591–10596 (2011).
Seebacher, F., White, C. R. & Franklin, C. E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66 (2014).
Dillon, M. E., Wang, G. & Huey, R. B. Global metabolic impacts of recent climate warming. Nature 467, 704–706 (2010).
Deutsch, C. A. et al. Increase in crop losses to insect pests in a warming climate. Science 361, 916–919 (2018).
Jørgensen, L. B., Malte, H. & Overgaard, J. How to assess Drosophila heat tolerance: unifying static and dynamic tolerance assays to predict heat distribution limits. Funct. Ecol. 33, 629–642 (2019).
Hollingsworth, M. J. Temperature and length of life in Drosophila. Exp. Gerontol. 4, 49–55 (1969).
Fry, F. E. J., Hart, J. S. & Walker, K. F. Lethal Temperature Relations for a Sample of Young Speckled Trout, Salvelinus fontinalis 9–35 (Univ. Toronto, 1946).
MacLean, H. J. et al. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Philos. Trans. R. Soc. B 374, 20180548 (2019).
Pörtner, H.-O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008).
Ørsted, M., Jørgensen, L. B. & Overgaard, J. Finding the right thermal limit: a framework to reconcile ecological, physiological, and methodological aspects of CTmax in ectotherms. J. Exp. Biol. 225, jeb244514 (2022).
Brown, J. H., Gillooly, J. F., Alle, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Munch, S. B. & Salinas, S. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc. Natl Acad. Sci. USA 106, 13860–13864 (2009).
Jørgensen, L. B., Malte, H., Ørsted, M., Klahn, N. A. & Overgaard, J. A unifying model to estimate thermal tolerance limits in ectotherms across static, dynamic and fluctuating exposures to thermal stress. Sci. Rep. 11, 12840 (2021).
Rezende, E. L., Castañeda, L. E. & Santos, M. Tolerance landscapes in thermal ecology. Funct. Ecol. 28, 799–809 (2014).
Bowler, K. Heat death in poikilotherms: is there a common cause? J. Therm. Biol. 76, 77–79 (2018).
Somero, G. N. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (2010).
Buckley, L. B., Huey, R. B. & Kingsolver, J. G. Asymmetry of thermal sensitivity and the thermal risk of climate change. Glob. Ecol. Biogeogr. 31, 2231–2244 (2022).
Overgaard, J., Kearney, M. R. & Hoffmann, A. A. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Glob. Change Biol. 20, 1738–1750 (2014).
Pinsky, M. L., Eikeset, A. M., McCauley, D. J., Payne, J. L. & Sunday, J. M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019).
Huey, R. B. et al. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B 367, 1665–1679 (2012).
Kearney, M., Shine, R. & Porter, W. P. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 (2009).
Woods, H. A., Dillon, M. E. & Pincebourde, S. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J. Therm. Biol 54, 86–97 (2015).
Stevenson, R. D. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 126, 362–386 (1985).
Chen, I., Hill, J. K., Ohlemüller, R., Roy, D. B. & Thomas, C. D. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011).
Buckley, L. B. & Kingsolver, J. G. Functional and phylogenetic approaches to forecasting species’ responses to climate change. Annu. Rev. Ecol. Evol. Syst. 43, 205–226 (2012).
Roeder, K. A., Bujan, J., de Beurs, K. M., Weiser, M. D. & Kaspari, M. Thermal traits predict the winners and losers under climate change: an example from North American ant communities. Ecosphere 12, e03645 (2021).
Penick, C. A., Diamond, S. E., Sanders, N. J. & Dunn, R. R. Beyond thermal limits: comprehensive metrics of performance identify key axes of thermal adaptation in ants. Funct. Ecol. 31, 1091–1100 (2017).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (2008).
Huey, R. B. & Stevenson, R. D. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Integr. Comp. Biol. 19, 357–366 (1979).
Sinclair, B. J. et al. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372–1385 (2016).
Tewksbury, J. J., Huey, R. B. & Deutsch, C. A. Putting the heat on tropical animals the scale of prediction. Science 320, 1296–1297 (2008).
Kingsolver, J. G., Diamond, S. E. & Buckley, L. B. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415–1423 (2013).
Kingsolver, J. G. & Woods, H. A. Beyond thermal performance curves: modeling time-dependent effects of thermal stress on ectotherm growth rates. Am. Nat. 187, 283–294 (2016).
Kingsolver, J. G., Higgins, J. K. & Augustine, K. E. Fluctuating temperatures and ectotherm growth: distinguishing non-linear and time-dependent effects. J. Exp. Biol. 218, 2218–2225 (2015).
Clusella-Trullas, S., Garcia, R. A., Terblanche, J. S. & Hoffmann, A. A. How useful are thermal vulnerability indices? Trends Ecol. Evol. 36, 1000–1010 (2021).
Pincebourde, S. & Casas, J. Narrow safety margin in the phyllosphere during thermal extremes. Proc. Natl Acad. Sci. USA 116, 5588–5596 (2019).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Moss, R. H. et al. The next generation of scenarios for climate change research and assessment. Nature 463, 747–756 (2010).
Hausfather, Z. & Peters, G. P. Emissions—the ‘business as usual’ story is misleading. Nature 577, 618–620 (2020).
Tollefson, J. How hot will Earth get by 2100? Nature 580, 443–445 (2020).
Assis, J. et al. Bio‐ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2018).
Tyberghein, L. et al. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 21, 272–281 (2012).
Jørgensen, L. B., Ørsted, M., Malte, H., Wang, T. & Overgaard, J. Data from: Extreme escalation of heat failure rates in ectotherms with global warming. Zenodo https://doi.org/10.5281/zenodo.6979789 (2022).
Grove, T. J., McFadden, L. A., Chase, P. B. & Moerland, T. S. Effects of temperature, ionic strength and pH on the function of skeletal muscle myosin from a eurythermal fish, Fundulus heteroclitus. J. Muscle Res. Cell Motil. 26, 191–197 (2005).
Doudoroff, P. The resistance and acclimatization of marine fishes to temperature changes. II. Experiments with Fundulus and Atherinops. Biol. Bull. 88, 194–206 (1945).
Sirikharin, R., Söderhäll, I. & Söderhäll, K. Characterization of a cold-active transglutaminase from a crayfish, Pacifastacus leniusculus. Fish Shellfish Immunol. 80, 546–549 (2018).
Becker, C. D. & Genoway, R. G. Resistance of crayfish to acute thermal shock: preliminary studies. in Proc. Thermal Ecology NTIS Conf. 730505 (eds Gibbons, J. W. & Sharitz, R. R.) 146–150 (NTIS, 1974).
Widdows, J. Effect of temperature and food on the heart beat, ventilation rate and oxygen uptake of Mytilus edulis. Mar. Biol. 20, 269–276 (1973).
Wallis, R. L. Thermal tolerance of Mytilus edulis of eastern Australia. Mar. Biol. 30, 183–191 (1975).
Gray, J. The mechanism of ciliary movement. III. The effect of temperature. Proc. R. Soc. B 95, 6–15 (1923).
Shertzer, R. H., Hart, R. G. & Pavlick, F. M. Thermal acclimation in selected tissues of the leopard frog Rana pipiens. Comp. Biochem. Physiol. A 51, 327–334 (1975).
Orr, P. R. Heat death. II. Differential response of entire animal (Rana pipiens) and several organ systems. Physiol. Zool. 28, 294–302 (1955).
Lighton, J. R. B. & Duncan, F. D. Energy cost of locomotion: validation of laboratory data by in situ respirometry. Ecology 83, 3517–3522 (2002).
Heatwole, H. & Harrington, S. Heat tolerances of some ants and beetles from the pre-Saharan steppe of Tunisia. J. Arid Environ. 16, 69–77 (1989).
Acknowledgements
We thank our colleagues at the Section for Zoophysiology, Aarhus University for the many discussions on temperature biology of animals. This work was funded by The Danish Council for Independent Research—Natural Sciences (to J.O.) and The Danish Council for Independent Research—Technology and Production Sciences (to M.Ø.).
Author information
Authors and Affiliations
Contributions
L.B.J., M.Ø. and J.O. conceptualized the study and all of the authors participated in its design. L.B.J., M.Ø., H.M. and J.O. collected the data and performed the analysis. L.B.J. curated the data. L.B.J., M.Ø., T.W. and J.O. wrote and visualized the original draft, and all of the authors contributed to the review and editing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Raymond Huey, David Vasseur and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Current and projected change in mean and maximum temperature under climate warming.
a, Current mean annual temperature described by BIO1 or SSTmean for terrestrial and aquatic environments, respectively. b, Current maximum temperature described by BIO5 or SSTmax for terrestrial and aquatic environments, respectively. (a, b) share the legend immediately below. c–d, Projected change in (c) mean annual temperature and (d) maximum temperature under the SSP1-2.6 scenario. e–f, Projected change in (e) mean annual temperature and (f) maximum temperature under the SSP2-4.5 scenario. g–h, Projected change in (g) mean annual temperature and (h) maximum temperature under the SSP5-8.5 scenarios. (c–h) share the bottom legend and the future period is 2081-2100 for terrestrial environments and 2090-2100 for aquatic environments, as they appear in WorldClim 2.149 and Bio-ORACLE 2.053,54, respectively. White areas indicate that temperature data were not available.
Extended Data Fig. 2 Projected increase in biological rates of permissive processes under climate warming.
Increase in biological rates (in %) of permissive processes for both terrestrial (Ea = 0.57 eV) and aquatic species (Ea = 0.44 eV) resulting from changes in annual mean temperature under the (a) SSP1-2.6, (b) SSP2-4.5 and (c) SSP5-8.5 scenario. The future period is 2081-2100 for terrestrial environments and 2090-2100 for aquatic environments, as they appear in WorldClim 2.149 and Bio-ORACLE 2.053,54, respectively. White areas indicate that temperature data were not available to calculate the increase in biological rate.
Extended Data Fig. 3 Risk of exposure to environmental temperatures above Tc for Pheidole megacephala.
Global risk analysis evaluating exposure to environmental (air) temperatures beyond the critical temperature Tc (separating the permissive and stressful temperature range, here calculated as the temperature causing heat failure in 24 h) in current and future climates (2081-2100, SSP2-4.5). Occurrence locations in the global distribution of P. megacephala are coloured according to the comparison of Tc to maximal air temperature (Tair max). Grey, Tc > Tair max in current and future climates; red, Tc < Tair max in the future climate scenarios; maroon, Tc < Tair max in the current climate. Occurrence records were aggregated to 184 km cells for increased visibility, and a section of the distribution found in Southern Africa is shown in Fig. 3b, with slight discrepancies due to different spatial resolutions of occupied cells.
Extended Data Fig. 4 Projected increase in heat failure rates under climate warming.
Increase in heat failure rates (in %) for both terrestrial (Ea = 5.53 eV) and aquatic species (Ea = 6.69 eV) resulting from changes in maximum temperature under the (a) SSP1-2.6, (b) SSP2-4.5 and (c) SSP5-8.5 scenario. The future period is 2081-2100 for terrestrial environments and 2090-2100 for aquatic environments, as they appear in WorldClim 2.149 and Bio-ORACLE 2.053,54, respectively. White areas indicate that temperature data were not available to calculate the heat failure rate increase.
Extended Data Fig. 5 Summary of increases in heat failure rate across latitudes.
Boxplots of terrestrial and aquatic heat failure rates under the SSP2-4.5 warming scenario across five latitudinal clines summarizing the results reported in Extended Data Fig. 4b. The boxplot midline represents the median, the lower and upper line of the box represents the 1st and 3rd quartile, respectively (with whiskers extending up to 1.5 times this range), outliers not shown.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jørgensen, L.B., Ørsted, M., Malte, H. et al. Extreme escalation of heat failure rates in ectotherms with global warming. Nature 611, 93–98 (2022). https://doi.org/10.1038/s41586-022-05334-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05334-4
- Springer Nature Limited
This article is cited by
-
Parabacteroides distasonis regulates the infectivity and pathogenicity of SVCV at different water temperatures
Microbiome (2024)
-
Acceleration of Drosophila subobscura evolutionary response to global warming in Europe
Nature Climate Change (2024)
-
Variation in temperature of peak trait performance constrains adaptation of arthropod populations to climatic warming
Nature Ecology & Evolution (2024)
-
Metabolic plasticity drives mismatches in physiological traits between prey and predator
Communications Biology (2024)
-
How to quantify the regional effects of ocean temperature rise due to climate change: implications of Octopus maya ecophysiology on food security of the Yucatan shelf artisanal fishermen
Regional Environmental Change (2024)