Abstract
Mutations in the death receptor FAS1,2 or its ligand FASL3 cause autoimmune lymphoproliferative syndrome, whereas mutations in caspase-8 or its adaptor FADD—which mediate cell death downstream of FAS and FASL—cause severe immunodeficiency in addition to autoimmune lymphoproliferative syndrome4,5,6. Mouse models have corroborated a role for FADD–caspase-8 in promoting inflammatory responses7,8,9,10,11,12, but the mechanisms that underlie immunodeficiency remain undefined. Here we identify NEDD4-binding protein 1 (N4BP1) as a suppressor of cytokine production that is cleaved and inactivated by caspase-8. N4BP1 deletion in mice increased the production of select cytokines upon stimulation of the Toll-like receptor (TLR)1–TLR2 heterodimer (referred to herein as TLR1/2), TLR7 or TLR9, but not upon engagement of TLR3 or TLR4. N4BP1 did not suppress TLR3 or TLR4 responses in wild-type macrophages, owing to TRIF- and caspase-8-dependent cleavage of N4BP1. Notably, the impaired production of cytokines in response to TLR3 and TLR4 stimulation of caspase-8-deficient macrophages13 was largely rescued by co-deletion of N4BP1. Thus, the persistence of intact N4BP1 in caspase-8-deficient macrophages impairs their ability to mount robust cytokine responses. Tumour necrosis factor (TNF), like TLR3 or TLR4 agonists, also induced caspase-8-dependent cleavage of N4BP1, thereby licensing TRIF-independent TLRs to produce higher levels of inflammatory cytokines. Collectively, our results identify N4BP1 as a potent suppressor of cytokine responses; reveal N4BP1 cleavage by caspase-8 as a point of signal integration during inflammation; and offer an explanation for immunodeficiency caused by mutations of FADD and caspase-8.
Similar content being viewed by others
Main
Caspase-8 mediates apoptosis by death receptors that include TNFR1 and FAS14. Mutations in FAS1,2 or FASL3 cause autoimmune lymphoproliferative syndrome in humans, as a result of the aberrant culling of T lymphocytes15. By contrast, mutations in caspase-8 or its adaptor FADD cause more complex diseases that—in addition to autoimmune lymphoproliferative syndrome—include increased susceptibility to recurrent viral and bacterial infections, and very early onset inflammatory bowel disease4,5,6. FADD- or caspase-8-deficiency in mouse tissues also causes intestinal inflammation16,17, increased susceptibility to infection and diminished responses to TLR stimuli8,10,11,13. Under certain conditions, TLR3 and TLR4 activate caspase-8 via the adaptor TRIF18,19,20, but in general TLR3 and TLR4 signalling does not cause apoptosis. Moreover, substrates of caspase-8, the cleavage of which might be crucial for normal TLR responses and innate immunity, remain unknown.
N4BP1 as a caspase-8 cleavage substrate
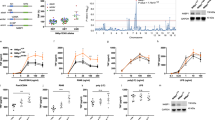
To identify substrates of caspase-8 during TLR4 signalling, we compared untreated mouse bone-marrow-derived macrophages (BMDMs) with BMDMs that were stimulated for 1 h with the TLR4 agonist lipopolysaccharide (LPS), either with or without the pan-caspase inhibitor emricasan. Cell lysates were enzymatically digested with trypsin, and then peptides with a C-terminal aspartic acid residue were immunoprecipitated to enrich for substrates of caspases21,22 (Fig. 1a). Mass spectrometry revealed that the two most abundant peptides specific to treatment with LPS alone were from N4BP1, ending at residues Asp488 and Asp200 (Fig. 1b, c). Residues of N4BP1 that precede Asp488 and Asp200 closely match consensus sequences of predicted caspase substrates and are conserved in human N4BP123 (Extended Data Fig. 1a, b). Western blotting confirmed that treatment with LPS increased levels of an approximately 54-kDa N4BP1 fragment within 30 min, which would be consistent with cleavage after Asp488 (Extended Data Fig. 1c).
a, Experimental workflow for results shown in b, c. Mouse BMDMs from wild-type C57BL/6N mice were treated for 1 h with LPS or with LPS and emricasan. Lysates from collected cells were trypsin-digested and peptides were immunoprecipitated with an antibody that recognizes C-terminal aspartic acid residues. Immunoprecipitates were subjected to mass spectrometric analysis. LC–MS/MS, liquid chromatography with tandem mass spectrometry. b, c, Volcano plots showing the peptides that are enriched in LPS-treated versus unstimulated BMDMs (b) and in BMDMs treated with LPS versus LPS and emricasan (c). The x axis shows log2-transformed fold change; y-axis shows −log10-transformed adjusted P value, calculated using a linear mixed-effects model adjusted for multiple hypothesis testing using the Benjamini–Hochberg procedure. Dotted lines are at P = 0.05 and log2-transformed fold change of 1. Results are from three independent biological replicates for each condition. d, Wild-type (WT), Mlkl−/− and Mlkl−/−Casp8−/− BMDMs were stimulated for 1 h with the indicated TLR agonists, followed by immunoblot analysis. The long form (-L), short form (-S) and cleavage product of N4BP1 are highlighted by arrowheads. Pam3, Pam3csk4. e, Western blots of HEK293T cells transfected with the indicated N4BP1 constructs and treated for 4 h with TNF. Representative of three independent experiments (d, e).
A third N4BP1 peptide ending at Asp325 was found in unstimulated BMDMs, but it was not induced further by LPS (Extended Data Fig. 1a). Cleavage at Asp325 may give rise to the approximately 60-kDa form of N4BP1 in unstimulated BMDMs that is detected with an antibody raised against N4BP1 residues 342–390 (N4BP1-S) (Extended Data Fig. 1c). Although RNA sequencing of BMDMs yielded no evidence of alternative splicing of N4bp1 transcripts (GSE148066), the precise nature of N4BP1-S remains to be determined.
Given that TLR3 and TLR4 stimulation can activate caspase-818,19,20, we tested whether FADD and caspase-8 are required for cleavage of N4BP1 using Mlkl−/−Fadd−/− and Mlkl−/−Casp8−/− BMDMs, respectively. The contribution of FADD or caspase-8 could not be ascertained in isolation because Fadd−/− and Casp8−/− mice are embryonically lethal, owing to aberrant necroptosis24,25,26,27. In contrast to wild-type and Mlkl−/− BMDMs, which showed robust cleavage of N4BP1 upon stimulation with the TLR3 agonist polyinosinic:polycytidylic acid (poly(I:C)), LPS, TNF or FASL, both Mlkl−/−Casp8−/− and Mlkl−/−Fadd−/− BMDMs did not cleave N4BP1 (Fig. 1d, Extended Data Fig. 1d–f). Autocrine TNF production appeared to mediate low-level, basal cleavage of N4BP1 in unstimulated wild-type BMDMs because this cleavage was not present in Tnfr1−/− (Tnfr1 is also known as Tnfrsf1a) BMDMs (Extended Data Fig. 1g). Consistent with FADD–caspase-8 mediating cleavage of N4BP1, treatment with LPS induced caspase-8 enzymatic activity, coincident with N4BP1 cleavage (Extended Data Fig. 1h). In addition, Mlkl−/−Casp8C362A/C362A mouse embryonic fibroblasts that express caspase-8(C362A), which is catalytically inactive28, were defective at LPS-induced N4BP1 cleavage when compared with Mlkl−/− mouse embryonic fibroblasts (Extended Data Fig. 1i). Thus, FADD and the catalytic activity of caspase-8 are essential for the LPS-induced cleavage of N4BP1.
Agonists of TLRs that signal exclusively via the adaptor MyD88 (referred to hereafter as TRIF-independent TLRs)—including Pam3csk4 (a TLR1/2 agonist), R837 (imiquimod; a TLR7 agonist) and CpG-B DNA (a TLR9 agonist)—did not induce detectable N4BP1 cleavage within 1 h (Fig. 1d). Consistent with TLR3 and TLR4 engaging TRIF to trigger caspase-8-dependent cleavage of N4BP1, Trif−/− (Trif is also known as Ticam1) BMDMs showed N4BP1 cleavage in response to TNF or FASL, but not in response to LPS or poly(I:C) (Extended Data Fig. 1j). TNF treatment of HEK293T cells that express ectopic wild-type N4BP1, but not those that express mutant N4BP1(D488A), also produced the 54-kDa N4BP1 cleavage product (Fig. 1e), and this was blocked by emricasan or the pan-caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD) (Extended Data Fig. 1k). Thus, Asp488 appears to be the dominant, but not exclusive, cleavage site in N4BP1. Caspase-8 probably cleaved N4BP1 in the cytosol, as both proteins were detected in cytoplasmic, but not nuclear, extracts (Extended Data Fig. 1l). Consistent with direct cleavage, recombinant caspase-8 rapidly cleaved recombinant N4BP1 residues 286–893 (Extended Data Fig. 1m). Collectively, these data indicate that caspase-8 cleaves N4BP1 downstream of multiple caspase-8-activating receptors.
N4BP1 suppresses select cytokine responses
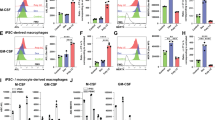
Previous work has described N4BP1 as an interferon-inducible viral restriction factor29, but whether N4BP1 has other roles was unclear. Given the intriguing pattern of TLR-induced N4BP1 cleavage, we tested whether N4BP1 regulates TLR-induced cytokine production. Wild-type BMDMs were CRISPR-edited with recombinant Cas9 and control or N4bp1-targeting guide RNAs (gRNAs)30,31 (Extended Data Fig. 2a). Control and N4bp1-deficient BMDMs treated with poly(I:C), LPS or TNF secreted comparable amounts of chemokines and cytokines (Fig. 2a, Extended Data Fig. 2b, c). By contrast, N4bp1-deficient BMDMs produced significantly greater amounts of select chemokines and cytokines than control BMDMs upon stimulation of TLR1/2, TLR7 or TLR9 (Fig. 2a, Extended Data Fig. 2d, e). Most notable among the affected cytokines were IL-6 and G-CSF, although TNF and chemokines such as CXCL1, CCL3 and CCL4 were also elevated. In addition, CRISPR-mediated deletion of N4BP1 in human macrophages derived from peripheral blood monocytes increased R848 (a TLR7 and TLR8 agonist)- or Pam3csk4-induced production of IL-6 and G-CSF, whereas LPS-induced cytokine production was largely unaltered (Extended Data Fig. 2f, g). Thus, N4BP1 potently suppresses select cytokine and chemokine responses by TRIF-independent TLRs.
a, Plots show TLR-induced cytokine secretion after 24 h, among BMDMs electroporated with Cas9 enzyme complexed with control gRNA or gRNA targeting N4bp1 (∆N4bp1). Bars represent mean values. Values for unstimulated BMDMs are the same among different graphs here and in Extended Data Fig. 2b–e and are replotted separately for clarity. Yellow shading denotes substantially increased cytokines secreted by ∆N4bp1 BMDMs. b, Model of differential regulation of TLRs by N4BP1. c, Plots show TNF (left) and IL-6 (right) secretion 24 h after LPS stimulation, among BMDMs of indicated genotypes electroporated with control or N4bp1-targeting (ΔN4) gRNAs. Bars represent mean values; three biological replicates per experiment in a and c, representative of three independent experiments for control and Mlkl−/−Casp8−/− BMDMs and one experiment for Mlkl−/−Fadd−/− BMDMs. P values calculated using unpaired, two-sided t-test (a) and one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test (c).
We hypothesized that TLR3- and TLR4-induced cytokine secretion appeared normal in N4BP1-deficient cells because TRIF-dependent cleavage of N4BP1 by caspase-8 inactivates its cytokine-suppressive function (Fig. 2b). This model predicts that caspase-8 deficiency blunts TLR3- and TLR4-induced cytokine responses owing to aberrant persistence of uncleaved N4BP1. Accordingly, Mlkl−/−Casp8−/− BMDMs produced less TNF and IL-6 than wild-type and Mlkl−/− BMDMs in response to LPS (Fig. 2c), and less IL-6 and CCL3 in response to poly(I:C) (Extended Data Fig. 2i). Importantly, CRISPR–Cas9-mediated deletion of N4BP1 in Mlkl−/−Casp8−/− BMDMs restored TLR4-induced—and, to a partial extent, TLR3-induced—cytokine responses, to levels similar to those of wild-type and Mlkl−/− BMDMs (Fig. 2c, Extended Data Fig. 2h, i). CRISPR-mediated deletion of N4BP1 also restored TLR4-induced cytokine production by Mlkl−/−Fadd−/− BMDMs (Fig. 2c). Thus, cleavage and inactivation of N4BP1 by caspase-8 is critical for normal TLR3- and TLR4-induced cytokine production.
Role of N4BP1 in vivo
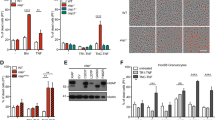
To explore these findings in vivo, we generated N4bp1−/− mice, which were viable, fertile and grossly normal (Extended Data Fig. 3a). Consistent with our findings using CRISPR–Cas9 gene deletion, N4bp1−/− BMDMs were hyper-responsive to TLR1/2, TLR7 or TLR9 stimulation, producing significantly more G-CSF, IL-6, TNF and CXCL1 than did wild-type BMDMs (Extended Data Fig. 3b). At 6–7 weeks of age, N4bp1−/− mice showed no overt pathology in any organ examined, nor did they have substantially altered serum cytokine levels when compared with wild-type littermates (Extended Data Fig. 3c–g). However, activated T cells, spontaneous germinal centre B cells and Ly6Chigh inflammatory monocytes were more frequent in the spleens, inguinal lymph nodes and mesenteric lymph nodes of N4bp1−/− mice when compared with wild-type littermates (Fig. 3a, Extended Data Figs. 3h–j, 4a–g). These cell types appeared even more prominent at 13–14 weeks of age, at which time N4bp1−/− mice developed mild lung inflammation with perivascular and peribronchiolar lymphocyte aggregates and increased levels of serum CXCL1 (Fig. 3a, b, Extended Data Fig. 4a–i). However, these effects were not associated with major organ pathology, splenomegaly or hypergammaglobulinaemia (Extended Data Fig. 4j–l). In addition, N4bp1−/− mice did not contain an expanded B220+CD3+ T cell population, as is characteristically observed in mice deficient in FADD–caspase-8-mediated cell death25,26 (Extended Data Fig. 5a, b). Thus, N4bp1−/− mice develop mild, age-dependent inflammation and immune dysregulation.
a, Mean fraction of activated (CD44+CD62L−) CD4+ T cells and germinal centre (CD38−FAS+) B cells in the spleens of mice at indicated ages. Dots represent individual mice. Results are pooled from three independent experiments. n = 7 mice per genotype at 6–7 weeks old and n = 8 mice per genotype at 13–14 weeks old; lines represent mean. b, Representative histological images of lungs for N4bp1−/− (n = 5) and N4bp1+/+ (n = 6) mice at 13–14 weeks old. Scale bars, 200 μm. Red arrow highlights a representative lymphocyte aggregate. Plot to the right shows the total number of perivascular and peribronchiolar lymphoid aggregates enumerated per histological section containing two lung lobes. Each dot represents the number of lymphoid aggregates from one section from one mouse; lines represent mean; error bars represent s.d. c, Total histological skin scores for the indicated mouse genotypes after daily topical treatment with imiquimod; n = 10 mice per genotype. Lines represent mean. d, e, Representative histological images (d) and Gr-1 immunohistochemistry (e) of skin from the experiment in c. Scale bars, 100 μm. f–h, Mice of the indicated genotypes were injected intraperitoneally with 30 mg kg−1 of Pam3csk4. Plots show mean rectal temperatures (f), survival (g) and serum cytokine responses at 24 h (h); n = 10 mice per genotype (f, g); for h, n = 6 untreated wild-type mice; n = 8 Pam3csk4-treated wild-type mice; n = 4 untreated N4bp1−/− mice; and n = 9 Pam3csk4-treated N4bp1−/− mice. Error bars in f represent s.e.m. Lines represent mean (f, h). P values calculated using one-way ANOVA with Bonferroni’s multiple comparisons test (a), unpaired two-sided t-test (f, h), two-tailed Mann–Whitney test (c) and two-sided log-rank test (g).
To test whether N4BP1 suppresses inflammation in TLR-dependent models of inflammatory disease, we used a TLR7-dependent model of psoriasis in which imiquimod (R837) is applied to the skin daily for 4 days. Histological evaluation revealed that N4bp1−/− mice developed exacerbated psoriatic lesions compared to wild-type mice, marked by worsened serocellular crusting, epidermal hyperplasia and inflammatory cell infiltration (Fig. 3c–e). N4bp1−/− mice also developed worse anaemia and had slightly higher levels of serum CXCL1 than wild-type mice (Extended Data Fig. 5c, d). Next, we tested whether N4bp1−/− mice were hyper-responsive in a TLR1/2-dependent model of acute septic shock. Wild-type mice injected with Pam3csk4 maintained their core body temperature, whereas N4bp1−/− mice lost about 4 °C of body temperature over the course of the first 23 h—after which only some of the mice recovered (Fig. 3f, g). In addition, N4bp1−/− mice produced significantly more IL-6, TNF and CXCL1 than wild-type mice at 24 h after injection of Pam3csk4 (Fig. 3h). Thus, N4BP1 deficiency exacerbates disease in TLR7-dependent and in TLR1/2-dependent models of inflammatory disease.
Insights into N4BP1-mediated cytokine suppression
We examined whether N4BP1 suppresses cytokine responses by affecting TLR-induced signal transduction. Control and N4BP1-deficient BMDMs stimulated with R837 or LPS showed comparable phosphorylation and degradation of IκBα as well as comparable phosphorylation of p65, p38, JNK and ERK (Extended Data Fig. 6a). Accordingly, wild-type and N4bp1−/− BMDMs had comparable gene-expression profiles before and after 4 h of stimulation with R837 (Extended Data Fig. 6b, c). However, at 8 and 16 h after TLR7 stimulation, genes involved in ‘cytokine–cytokine receptor interactions’ were expressed at higher levels in N4bp1−/− BMDMs than in wild-type BMDMs (Extended Data Fig. 6b, d, e). Thus, N4BP1 may regulate cytokine transcripts after their initial transcriptional induction.
We also explored the role of N4BP1 in defective LPS-induced gene transcription in caspase-8-deficient BMDMs11,12. We identified 214 LPS-induced genes in Mlkl−/− and wild-type BMDMs, the expression of which was significantly decreased in Mlkl−/−Casp8−/− BMDMs (Extended Data Fig. 7a–c). CRISPR-mediated deletion of N4BP1 in Mlkl−/−Casp8−/− BMDMs restored transcription of 45% of these genes (97 out of 214), whereas it increased transcription of only 8% of the non-LPS-responsive genes (8 out of 96) that were downregulated by caspase-8 deficiency (P < 0.0001) (Extended Data Fig. 7c). By contrast, N4BP1 deletion in Mlkl−/− BMDMs did not affect expression of LPS-stimulated genes (Extended Data Fig. 7a). The caspase-8-dependent genes whose expression was restored by N4BP1 deletion were enriched in cytokine signalling genes (Extended Data Fig. 7d). Restoration of LPS-induced gene expression was not explained by changes in NF-κB signal transduction12, because LPS-induced phosphorylation of IKKα, IKKβ and IκBα—plus the subsequent degradation of IκBα—were largely unaffected by deficiency in N4BP1 (Extended Data Fig. 7e). These findings support the notion that aberrantly persistent N4BP1 impairs inflammatory cytokine and gene expression in LPS-stimulated Mlkl−/−Casp8−/− BMDMs, possibly at a post-transcriptional level. Future work should address whether N4BP1 suppresses gene expression using its RNA-binding K homology domain, NYN RNase domain and/or its ubiquitin-binding domains29,32,33,34 (Extended Data Fig. 7f).
TNF licensing of TRIF-independent TLR responses
During pathogenic infections in vivo, macrophages may encounter both TNF and TLR ligands. Given that TNF induces caspase-8-dependent cleavage of N4BP1, we wondered whether TNF might license cytokine and chemokine production downstream of TRIF-independent TLRs35 (Fig. 4a). Indeed, TNF co-treatment of wild-type BMDMs stimulated with R837, CpG or Pam3csk4 augmented secretion of IL-6, G-CSF, CXCL1 and CCL3 (Fig. 4b, Extended Data Fig. 8a, b). By contrast, wild-type BMDMs stimulated with poly(I:C) or LPS—which already exhibit caspase-8-mediated N4BP1 cleavage—showed no increase in IL-6, G-CSF and CXCL1 production upon co-treatment with TNF, and only a modest increase in CCL3 (Fig. 4b, Extended Data Fig. 8c). Consistent with caspase-8 mediating TNF licensing of TRIF-independent TLR responses, TNF enhanced R837-, CpG- and Pam3csk4-induced IL-6 or G-CSF production in wild-type and Mlkl−/− BMDMs, but had a much smaller effect on Mlkl−/−Casp8−/− BMDMs (Fig. 4c).
a, Model for TNF licensing of TRIF-independent TLRs. b–d, Plots show cytokine secretion among BMDMs treated for 24 h with the indicated TLR agonists and/or TNF. Unstimulated and TNF-stimulated values are the same among graphs in b, and are replotted separately for clarity. Bars represent mean values; b and c are representative of three or two independent experiments, respectively, with three biological replicates each; d represents pooled results of three independent experiments with three biological replicates each. e, Survival plots of mice of the indicated genotypes infected with approximately 2 × 103 colony-forming units of S. pneumoniae via the intranasal route and injected intraperitoneally with anti-gp120 or TNFRII-Fc. Results are pooled from two independent experiments. P values calculated using two-sided unpaired t-test (b, c), one-way ANOVA with Bonferroni’s multiple comparisons test (d) or two-sided log-rank test (e).
To directly test whether caspase-8 licensed TRIF-independent TLR responses by cleaving N4BP1, we engineered knock-in mice that express cleavage-resistant N4BP1(D488A). At 10–12 weeks old, N4bp1D488A/D488A mice displayed no substantial organ pathology or immune dysregulation (Extended Data Fig. 8d–l). Consistent with Asp488 being the predominant site at which N4BP1 is cleaved by caspase-8, N4bp1D488A/D488A BMDMs treated with poly(I:C), LPS, TNF or FASL did not contain the approximately 54-kDa N4BP1 fragment that is produced in wild-type BMDMs (Extended Data Fig. 9a–c). An alternative, approximately 70–75-kDa cleavage product arose in LPS-, poly(I:C)- and TNF-stimulated N4bp1D488A/D488A BMDMs (Extended Data Fig. 9a–c), possibly representing the result of enhanced cleavage at Asp200 and/or other sites. Cleavage of full-length N4BP1 was nevertheless impaired in N4bp1D488A/D488A BMDMs. As with Mlkl−/−Casp8−/− BMDMs, N4bp1D488A/D488A BMDMs showed impaired IL-6 production in response to poly(I:C) or LPS (Extended Data Fig. 9d). TNF licensing of TRIF-independent TLR responses was also greatly diminished in N4bp1D488A/D488A BMDMs (Fig. 4d); the incomplete prevention of TNF licensing in these cells may reflect cleavage of N4BP1 at sites other than Asp488 (Extended Data Fig. 9a–c). Consistent with this, the inability of TNF to license TRIF-independent TLR responses by Mlkl−/−Casp8−/− BMDMs was due to N4BP1, as Mlkl−/−Casp8−/− ∆N4bp1 BMDMs showed fully ‘licensed’ IL-6 production whether or not they were co-stimulated with TNF (Extended Data Fig. 9e). Doses of FASL that did not kill BMDMs also licensed IL-6 and G-CSF production by TRIF-independent TLRs, which was diminished in N4bp1D488A/D488A and Mlkl−/−Casp8−/− BMDMs (Extended Data Fig. 10). Thus, cleavage of N4BP1 by caspase-8 provides a critical point of signal integration during inflammation, enabling death receptors such as TNFR1 and FAS to inactivate N4BP1 and thereby unleash heightened cytokine responses by TRIF-independent TLRs (Fig. 4a).
TNF blocking agents are widely used in the treatment of inflammatory diseases, but are associated with an increased risk of infection36. We speculated that TNF blockade might decrease protection from infection by preventing TNF-induced inactivation of N4BP1. To test this possibility, we infected wild-type and N4bp1−/− mice intranasally with Streptococcus pneumoniae and then treated the mice with TNFRII-Fc to block TNF signalling. As expected, wild-type mice treated with TNFRII-Fc had significantly increased mortality when compared to wild-type mice treated with an irrelevant anti-gp120 antibody37 (Fig. 4e). By contrast, N4bp1−/− mice largely survived infection, even upon TNF blockade (Fig. 4e). This finding is consistent with the notion that inactivation of N4BP1 is an important function of TNF during bacterial infection; however, whether this observation represents enhanced resistance or increased tolerance of N4bp1−/− mice to S. pneumoniae remains unknown.
Discussion
Collectively, our results identify N4BP1 as an important negative regulator of select cytokine and chemokine responses elicited by TRIF-independent TLRs. Inactivation of N4BP1 by receptors that engage caspase-8 point to a model in which N4BP1 limits inflammatory cytokine responses to minor insults. For example, N4BP1 may prevent isolated encounters with low levels of TLR ligands—whether from endogenous or microbial sources—from eliciting over-exuberant cytokine responses. Consistent with this idea, N4bp1−/− mice develop mild inflammation and immune dysregulation in multiple tissues that becomes more pronounced with age. By contrast, encounters with more threatening pathogens that trigger TNF production would induce N4BP1 cleavage and thereby license TRIF-independent TLRs to produce inflammatory cytokines, such as IL-6, at a level commensurate with the threat. As illustrated by our experiments with S. pneumoniae and TNFRII-Fc, this model suggests that inhibition of the TNF–caspase-8–N4BP1 axis may—at least in part—explain how therapeutic TNF blockade ameliorates inflammatory disease while heightening susceptibility to infections. Finally, in patients and animal models with FADD or caspase-8 deficiency, an inability to cleave N4BP1 may aberrantly constrict cytokine responses and contribute to their long-observed, but enigmatic, immunodeficiencies.
Methods
Mice
The Genentech institutional animal care and use committee responsible for ethical compliance approved all animal protocols. Mlkl−/−38, Ripk3−/−39, Casp8−/−40, Trif−/−41, Tnfr1−/−42 and Fadd−/−43 mice have previously been described. C57BL6/N mice (Charles Rivers laboratories) were used as wild-type controls in Extended Data Fig. 5b and as a source of wild-type BMDMs for experiments depicted in Figs. 1b, c, 2a, 4b, Extended Data Figs. 1c, l, 2a–e, 6a, 8a–c.
N4bp1fl/+ mice were generated by Ozgene using C57BL/6 embryonic stem cells. The N4bp1 floxed region corresponds to genomic position: chr8: 86,860,318–86,863,542 (GRCm38). N4bp1+/− mice were derived from N4bp1fl/+ mice by excision of exon 2 using HTN.Cre treatment of 1-cell embryos during in vitro fertilization44. Mice were maintained on a C57BL/6N background.
To generate N4bp1D488A/+ mice, a synthetic single-guide RNA (sgRNA) was generated that used as a guide target: 5′ ACTGTCGACCTGGAAACTGA 3′; PAM: TGG with predicted cut sites in chr8: 86860848– 6860849. For sgRNA selection, we used CRISPR design tools CRISPOR (http://www.crispor.tefor.net/) and Benchling, which use a previously described algorithm45 to provide ‘MIT’ and ‘CFD’ specificity scores for each sgRNA, as well as the top 30 predicted off-target loci and corresponding MIT and CFD off-target scores.
The following oligonucleotide donor was used to introduce the D488A mutation in exon 2: 5′ACAGTGCCAGTAGGACAAAAACAGGAAATCTGGGGTTCAAAGCAGAACTCAAGTTGTACTGTCGAtCTGGAgACcGccGGCCACTCGGCCTCTGCCGCCTCTGCCAGCCCTAAAGACATCAGTTTTGTTTCAAGGGGAGCTTCAGGGCACCAA 3′.
Reagent concentrations for two-cell electroporation were as follows: 500 ng/μl HIFI Cas9 protein (IDT; 1081060), 250 ng/μl sgRNA (Synthego) and oligonucleotide donor (200 ng/μl; IDT). After electroporation and embryo transfer, genomic DNA was prepared from tail tip biopsies of potential G0 founders. G0 mice were screened by droplet digital PCR46, with targeted sequencing confirming on-target mutation and excluding off-target mutation of the 30 most likely predicted off-target sites. Mice were maintained on a C57BL/6N background.
For the experiment in Fig. 4e, mice of the indicated genotypes were inoculated intranasally with 2 × 103 colony-forming units of S. pneumoniae (ATCC 6303). TNF blockade by TNF-RII Fc (Genentech) or anti-gp120 (Genentech) were administered through intraperitoneal injection 4 h before infection and then 3 times per week at 10 mg/kg. Mice were monitored for health status at least twice per day for the first 7 days post-infection, then at least once per day. Data in Fig. 4e are pooled from 2 independent experiments, which lasted a total of either 9 or 17 days after infection.
For imiquimod-induced psoriasis experiments, treated skin on ear and back was shaved before topical application of 25 mg of Aldara (imiquimod) cream (5%, 3M Pharmaceuticals) at each site daily for 4 consecutive days. Lesions were evaluated histologically 24 h after the last application. Histological images of skin were evaluated for epidermal hyperplasia (scored 1–4), crusting and ulceration (scored 1–4), and dermatitis and panniculitis (scored 1–4), with greater scores indicating worse disease. The total clinical score was calculated by adding these individual scores. For Pam3csk4 sepsis model, mice were injected intraperitoneally with 30 mg/kg of Vaccigrade Pam3csk4 (Invivogen) in PBS, after which mice were closely monitored and body temperature was measured at indicated time points using a lubricated digital rectal probe. Haematology assays were run on a Sysmex XT-2000iV automated haematology analyser.
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Immunohistochemistry and histology
Rat anti-Ly-6G (Gr-1) antibody (BD Biosciences, clone RB6-8C5) was used for immunohistochemistry at a working concentration of 15 μg/ml with Target antigen retrieval (Dako). Immunohistochemistry and histology images were acquired with Leica Application Suite v.4.6.0. Immunolabelling was detected with biotinylated donkey ant-rat IgG secondary antibody (Jackson Laboratories), and ABC-Peroxidase Elite detection (Vector Laboratories). Hydrogen peroxide, Scytek biotin block and donkey serum were used to block non-specific signal.
Cell culture
Primary mouse embryonic fibroblasts were previously generated from mice of Mlkl−/−, Mlkl−/−Casp8−/− and Mlkl−/−Casp8C362A/C362A genotypes28, isolated from embryos at embryonic day (E)14.5 and excluding the head, intestine and liver. Mouse embryonic fibroblasts were grown in high-glucose Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutaMAX (Gibco), 1× non-essential amino acids solution (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) on tissue culture dishes pre-coated with 0.1% gelatin in PBS.
HEK293T cells (ATCC CRL-3216; tested for mycoplasma contamination but not authenticated) were cultured in the high glucose version of DMEM supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 1× non-essential amino acids solution, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were transfected for 48 h with plasmids encoding wild-type or D488A mouse N4BP1 constructs containing N-terminal 3×Flag tags. Plasmids were transfected using Lipofectamine 3000 (Thermo Fisher Scientific) or Fugene (ProMega) according to manufacturer’s protocol.
Primary BMDMs were differentiated from bone marrow for 7 days in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 50 μM 2-mercaptoethanol (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco) and 50 ng/ml of recombinant M-CSF (R&D Systems).
CRISPR-mediated deletion of N4bp1 in mouse BMDMs of indicated genotypes was generated by Cas9 ribonucleoprotein electroporation. In brief, BMDMs were differentiated for 5 days in the above-mentioned RPMI medium supplemented on nontreated plates. BMDMs were collected and extensively washed with PBS, and then 5 × 106 BMDMs were electroporated with recombinant Cas9 protein v.3 (IDT) complexed with N4bp1-specific gRNAs (seed sequence/Alt-R crRNA: 5′-GCTGCAACTCCGCGGCGCGC-3′ and 5′-AGCAGTAACGGTTCTGTAGA-3′ annealed to Alt-R tracrRNA (IDT)) in nucleofector solution P3 (Lonza). The following GFP-targeting crRNA sequences were annealed to Alt-R tracrRNA (IDT) and were used as controls: 5′-GGTGGTGCAGATGAACTTCA-3′ and 5′-GGCATCGACTTCAAGGAGGA-3′. BMDMs were maintained in above-described RPMI medium for the following 7 days.
BMDMs were treated as indicated with poly(I:C) (2 μg/ml, Invivogen), LPS (from Escherichia coli K12, Invivogen; 100 ng/ml in all figures with the exception of 1 μg/ml in Extended Data Fig. 1i), R837 (2 μg/ml, Invivogen), Pam3csk4 (1 μg/ml, Invivogen), CpG-B (ODN 1826, 5 μM, Invivogen), R848 (500 ng/ml, Invivogen), TNF (100 ng/ml, Genentech), multimeric FASL (100 ng/ml, Adipogen), 20 μM Z-VAD-FMK (Promega) and emricasan (20 μM, Fisher Scientific). For cytokine secretion assays, stimulation of BMDMs with TLR agonists, and/or TNF or FASL, was performed in above-described DMEM with indicated stimuli in 96-well tissue culture plates with 100,000 cells per well. Caspase-8 activity was measured using the Caspase-Glo 8 assay (Promega); data were acquired with PerkinElmer EnVision Manager 1.13.3009.1401.
For preparation of human monocytes, peripheral blood was collected from healthy donors participating in the Genentech blood donor program after written, informed consent from the Western Institutional Review board. Peripheral blood mononuclear cells were isolated using Sepmate tubes (StemCell Technologies) and ACK (ammonium–chloride–potassium) lysing buffer (Thermo Fisher Scientific) following the manufacturer’s protocol from buffy coats. Human monocytes were isolated from the peripheral blood mononuclear cells using the EasySep Human Monocyte isolation kit (Stem Cell Technologies cat. no. 19359) according to the manufacturer’s protocol. Monocytes were either aliquoted, frozen in CryoStor Freeze Medium (cat. no. 2020102) and then thawed into non-tissue-culture treated 6-well plates (Thermo Fisher Scientific) at 106 cells per ml in macrophage medium (DMEM high glucose supplemented with 10% FBS (Gibco), 2 mM GlutaMAX, 100 U/ml penicillin, 100 μg/ml streptomycin, and M-CSF 100 ng/ml (Peprotech)) or plated immediately after isolation. Plated or thawed monocytes were cultured overnight.
For CRISPR-mediated gene editing in human monocyte-derived macrophages, Alt-R crRNA and tracrRNAs were synthesized and annealed as above. The annealed gRNA mixture was incubated with Cas9 protein v.3 at a 3:1 molar ratio. Cas9 RNPs were electroporated into 3 × 106 cells for each gRNA and human donor combination using a Lonza 4D-nucleofector system (4D-Nucleofector Core Unit: Lonza, AAF-1002B; 4D-Nucleofector X Unit: AAF-1002X) with previously optimized conditions47. Then, 150–180 μl of prewarmed macrophage medium was immediately added into each well of the nucleofector cassette strip after electroporation. On day 5 after electroporation, macrophages were stimulated for 24 h with TLR agonists at a density of 35,000–40,000 cells per well of a 96-well tissue culture plate. The following human N4BP1-targeting crRNA sequences were used in combination: 5′-GAGCTGCAGCCAGATGCGCG-3′ and 5′-GTAACCGAAGGTAACAGCTG-3′. The above-described GFP-targeting crRNA sequences were used as control.
Flow cytometry
Single-cell suspensions were prepared from organs, nonspecific antibody binding was minimized by anti-CD16/CD32 antibody (clone 93, eBioscience), and cells were stained in PBS containing 2% heat-inactivated fetal bovine serum and 1 mM EDTA at 4 °C. The following fluorophore-conjugated antibodies were used: CD4 (RM4-5, eBioscience), CD8α (53-6.7, eBioscience), CD3ε (145-2C11, BD Biosciences), B220 (RA3-6B2, BD Biosciences), CD11b (M1/70, eBioscience), Ly6C (HK1.4, Biolegend), Ly6G (1A8, Biolegend), TCRβ (H57-597, BD Biosciences), CD44 (IM7, Biolegend), CD62L (MEL-14, Biolegend), CD19 (1D3, BD Biosciences), CD38 (90, eBioscience) and FAS (Jo2, BD Biosciences). Dead cells were excluded using 7-AAD (eBioscience). Antibodies were used for flow cytometry at 1:400 dilution. Samples were acquired with BD FACSDiva software v.8.0 on FACSCantoII (BD Biosciences) and analysed with FlowJo software version 9.9.6. Representative gating is shown in Extended Data Figs. 3h–j, 5a.
Western blotting
Cells were lysed in 20 mM Tris HCl pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% (v/v) Triton X-100, 10% (v/v) glycerol and complete protease inhibitor cocktail. Insoluble material was removed by centrifugation at 20,000g before addition of LDS sample buffer followed by incubation at 70 °C for at least 10 min. Western blotting antibodies included: actin (C4, MP Biomedicals), tubulin (Abcam antibody (ab6046)), N4BP1 (EPNCIR118, Abcam), caspase-8 (1G12, Enzo Life sciences cat. no. ALX-804-447-C100), HSP90 (C45G5, Cell Signaling Technologies), c-Jun (60A8, Cell Signaling Technologies), IκBα (no. 9242, Cell Signaling Technologies), p-IκBα (14D4, Cell Signaling Technologies), p65 (D14E12, Cell Signaling Technologies), p-p65 (S536, Cell Signaling Technologies), p-ERK (no. 9101, Cell Signaling Technologies), p-JNK (81E11, Cell Signaling Technologies), p38 (D13E1, Cell Signaling Technologies), p-p38 (D3F9, Cell Signaling Technologies), p-IKKα/β (C84E11, Cell Signaling Technologies), IKKβ (D30C6, Cell Signaling Technologies), and Jackson Immunoresearch (HRP-anti-mouse, cat. no. 115-035-174; HRP-anti-rabbit, cat. no. 211-032-171). For western blots, antibody for actin was used at a 1:10,000 dilution; antibody for tubulin was used at 1:5,000 dilution; antibodies for N4BP1, caspase-8, c-Jun and HSP90 were used at 1:4,000 dilution; secondary anti-mouse and ant-rabbit antibodies were used at 1:10,000 dilution; all other antibodies were used at 1:1,000 dilution. For fractionation of cytoplasmic and nuclear extracts, subcellular protein fractionation kit (ThermoFisher, no. 78840) was used according to manufacturer’s protocol.
RNA-sequencing data analysis
GSNAP (version 2013-11-10)48 was used to align raw FASTQ reads to the mouse reference genome (GRCm38/mm10) with following parameters: ‘-M 2 -n 10 -B 2 -i 1 -N 1 -w 200000 -E 1–pairmax-rna=200000–clip-overlap’. Reads were filtered to include only the uniquely mapped reads. Differential expression analysis was performed using R package Limma49. For Extended Data Fig. 6, genes were considered differentially expressed if the log2-transformed fold change was either >1 or <−1 and the adjusted P value < 0.05. The R package EnhancedVolcano (version 1.0.1; https://github.com/kevinblighe/EnhancedVolcano) was used to create the volcano plot. Gene ontology analysis was performed using Metascape50 with species as ‘M. musculus’, P value cutffoff of 10−5 and minimum overlap set to 25. The list of NF-κB genes was derived from MSigDB database (26771021) and mouse cytokines were obtained from a published source51.
For Extended Data Fig. 7, caspase-8-dependent downregulated genes were defined as the genes that go down significantly (log2-transformed fold change < 0 and adjusted P value <0.05) in Mlkl−/−Casp8−/− BMDMs treated with LPS for 8 h versus Mlkl−/− BMDMs treated with LPS for 8 h and Mlkl−/−Casp8−/− BMDMs treated with LPS for 8 h versus wild-type BMDMs treated with LPS for 8 h. LPS-induced genes were defined as those that were significantly upregulated in wild-type BMDMs treated with LPS for 8 h versus untreated wild-type BMDMs (log2-transformed fold change > 0, and adjusted P value < 0.05). Pathway enrichment was performed using online version of GSEA (https://www.gsea-msigdb.org/gsea/msigdb/annotate.jsp) using the ‘Reactome gene sets’.
Recombinant N4BP1 production and in vitro cleavage reaction
Mouse N4BP1 S286–D893 containing the mutation F705E was expressed in Sf9 cells as a His-Avi fusion construct using baculovirus. The cell pellet was collected after 72 h and resuspended in buffer A (50 mM Tris pH 7.5, 200 mM NaCl, 5% glycerol, 5 mM MgCl2 and 1 mM TCEP). Cells were lysed using a microfluidizer and the lysate was clarified by centrifugation (40,000 rpm for 1 h) and filtration. The lysate was equilibrated with NI-NTA Superflow resin (Qiagen) for 1 h at 4 °C. The resin was washed with 1 l of buffer A and eluted with buffer A supplemented with 250 mM imidazole. Protein-containing fractions were pooled, dialysed against buffer A overnight and further purified by size exclusion chromatography pre-equilibrated in buffer B (25 mM Tris pH 7.5, 200 mM NaCl, 5% glycerol, 1 mM MgCl2 and 1 mM TCEP). Fractions were analysed by SDS–PAGE and fractions containing pure protein were combined, concentrated, flash-frozen in liquid nitrogen and stored at −80 °C.
For in vitro cleavage reaction in Extended Data Fig. 1m, 12.5 ng/μl recombinant N4BP1 was incubated with fourfold increasing concentrations of recombinant active mouse caspase-8 (Enzo Life Sciences). The highest concentration of active caspase-8 used was 12.5 ng/μl; the lowest concentration used was 48.8 pg/μl. Reactions containing the highest concentration of active caspase-8 were incubated in the absence or presence of 20 μM of emricasan (Fisher Scientific). Reactions were incubated for 30 min at 37 °C in 20-μl volumes in buffer containing 50 mM HEPES, pH 7.2, 50 mM sodium chloride, 0.1% CHAPS, 10 mM EDTA, 5% glycerol and 10 mM dithiothreitol (DTT). Reactions were stopped by addition of LDS sample buffer and boiling of samples.
Proteomic sample preparation
Cells were collected and lysed in 20 mM HEPES pH 8.0, containing 9 M urea,1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate containing 1× Halt protease inhibitor cocktail, EDTA-free (Thermo Fisher Scientific). Lysates from three conditions (untreated, LPS-treated and LPS + emricasan) comprising three independent biological replicates per condition (a total of nine samples) were processed for proteomic analysis. Samples were sonicated using a Misonix Microson XL sonicator followed by centrifugation at 20,000g for 20 min at 15 °C. Protein concentration was measured using Bradford assay (BioRad). Equal amounts of proteins per sample were subjected to reduction with 5 mM DTT at 37 °C for 1 h followed by alkylation with 15 mM iodoacetamide at room temperature for 20 min in the dark. Samples were diluted to a final concentration of 2 M urea before digestion with Lys-C (Wako) at an enzyme:substrate ratio of 1:50 at 37 °C for 4 h followed by tryptic (Promega) digestion at an enzyme:substrate ratio of 1:50 at 37 °C overnight. The peptide solution was acidified with 20% trifluoroacetic acid (TFA) before solid-phase extraction using a C18 cartridge (500 mg absorbent) from Waters. Peptides were eluted with 3× 2.0 ml of 0.1% TFA and 60% acetonitrile (ACN) in water followed by peptide concentration measurement using a quantitative colorimetric peptide assay kit (Thermo Fisher Scientific). Equal amounts per condition (16 mg/condition) were subjected to lyophilization overnight.
Immunoaffinity capture of C-terminal aspartic acid peptides and TMT labelling
Immunoaffinity enrichment of peptides containing C-terminal aspartic acid residues was performed using an antibody that recognizes caspase 6/8, 2/3/7 cleavage motifs according to the PTMScan protocol21,22. PTMScan Proteomics System for cleaved caspase substrates and the antibody included in this kit were used for immunoaffinity enrichment and were in-licensed from Cell Signaling Technologies (http://www.cellsignal.com/services/peruse_licensing.html, catalogue no. 9817BC). The enriched peptide mixture was dried down completely followed by chemical labelling with tandem mass tag (TMT) (Thermo Fisher Scientific) for multiplexing. The dried peptide mixture was reconstituted in 100 μl of HEPES (200 mM, pH 8.5) and 300 μl of ACN. TMT reagent (10 μl) was added to each of the 9 samples (each vial of TMT reagent was reconstituted in 40 μl of ACN). Labelling was performed at room temperature for 1.5 h. A small amount (2 μl) from each condition was mixed, desalted and analysed to determine labelling efficiency. The reaction was quenched with 5% hydroxylamine once labelling efficiency was calculated to be at least 95%. Samples were combined at equal amounts followed by desalting and drying to completion. The dried peptide mixture from the 9-plex was subjected to a second round of immunoaffinity enrichment to minimize nonspecificity. The resulting enriched peptide mixture was desalted, dried to completion and was ready for mass spectrometry analysis. TMT reagents and reporter ions used were as follows: untreated, replicate 1: TMT10–126, 126.1277; untreated, replicate 2: TMT10–127N, 127.1246; untreated, replicate 3: TMT10–127C, 127.1309; LPS, replicate 1: TMT10–128N, 128.1281; LPS, replicate 2: TMT10–128C, 128.1341; LPS, replicate 3: TMT10–129N, 129.1317; LPS and emricasan, replicate 1: TMT10–129C, 129.1376; LPS and emricasan, replicate 2: TMT10–130N, 130.1348; and LPS and emricasan, replicate 3: TMT10–130C, 130.1409.
Mass spectrometry analysis
Desalted peptides were reconstituted in 0.1% formic acid and 2% ACN in water, and loaded onto an Aurora series 25 cm × 75 μm I.D. column (IonOpticks) using a Dionex Ultimate 3000 RSLC system (Thermo Fisher Scientific). Peptide separation was performed at 0.3 μl/min using a 150-min 2-step linear gradient in which solvent B (0.1% formic acid and 2% water in ACN) was increased from 2% to 30% over 135 min and then from 30% to 50% over 15 min with a total analysis time of 185 min. Duplicate injections were made for technical replicates. Peptides were analysed using an Orbitrap Fusion Lumos instrument (Thermo Fisher Scientific). Precursor ions (MS1) were analysed in the Orbitrap (AGC target 1 × 106, 120,000 mass resolution, 50-ms maximum injection time) and 10 most abundant species were selected for fragmentation (MS2). Ions were filtered on the basis of charge state ≥ 2 (z = 2, 3 and 4–6) and monoisotopic peak assignment and dynamic exclusion (45 s ± 10 ppm) was enabled. Each precursor ion was isolated at a mass width of 0.5 Th followed by fragmentation using collision-induced dissociation (CID at 35 NCE), MS2 AGC target was set at 1.5 × 104 with a maximum injection time of 100 ms. Multiple fragment ions were isolated using synchronous precursor selection (SPS) before HCD (55 NCE, SPS notches = 8, AGC target = 1.5 × 105, maximum injection time of 150 ms) MS3 fragmentation and Orbitrap analysis at 50,000 resolution.
Mass spectrometry bioinformatics and statistical analysis
MS/MS data were searched using the Mascot search algorithm (Matrix Sciences) against a concatenated forward–reverse target–decoy database (UniProtKBconcat 2016_06) consisting of mouse proteins and common contaminant sequences. Spectra were assigned using a precursor mass tolerance of 50 ppm and fragment ion tolerance of 0.8 Da. Static modifications included carbamidomethyl cysteine (+57.0215 Da), TMT (229.1629 Da) on both the N terminus of the peptides and lysine residues, and variable modification included oxidized methionine (+15.994 Da). Up to 3 mis-cleavages C-terminal of lysine, arginine and aspartic acid residues were allowed. Peptide spectral matches were filtered at 5% false-discovery rate (FDR)52 followed by protein filtering at 2% FDR. Peptides containing the C-terminal aspartic acid were filtered using the regular expression ‘[D][.][^-]’.
Quantification and statistical testing of the TMT proteomics data was performed by MSstatsTMT v.1.2.7. Before MSstatsTMT analysis, the peptide spectral matches (PSMs) were filtered as follows. PSMs were filtered out if they were (1) from decoy proteins, (2) from peptides with length less than 7, (3) with isolation specificity less than 70%, (4) with reporter ion intensity less than 256 and (5) with summed reporter ion intensity (across all 9 channels) lower than 30,000. In the case of redundant PSMs (that is, multiple PSMs in one mass spectrometry run that map to the same peptide), the PSMs were summarized by summing the reporter ion intensities per peptide and channel. Next, MSstatsTMT summarized the peptides to the protein level using Turkey median polish summarization and global median normalization. This normalization equalized the medians of the reporter ion intensities across all nine channels. As a final step, the differential abundance analysis between conditions was performed in MSstatsTMT based on a linear mixed-effects model per protein. The inference procedure was adjusted by applying an empirical Bayes shrinkage. P values from the linear mixed-effects model were also adjusted for multiple hypothesis testing by using the Benjamini–Hochberg procedure.
Cytokine, chemokine and immunoglobulin detection
Sera from adult mice and cell supernatants were analysed using Milliplex MAP Mouse Cytokine/Chemokine Magnetic Bead Panel – Premixed 32-plex – Immunology Multiplex Assay (Millipore); human cytokines in cell supernatants were measured with a cytokine luminex panel (Millipore). Immunoglobulin isotypes were quantified by Milliplex MAP Mouse Immunoglobulin Isotyping Magnetic Bead Panel - Isotyping Multiplex Assay (Millipore). Luminex data were collected on luminex Flex-Map 3D (Luminex).
Statistics and figure preparation
Statistical analysis of results was performed by unpaired, two-tailed t-test, Mann–Whitney test, one-way ANOVA with Bonferroni’s multiple comparison’s test or log-rank test. P < 0.05 was considered significant. P value calculations for mass spectrometry analysis in Fig. 1b, c were performed as described above. All other statistical analyses were performed using GraphPad Prism 6. Models in Figs. 1a, 2b, 4a were created using BioRender (https://biorender.com/).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The datasets generated during the current study are available from the corresponding authors on reasonable request. The RNA-sequencing data (Extended Data Figs. 6b–e, 7a–d) are available at the Gene Expression Omnibus (GEO) under accession code GSE148066. Proteomics raw data files have been deposited to the UCSD MassIVE database (https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=6b442a4570b747b296e25a6b28c94613). Source data are provided with this paper.
Code availability
All custom code can be accessed at https://github.com/rreja/GitlinA_manuscript.
References
Rieux-Laucat, F. et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268, 1347–1349 (1995).
Fisher, G. H. et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81, 935–946 (1995).
Del-Rey, M. et al. A homozygous Fas ligand gene mutation in a patient causes a new type of autoimmune lymphoproliferative syndrome. Blood 108, 1306–1312 (2006).
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002).
Lehle, A. S. et al. Intestinal inflammation and dysregulated immunity in patients with inherited caspase-8 deficiency. Gastroenterology 156, 275–278 (2019).
Bolze, A. et al. Whole-exome-sequencing-based discovery of human FADD deficiency. Am. J. Hum. Genet. 87, 873–881 (2010).
Peterson, L. W. et al. Cell-extrinsic TNF collaborates with TRIF signaling to promote Yersinia-induced apoptosis. J. Immunol. 197, 4110–4117 (2016).
Weng, D. et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl Acad. Sci. USA 111, 7391–7396 (2014).
Kang, S. et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 6, 7515 (2015).
Mandal, P. et al. Caspase-8 collaborates with caspase-11 to drive tissue damage and execution of endotoxic shock. Immunity 49, 42–55.e6 (2018).
Philip, N. H. et al. Activity of uncleaved caspase-8 controls anti-bacterial immune defense and TLR-induced cytokine production independent of cell death. PLoS Pathog. 12, e1005910 (2016).
DeLaney, A. A. et al. Caspase-8 promotes c-Rel-dependent inflammatory cytokine expression and resistance against Toxoplasma gondii. Proc. Natl Acad. Sci. USA 116, 11926–11935 (2019).
Allam, R. et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 15, 982–990 (2014).
Varfolomeev, E. E. et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Watanabe-Fukunaga, R., Brannan, C. I., Copeland, N. G., Jenkins, N. A. & Nagata, S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356, 314–317 (1992).
Günther, C. et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 (2011).
Welz, P. S. et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
He, S., Liang, Y., Shao, F. & Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl Acad. Sci. USA 108, 20054–20059 (2011).
Kaiser, W. J. et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
Pham, V. C. et al. Complementary proteomic tools for the dissection of apoptotic proteolysis events. J. Proteome Res. 11, 2947–2954 (2012).
Rush, J. et al. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23, 94–101 (2005).
Kumar, S., van Raam, B. J., Salvesen, G. S. & Cieplak, P. Caspase cleavage sites in the human proteome: CaspDB, a database of predicted substrates. PLoS ONE 9, e110539 (2014).
Zhang, H. et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373–376 (2011).
Oberst, A. et al. Catalytic activity of the caspase-8–FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Dillon, C. P. et al. Survival function of the FADD–CASPASE-8–cFLIPL complex. Cell Rep. 1, 401–407 (2012).
Newton, K. et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682 (2019).
Yamasoba, D. et al. N4BP1 restricts HIV-1 and its inactivation by MALT1 promotes viral reactivation. Nat. Microbiol. 4, 1532–1544 (2019).
Heger, K. et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature 559, 120–124 (2018).
Oh, S. A., Seki, A. & Rutz, S. Ribonucleoprotein transfection for CRISPR/Cas9-mediated gene knockout in primary T cells. Curr. Protoc. Immunol. 124, e69 (2019).
Nepravishta, R. et al. CoCUN, a novel ubiquitin binding domain identified in N4BP1. Biomolecules 9, 284 (2019).
Zhang, X. et al. An interaction landscape of ubiquitin signaling. Mol. Cell 65, 941–955 (2017).
Fenner, B. J., Scannell, M. & Prehn, J. H. M. Identification of polyubiquitin binding proteins involved in NF-κB signaling using protein arrays. Biochim. Biophys. Acta 1794, 1010–1016 (2009).
Deguine, J., Wei, J., Barbalat, R., Gronert, K. & Barton, G. M. Local TNFR1 signaling licenses murine neutrophils for increased TLR-dependent cytokine and eicosanoid production. J. Immunol. 198, 2865–2875 (2017).
Singh, J. A. et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 386, 258–265 (2015).
van der Poll, T., Keogh, C. V., Buurman, W. A. & Lowry, S. F. Passive immunization against tumor necrosis factor-α impairs host defense during pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 155, 603–608 (1997).
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Newton, K. et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 (2014).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Peschon, J. J. et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160, 943–952 (1998).
Newton, K. et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574, 428–431 (2019).
Ryder, E. et al. Rapid conversion of EUCOMM/KOMP-CSD alleles in mouse embryos using a cell-permeable Cre recombinase. Transgenic Res. 23, 177–185 (2014).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Hindson, B. J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604–8610 (2011).
Freund, E. C. et al. Efficient gene knockout in primary human and murine myeloid cells by non-viral delivery of CRISPR-Cas9. J. Exp. Med. 217, e20191692 (2020).
Wu, T. D. & Watanabe, C. K. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics 21, 1859–1875 (2005).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Turner, M. D., Nedjai, B., Hurst, T. & Pennington, D. J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 1843, 2563–2582 (2014).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for mass spectrometry-based proteomics. Methods Mol. Biol. 604, 55–71 (2010).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protocols 10, 845–858 (2015).
Acknowledgements
We thank members of the Dixit and D. Vucic laboratories for advice, discussions and reagents—in particular, K. Wickliffe, B. Lee and A. Maltzman; the Genentech genetic analysis, histology, necropsy, immunohistochemistry, clinical pathology and FACS groups for technical assistance; R. Asuncion, B. Torres, B. Hough, M. Long, E. Lu and M. Dempsey for animal husbandry; and B. Pulendran, S. Galli and T. Montine for support.
Author information
Authors and Affiliations
Contributions
A.D.G., K.H., K.N., V.M.D., B.H., B.S.M., M.X. and W.P.L. designed, planned and/or supervised the study; A.D.G., K.H., A.F.S., D.Y., E.S., J.Z., Y.C.K., E.C.F. and J.K. performed experiments; V.C.P. and T.H. performed and analysed mass spectrometry experiments, under supervision of J.R.L.; R.R. analysed RNA-sequencing data; A.P., R.C., N.B. and M.R.-G. generated mice; M.D. and J.D.W. generated histological and immunohistochemical data; A.D.G. wrote the paper with edits from K.N. and input from co-authors.
Corresponding authors
Ethics declarations
Competing interests
All of the authors, except for A.D.G., are employees of Genentech, Inc. A.D.G. is a Visiting Scientist at Genentech, Inc.
Additional information
Peer review information Nature thanks Igor Brodsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Caspase-8-dependent cleavage of N4BP1.
a, Amino acid sequence of N4BP1 showing peptide sequences detected by mass spectrometry, from experiment depicted in Fig. 1a–c. b, Sequence alignment of mouse and human N4BP1 at indicated amino acid residues. c, Western blot of mouse BMDMs treated with LPS for indicated amounts of time. Representative of two independent experiments. d–f, Western blots of BMDMs of indicated genotypes treated with agonists of TLRs, TNF or FASL. Results representative of three independent experiments for wild-type, Mlkl−/− and Mlkl−/− Casp8−/− genotypes treated with TLR ligands; two experiments with TNF and FASL treatments; one experiment for Mlkl−/− Fadd-/- genotype. g, Western blot of wild-type and Tnfr1−/− BMDMs. Results are representative of three independent experiments. h, Graph depicts cleavage of a proluminogenic caspase-8 substrate in BMDMs of indicated genotypes following LPS stimulation. Dots represent mean fold change in luminescence relative to unstimulated cells of the same genotype from three independent experiments; error bars represent s.e.m. i, Western blots of Mlkl−/−, Mlkl−/−Casp8C362A/C362A and Mlkl−/−Casp8−/− primary mouse embryonic fibroblasts stimulated with LPS for indicated amounts of time. Representative of three independent experiments. j, Western blots of wild-type and Trif−/− BMDMs stimulated with indicated agonists for 1 h. k, Western blots of HEK293T cells transfected with a construct encoding N4BP1 and treated with TNF, z-VAD and emricasan for 4 h, as indicated. l, Western blot of cytoplasmic and nuclear extracts from BMDMs stimulated with LPS for indicated periods of time. m, Western blot of 30-min in vitro cleavage reaction containing 250 ng of recombinant N4BP1 (residues 286–893) and fourfold serial dilutions of recombinant active mouse caspase-8, starting from a highest concentration of 12.5 ng/μl. Co-incubation with 20 μM emricasan was used as a control together with the highest concentration of caspase-8. Arrowhead highlights band corresponding to cleavage at Asp488 (data not shown). Results in j–m represent two independent experiments each. Long form (L), short form (S) and cleavage product of N4BP1 are highlighted by arrowheads.
Extended Data Fig. 2 N4BP1 regulates cytokine responses and is inhibited by caspase-8.
a, Western blot of BMDMs electroporated with Cas9 enzyme complexed with control or N4bp1-targeting (∆N4bp1) gRNA. b–e, Plots show cytokine secretion of BMDMs 24 h after stimulation with poly(I:C) (b), TNF (c), Pam3csk4 (d) or CpG (e) and electroporated as in a. Values for unstimulated BMDMs in b–e are the same as those in Fig. 2a and are replotted separately here for clarity. Bars represent mean values; representative of three independent experiments with three biological replicates each. f, g, Plots show IL-6 (f) and G-CSF (g) secretion at 24 h after stimulation with indicated TLR agonists from human peripheral blood monocyte-derived macrophages that were electroporated with indicated gRNAs. Macrophages derived from three healthy human donors are shown, performed in either duplicate or triplicate wells for each condition per donor. Values for unstimulated cells replotted separately for clarity. Bars represent mean values. h, Western blot of wild-type, Mlkl−/− and Mlkl−/−Casp8−/− BMDMs electroporated with control or N4bp1-targeting gRNAs as in a. Representative of three independent experiments. i, Plots show cytokine secretion 24 h after poly(I:C) stimulation from BMDMs electroporated with indicated gRNAs as in h. Lines represent mean values; representative of three independent experiments with three biological replicates each. Values for unstimulated cells were the same as those in Fig. 2c and are replotted here separately for clarity. P values calculated using unpaired, two-tailed t-test (d–g) or one-way ANOVA with Bonferroni’s multiple comparisons test (i).
Extended Data Fig. 3 Characterization of N4bp1−/− mice.
a, Top, schematic depicting N4bp1fl allele and N4bp1−allele following germline Cre-mediated recombination of loxP sites (red triangles). Boxes represent exons, with translated regions in white and untranslated regions in grey. Green and blue lines represent recognition sequences for flp recombinase (blue, F3; green, FRT). Exons and introns not depicted to scale. Bottom, western blot of BMDMs from N4bp1+/+, N4bp1+/− and N4bp1−/− mice. Each lane represents BMDMs from an individual mouse; representative of three independent experiments. b, Plots show cytokine secretion from BMDMs of indicated genotypes 24 h after stimulation with indicated TLR agonists. Lines represent mean values; representative of three independent experiments with three biological replicates each. c–f, Histological images of liver (c), lung (d), spleen (e) and small intestines (f) from N4bp1+/+ and N4bp1−/− mice at 6–7 weeks of age. Bars represent 200 μm for c–e and 100 μm for f. Representative of n = 7 mice for each genotype. g, Serum cytokines from mice of indicated genotypes at 6–7 weeks of age. Lines represent mean values; n = 9 for N4bp1−/−, n = 9 for N4bp1+/−, and n = 5 for N4bp1+/+ mice. P values calculated using two-sided unpaired t-test (b, g). h–j, Representative flow cytometric gating for analysis of germinal centre B cells (h), T cells (i) or Ly6Chigh monocytes (j), pertaining to experiments depicted in Fig. 3a, Extended Data Figs. 4a–g, 8h–j.
Extended Data Fig. 4 Age-dependent immune dysregulation in N4bp1−/− mice.
a–g, Plots show fraction of CD44+ cells among spleen CD8+ T cells (a); number of Ly6Chigh monocytes among 105 live splenocytes (b); fraction of germinal centre (CD38−FAS+) cells among B cells of mesenteric lymph nodes (LNs) (c) and inguinal lymph nodes (d); fraction of CD44+CD62L− cells among inguinal lymph node CD4+ T cells (e); fraction of CD44+ cells among inguinal lymph node CD8+ T cells (f); and total numbers of indicated spleen cell populations (g). Lines represent mean values. For a, b, d–f, n = 7 for each genotype at 6–7 weeks old and n = 8 for each genotype at 13–14 weeks old. For c, n = 4 for each genotype at 6–7 weeks old and n = 8 for each genotype at 13–14 weeks old. For g, n = 4 for each genotype at 6–7 weeks old, n = 6 for N4bp1+/+ at 13–14 weeks old, and n = 5 for N4bp1−/− at 13–14 weeks old. h, Histological images of spleen at 13–14 weeks old. Bar represents 200 μm. Representative of n = 6 N4bp1+/+ and n = 5 N4bp1−/− mice. i, Serum cytokine levels among N4bp1+/+ and N4bp1−/− mice at 12–14 weeks old. Lines represent mean values; n = 9 for N4bp1+/+ and n = 12 for N4bp1−/− mice for G-CSF, IL-6 and CXCL1; n = 8 for N4bp1+/+ and n = 12 for N4bp1−/− mice for CCL3 measurement. j–l, White blood cell counts and haemoglobin levels (j), spleen weights (k) and serum immunoglobulin levels (l) among 13–14-week-old N4bp1+/+ (n = 6) and N4bp1−/− (n = 5) mice. Lines represent mean values. P values for a–g and i were calculated using two-sided unpaired t-test.
Extended Data Fig. 5 Absence of CD3+B220+ cell population among N4bp1−/− mice, and imiquimod-induced psoriasis model.
a, Representative flow cytometric gating for analysing CD3+B220+ cells in spleen and mesenteric lymph nodes of mice of indicated genotypes. b, Plots show per cent of CD3+B220+ cells among live splenocytes (left) or live mesenteric lymph node cells (right). Bars represent mean values; each dot represents one mouse; n = 3 wild-type mice and n = 4 N4bp1−/− mice, aged 22–27 weeks; and n = 3 Ripk3−/−Casp8−/− mice, aged 12 weeks. c, d, Haemoglobin (c) and serum cytokine levels (d) among N4bp1+/+ (n = 10) and N4bp1−/− (n = 10) mice following 4 days of daily topical imiquimod treatment from experiment depicted in Fig. 3c–e. Lines represent mean values. P values were calculated using two-sided unpaired t-test.
Extended Data Fig. 6 Signal transduction and RNA sequencing of N4bp1+/+ and N4bp1−/− BMDMs.
a, Western blots of BMDMs that were electroporated with control or N4bp1-targeting (∆N4bp1) gRNAs and stimulated with either R837 or LPS for the indicated lengths of time; representative of two independent experiments. b, Volcano plots depict transcriptional profiles of N4bp1+/+ versus N4bp1−/− BMDMs either untreated or stimulated with R837 for the indicated lengths of time. c, Four-way plot; y-axis depicts log2-transformed fold change in gene expression of N4bp1+/+ BMDMs stimulated with R837 for 4 h relative to unstimulated N4bp1+/+ BMDMs; x-axis depicts log2-transformed fold change in gene expression of N4bp1−/− BMDMs stimulated with R837 for 4 h relative to unstimulated N4bp1−/− BMDMs. Blue dots represent NF-κB target genes; red dots represent select cytokine genes. Pearson correlation coefficient shown in top-left corner of plot. d, e, Gene set enrichment analysis of upregulated genes in N4bp1−/− BMDMs relative to N4bp1+/+ BMDMs stimulated with R837 for 8 (d) or 16 (e) hours. RNA-sequencing data represent three independent biological replicates for each genotype and each condition.
Extended Data Fig. 7 Role of N4BP1 in LPS-induced transcriptional program of Mlkl−/−Casp8−/− BMDMs.
a, b, Four-way plots; y-axis shows log2-transformed fold change of Mlkl−/−Casp8−/− BMDMs relative to Mlkl−/− BMDMs, stimulated with LPS for 8 h (a, b); in a, x-axis shows log2-transformed fold change of Mlkl−/− ∆N4bp1 BMDMs relative to Mlkl−/− BMDMs stimulated with LPS for 8 h; in b, x-axis shows log2 fold change of Mlkl−/−Casp8−/− ∆N4bp1 BMDMs relative to Mlkl−/−Casp8−/− BMDMs stimulated with LPS for 8 h. Blue and red dots highlight the indicated groups of genes. c, Pie charts show the fraction of genes increased in Mlkl−/−Casp8−/− ∆N4bp1 relative to Mlkl−/−Casp8−/− BMDMs at 8 h after LPS stimulation among LPS-induced and -noninduced genes that are downregulated by caspase-8-deficiency. LPS-induced genes were those that were significantly increased in expression (P < 0.05) in wild-type BMDMs treated with LPS for 8 h relative to untreated wild-type BMDMs. Per cent of N4BP1-dependent genes and total genes analysed per group are indicated in circle within pie chart. P value calculated using two-tailed Fisher’s exact test. d, Gene set enrichment analysis of genes that are both decreased in LPS-stimulated Mlkl−/−Casp8−/− BMDMs relative to Mlkl−/− and wild-type BMDMs as well as increased in LPS-stimulated Mlkl−/−Casp8−/− ∆N4bp1 relative to Mlkl−/−Casp8−/− BMDMs. BMDMs of all genotypes in a–d were electroporated with control or N4bp1-targeting (∆N4bp1) gRNAs. RNA-sequencing data represent three independent biological replicates per condition and genotype. e, Western blots of BMDMs of indicated genotypes that were electroporated with control or N4bp1-targeting (∆N4bp1) gRNAs and stimulated with LPS for the indicated lengths of time; representative of two independent experiments. f, Protein domains of N4BP1, including its RNA-binding K homology (KH) domain, NYN RNase domain and ubiquitin chain-binding UBA- and CUE-like domains. Protein domains not depicted to size; approximate amino acid boundaries of individual protein domains shown beneath protein schematic. KH and NYN domain boundaries were determined on the basis of Interpro domain prediction (https://www.ebi.ac.uk/interpro/); UBA and CUE-like domain boundaries were determined by secondary structure alignment using Phyre253.
Extended Data Fig. 8 TNF licenses cytokine responses by TRIF-independent TLRs and N4bp1D488A/D488A mice.
a–c, Cytokine secretion among BMDMs stimulated for 24 h with TNF and/or CpG (a), Pam3csk4 (b) or poly(I:C) (c). For clarity, unstimulated and TNF-stimulated values are replotted here separately, and are the same as those from Fig. 4b. Bars represent mean values; representative of three independent experiments with three biological replicates each. P values were calculated using two-sided unpaired t-test. d–g, Representative histological images of lung (d), spleen (e), liver (f) and small intestine (g) from N4bp1D488A/D488A and N4bp1+/+ littermate controls at 10–12 weeks of age. Representative of n = 5 mice of each genotype. Bars represent 90 μm. h, i, Plots show fractions of CD44+CD62L− among CD4+ T cells in spleen or inguinal lymph nodes (h) and fraction of CD38−FAS+ germinal centre cells among B cells in spleen and mesenteric lymph nodes (i). j, Plots show absolute numbers of indicated cell populations in spleens of mice of indicated genotypes. k, l, Plots show spleen weights (k) and white blood cell counts and haemogloblin levels (l) in mice of indicated genotypes. Each dot in h–l represents one mouse; lines or bars in h–l represent mean values; n = 5 mice of each genotype.
Extended Data Fig. 9 Resistance of N4BP1(D488A) protein to TLR3-, TLR4-, TNF- and FASL-induced cleavage and TNF licensing in Mlkl−/− Casp8−/− BMDMs.
a–c, Western blots from N4bp1+/+ and N4bp1D488A/D488A BMDMs stimulated for 1 h with indicated stimuli (a, b) or with TNF for indicated lengths of time (c). Representative of three (a, b) or two (c) independent experiments. d, Plots show IL-6 secretion from BMDMs of indicated genotypes stimulated with indicated ligands. These results are the same as those presented in Fig. 4d and are replotted here separately for clarity; results represent three pooled independent experiments each with three biological replicates per condition. P values were calculated using two-sided unpaired t-test. e, Plots show IL-6 secretion from BMDMs of indicated genotypes that were electroporated with control or N4bp1-targeting (∆N4bp1) gRNAs and stimulated with TLR agonists and/or TNF for 24 h, as indicated. Values for TNF-stimulated BMDMs are the same and are replotted separately for clarity. Bars represent mean values; dots represent biological replicates; representative of two experiments.
Extended Data Fig. 10 FAS licenses cytokine responses by TRIF-independent TLRs.
a, b, Cytokine secretion among wild-type, Mlkl−/− and Mlkl−/−Casp8−/− BMDMs 24 h after stimulation with the indicated TLR agonists in the presence or absence of FASL co-stimulation. c, d, Cytokine secretion among wild-type and N4bp1D488A/D488A BMDMs 24 h after stimulation with the indicated TLR agonists in the presence or absence of FASL co-stimulation. Bars represent mean values; representative of three independent experiments with three biological replicates each. Values for cytokine secretion for BMDMs stimulated with TLR agonists alone are from the same experiments depicted in Fig. 4c, d and are replotted here separately for clarity. P values were calculated using two-sided unpaired t-test (a, b) or one-way ANOVA with Bonferroni’s multiple comparison’s test (c, d).
Supplementary information
Supplementary Figure
This file contains the uncropped gel source data.
Source data
Rights and permissions
About this article
Cite this article
Gitlin, A.D., Heger, K., Schubert, A.F. et al. Integration of innate immune signalling by caspase-8 cleavage of N4BP1. Nature 587, 275–280 (2020). https://doi.org/10.1038/s41586-020-2796-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2796-5
- Springer Nature Limited
This article is cited by
-
cFLIP in the molecular regulation of astroglia-driven neuroinflammation in experimental glaucoma
Journal of Neuroinflammation (2024)
-
N4BP1 functions as a dimerization-dependent linear ubiquitin reader which regulates TNF signalling
Cell Death Discovery (2024)
-
VCP/p97-associated proteins are binders and debranching enzymes of K48–K63-branched ubiquitin chains
Nature Structural & Molecular Biology (2024)
-
Phosphorylation of caspase-8 by RSKs via organ-constrained effects controls the sensitivity to TNF-induced death
Cell Death Discovery (2024)
-
Caspase 8 deletion causes infection/inflammation-induced bone marrow failure and MDS-like disease in mice
Cell Death & Disease (2024)