Abstract
Although two-dimensional (2D) atomic layers, such as transition-metal chalcogenides, have been widely synthesized using techniques such as exfoliation1,2,3 and vapour-phase growth4,5, it is still challenging to obtain phase-controlled 2D structures6,7,8. Here we demonstrate an effective synthesis strategy via the progressive transformation of non-van der Waals (non-vdW) solids to 2D vdW transition-metal chalcogenide layers with identified 2H (trigonal prismatic)/1T (octahedral) phases. The transformation, achieved by exposing non-vdW solids to chalcogen vapours, can be controlled using the enthalpies and vapour pressures of the reaction products. Heteroatom-substituted (such as yttrium and phosphorus) transition-metal chalcogenides can also be synthesized in this way, thus enabling a generic synthesis approach to engineering phase-selected 2D transition-metal chalcogenide structures with good stability at high temperatures (up to 1,373 kelvin) and achieving high-throughput production of monolayers. We anticipate that these 2D transition-metal chalcogenides will have broad applications for electronics, catalysis and energy storage.
Similar content being viewed by others
Main
Two-dimensional (2D) atomic-layer crystals have demonstrated many unique physical and chemical properties as well as broad applications in electronics2, sensors5, catalysts9 and batteries10,11. Generally, 2D structures such as graphene, boron nitride and transition-metal sulfides can be produced via a top-down approach, that is, by directly exfoliating the vdW counterparts through mechanical3, liquid-phase1 and electrochemical procedures2. In this manner, various vdW materials—such as metal oxides12, hydroxides13 and topological insulators14—can also be synthesized, enriching the 2D family of materials. In these 2D vdW nanocrystals, the elemental compositions, stoichiometric ratios and structural phases are usually inherited from their parent bulk counterparts, although 2D nanocrystals with phase-specific structures such as 1T and 2H phases are difficult to synthesize selectively6,7. Here we demonstrate an efficient topological conversion of non-vdW solids such as transition-metal carbides and nitrides under chalcogen vapours to 2D vdW transition-metal chalcogenide layers with identified 2H/1T phases, good stability at high temperatures (<1,373 K) and achieving high-throughput production of monolayers. We anticipate that the resultant transition-metal chalcogenide layers with favourable features would have broad applications for electronics, energy storage and conversions.
In the past decade, some unusual 2D nanocrystals have emerged from non-vdW solids such as haematite15 or bulk layered transition-metal carbides and nitrides16, namely MAX phases, greatly increasing the number of 2D material compositions accessible. In particular, the non-vdW MAX phases—where M represents a transition-metal element, A usually represents an element from groups 13–16 of the periodic table and X is carbon or nitrogen—have predominantly mixed covalent or ionic M–X bonds and metallic M–A bonds17,18. Because the M–A bonds are more chemically active than the M–X bonds, A species in MAX phases can be extracted using highly reactive solvents (hydrogen fluoride and strong bases)16,19, allowing few-layer-thick 2D transition-metal carbides, carbonitrides and nitrides—called MXenes—to be created. These 2D nanocrystals are usually terminated with defects and surface terminations of -OH, -O, -F or -Cl20,21,22. Owing to the very close atomic packing and strong chemical bonds in non-vdW solids, it remains a challenge to convert them to 2D nanocrystals with abundant exposed surfaces and identified phases.
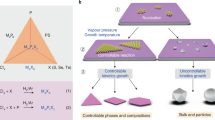
Here we demonstrate an efficient strategy that enables us to convert a family of non-vdW bulk solids such as MAX phases to 2D transition-metal chalcogenides with well-defined phases. As depicted in Fig. 1a, under chalcogen-containing vapours (HyZ, where Z represents sulfur, selenium or tellurium and y is 0 or 2) at high temperatures, non-vdW MAX phases and transition-metal borides, silicides and carbides (Supplementary Fig. 1) have high activities. In particular, the active M–A bonds in MAX phases react easily with chalcogen-containing gases, resulting in products of AZ and MZ compositions. Such reactions must produce an AZ intermediate product at high vapour pressure, which would allow rapid evaporation rates, thus boosting the continuous reaction into the bulk of the reactant material. Thermodynamically, if the reaction temperature were high enough, all the post-transition-metal A (Si, Al, Sn, Ge) species in MAX phases could be transformed to metal chalcogenide gases (Supplementary Figs. 2–5), which facilitates the conversion of MAX phases to 2D nanostructures. As an example, based on temperature–vapour pressure relationships (Fig. 1b and Supplementary Fig. 6)23,24, germanium chalcogenides (GeS, GeSe) have higher vapour pressures than other chalcogenide materials (SiS, Al2S3, SnS) at 1,073 K; from this we expect Ge-containing MAX phases to be easily converted to 2D transition-metal chalcogenides with vdW layers (Fig. 2c and Supplementary Fig. 7). According to the Clausius–Clapeyron equation25 (see equation (1) in Methods), upon increasing the temperature further to more than 1,100 K, other Si-, Sn- and Al-containing MAX phases should also serve as precursors for the generation of 2D transition-metal chalcogenides (Supplementary Fig. 8), owing to the increased vapour pressures of the products at higher reaction temperatures. Using this principle, we have synthesized 13 transition-metal chalcogenides (Supplementary Tables 1 and 2), including 7 binary chalcogenides (based on the Ti-, Nb-, Mo- and Ta-containing MAX phases and MXenes), 5 heteroatom-doped chalcogenides with selected 2H phase or 1T phase and one composite (based on quaternary MAX phases). This demonstrates that our synthetic protocol is versatile, enabling the efficient conversion of a large number of non-vdW bulk solids to 2D transition-metal chalcogenides. Notably, although the resulting 2D structures are derived from bulk MAX phases, their compositions and stoichiometric ratios are very different from the parent compositions and are also different from other products commonly derived from MAX phases, such as MXenes16,26.
a, Non-vdW solids such as MAX phases are progressively transformed to 2D transition-metal chalcogenides via a topological conversion reaction (MAX + HyZ (gas) → MZ + AZ), in which M represents an early-transition-metal element, A is an element from groups 13–16, X is C, N, B or Si, and Z refers to S, Se and Te, associated with volatile AZ products. b, Temperature–vapour pressure relationships for various AZ substances.
a, Schematic illustration of the conversion of MAX-Mo2GeC to accordion-like MoS2 under H2S gas at 1,073 K. b, X-ray diffraction patterns of MAX-Mo2GeC and accordion-like MoS2. c–e, Scanning electron microscope (c), sectional TEM (d) and high-resolution TEM (e) images of accordion-like MoS2. f, Crystalline structure of 1T TiSe2. g, Scanning electron microscope image and sectional TEM image (inset) of accordion-like TiSe2, indicating its highly expanded structure. h, Atomic-resolution STEM image of TiSe2 layers and the corresponding atomic configuration (inset) of the 1T phase. Purple and green balls represent Ti and Se atoms, respectively.
2D transition-metal chalcogenides (2H/1T)
As a proof of concept, we produced 2D transition-metal dichalcogenide (TMD)-MoS2 nanocrystals via engineering MAX-Mo2GeC under hydrogen disulfide gas at 1,073 K (Fig. 2a; see Methods). X-ray diffraction patterns reveal the disappearance of Mo2GeC peaks in the product (Fig. 2b). Instead, a series of diffraction peaks at 14.1°, 32.7°, 39.5° and 58.3° are well indexed to the (002), (100), (103) and (110) facets of hexagonal MoS2 (according to Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 37-1492), demonstrating the complete conversion and removal of Ge-layers from MAX-Mo2GeC during our synthetic process. The resulting product exhibits uniform structure with largely extended spacing in the whole scanning electron microscope image (Fig. 2c and Supplementary Fig. 9), similar to those reported for expanded graphite and MXenes16. Transmission electron microscopy (TEM) (Fig. 2d and Supplementary Fig. 10) and high-resolution TEM (Fig. 2e) confirm clearly the highly exfoliated nanocrystals with a uniform interplanar spacing of 0.28 nm, in good agreement with the spacing between the (100) facets of 2H MoS2 (ref. 2).
To identify the interior structure of the resultant MoS2, we conducted an ultrathin sectioning experiment. Many nanosheets with thicknesses from 0.4 nm to 4 nm are visible (Supplementary Fig. 11), indicating the co-existence of monolayers, bilayers and few layers in the sample. To inhibit the restacking of already-expanded 2D MoS2 and improve the fraction of monolayers, the converted samples were rapidly transferred to a low-temperature zone during our synthetic process. Thus, the fraction of monolayer MoS2 can be improved to 31% from 9% based on our standard conversion (Supplementary Figs. 12–16). Remarkably, when we directly converted thin non-vdW solid MXene-Mo2CTx in H2S gas, the fraction of monolayer MoS2 was up to about 91% (Supplementary Fig. 17). Raman spectra of the accordion-like MoS2 (Supplementary Figs. 18 and 19) show two typical peaks at 379 cm−1 and 405 cm−1, corresponding to the in-plane E2g1 and out-of-plane A1g vibrational modes of 2H MoS2 (Supplementary Fig. 67)2, respectively. On the basis of thermodynamic considerations, by enhancing the reaction temperatures to more than 1,100 K, accordion-like TiSe2 could also be derived from MAX-Ti3SiC2 by substituting H2S with selenium vapour (Fig. 2f–h, Supplementary Figs. 20–24), owing to the high vapour pressure of the SiSe product at such high temperatures (Supplementary Fig. 4). An atomic-resolution scanning transmission electron microscopy (STEM) image of TiSe2 (Fig. 2h) reveals the 1T superlattice with metal sites located at the centres of octahedral units. Such transformations suggest that our synthetic protocol can be generalized to convert non-vdW solids to vdW 2D nanocrystals with identified 2H/1T phases and high-throughput production of monolayers (Supplementary Figs. 25–36).
2D heteroatom-doped chalcogenides (2H/1T)
More than 70 ternary MAX phases27 and some new quaternary MAX phases28 such as (W2/3Y1/3)2AlC29 and (Ti1/2Nb1/2)2AlC30 have been explored, suggesting that it may be feasible to produce a series of transition-metal chalcogenides with multi-compositions via our topological conversion approach. One possibility is to produce accordion-like Y-doped WS2 with the 2H phase (Fig. 3a, b and Supplementary Figs. 37–39) based on a (W2/3Y1/3)2AlC precursor. After conversion with fast quenching, highly expanded accordion-like Y-doped WS2 can be obtained, in which the fraction of monolayers is up to 27% (Supplementary Figs. 40–45). High-resolution elemental mapping images (Fig. 3f) reveal that Y species are mainly monoatomically dispersed into the layers, associated with a few Y clusters, possibly accounting for the many Y–S bonds present (Supplementary Fig. 46). A typical 2H structure is visualized in an aberration-corrected TEM image (Fig. 3c), consistent with X-ray diffraction (Supplementary Fig. 38) and Raman analysis (Fig. 3h), indicating that the heteroatoms remain stable within the host 2D transition-metal chalcogenide structure. To further verify the Y–S bonds in Y-doped WS2, we conducted X-ray absorption near-edge fine structure (XANES) spectroscopy measurements. In the case of Y-doped WS2, the absorption edge in Y K-edge XANES spectra (Fig. 3i) is close to that of yttrium sulfide, suggesting that the Y is in the sulfide state. The Fourier transform spectra resulting from the analysis of Y-doped WS2 by extended X-ray absorption fine structure (EXAFS) spectroscopy (Fig. 3j) show a dominant peak at 2.2 Å, indexed to the Y–S bonds in comparison with yttrium sulfide. Theoretically, the energy of 2H WS2 is much lower than its 1T phase (>0.6 eV per formula unit). With increasing Y doping levels, the energy differences between 2H and 1T phases noticeably decrease, but it remains difficult to reverse the relative stability (Supplementary Fig. 68). Owing to the substitution of W (valency +4) in WS2 by low-valence Y (+3), the charge densities near the Fermi level tend to be localized around Y atoms, thus going against electron transfer (Supplementary Fig. 69). Moreover, the increase of defect scattering by Y dopants would also reduce the electrical conductivity. Thus, the electrical conductivity of Y-doped WS2 is experimentally measured to be 2.31 × 10−3 S cm−1, much lower than that of pure WS2 (8.13 × 10−2 S cm−1) (Supplementary Table 3). This is in contrast to those reported for d orbital electron-enriched metal (Re, Nb)-doped MoS231,32 and our Nb-doped TiSe2 (Supplementary Figs. 47–50), exhibiting substantial improvements in the electrical conductivities (Supplementary Figs. 51, 70 and Supplementary Table 3).
a, Schematic illustration of the conversion of quaternary MAX-(W2/3Y1/3)2AlC to Y-doped WS2. b, Typical scanning electron microscope image of Y-doped WS2, showing the expanded structure. c, d, High-resolution TEM (c) and high-angle annular dark-field (d) images of Y-doped WS2 layers. e–g, Elemental mapping images of W (e), Y (f) and S (g) species in Y-doped WS2 layers. h, Raman spectra of Y-doped WS2 and bulk WS2. i, j, Y K-edge XANES spectra (i) and Fourier transform spectra (j) of Y K-edge EXAFS for Y-doped WS2, demonstrating the presence of Y–S bonds in Y-doped WS2.
2D heteroatoms (Y, P) co-doped WS2 (1T)
While engineering the quaternary MAX phase (W2/3Y1/3)2AlC, other vapours such as phosphorus could be easily and simultaneously introduced into the synthetic system, creating both Y and P co-doped WS2 (Supplementary Figs. 52–56). As illustrated by the Raman spectra (Fig. 4d), for Y, P co-doped WS2 there are three dominant peaks at 130 cm−1 (J1), 258 cm−1 (J2) and 406 cm−1 (J3), corresponding to the vibration modes of the 1T phase6,33, as well as two peaks at 348 cm−1 and 414 cm−1, indexed to the E2g1 and A1g peaks of the 2H phase, respectively. The presence of the 1T phase in real space can be visualized via aberration-corrected STEM images (Fig. 4a and Supplementary Fig. 57). Combined with the intensities of cation and anion sites, the S and W elements can be identified using image contrast, as shown by the line intensity profile (Fig. 4c) acquired along the highlighted arrow in Fig. 4a. All the metal W atoms are located at the centres of octahedral units, in accordance with the atomic models of the 1T phase4,34 (Fig. 4b). Y elemental mapping images (Supplementary Figs. 58a and 59a) show that all the Y atoms are located at the centres of octahedral units, occupying the W sites in WS2. This can be further demonstrated via the line intensity profiles (Supplementary Figs. 58c, 58e and 59c), in which the intensities of the Y sites are weaker than those of the W sites and stronger than those of the S sites, directly demonstrating the presence of Y–S bonds in Y, P co-doped WS2. By carefully analysing the Y, P elemental mapping images and the line intensity profiles (Supplementary Figs. 58f, 58g, 59d–i), it is clear that the P atoms are located on the top of both Y and S sites, verifying the presence of Y–P and P–S bonds in the sample. These bonds can be further demonstrated via Fourier transform spectra of EXAFS (Fig. 4e and Supplementary Fig. 60), where there are two dominant peaks between 1.3 Å and 2.8 Å, corresponding to the overlap of Y–O, Y–S and Y–P bonds at 1.7 Å, 2.2 Å and 2.4 Å, respectively. In the P K-edge XANES spectra of Y, P co-doped WS2 (Fig. 4f), there is one prominent peak centred at 2,158 eV, attributed to the overlap of P–Y bonds (2,157 eV) and P–S bonds (2,159 eV), in good agreement with the above EXAFS, aberration-corrected STEM images and the corresponding elemental mapping analysis.
a, Atomic-resolution STEM image and its corresponding fast Fourier transform patterns (inset). b, Atomic configuration of Y, P co-doped WS2, exhibiting the 1T phase. c, Line intensity profile along the highlighted arrow in a. d, Raman spectra of Y, P co-doped WS2. e, Fourier transform spectra of Y K-edge EXAFS. f, P K-edge XANES spectra for Y, P co-doped WS2. g, Current versus voltage curves of Y, P co-doped WS2, Y-doped WS2 and exfoliated WS2.
Density functional theory calculations confirm that as P atoms adsorb on the hollow site 2 (h2), sulfur site 2 (S2) and tungsten site (W), the energy differences between 2H and 1T WS2 are negligible. However, when P atoms adsorb on the top of the Y atoms and their neighbours such as hollow site 1 (h1) and sulfur site 1 (S1), the energies of the 1T WS2 are surprisingly lower than when in the 2H phase, revealing that there is a unique yttrium–phosphorus (Y–P) joint effect that stabilizes the configuration of the 1T phase (Supplementary Figs. 71 and 72). Furthermore, it is difficult to reverse the relative energy stability between 2H and 1T WS2 by independent Y-doping or P-adsorption (Supplementary Figs. 61 and 73). Even after storage under ambient conditions for about one year, the 1T-containing WS2 remains stable (Supplementary Fig. 62), unlike the 1T transition-metal dichalcogenides produced via traditional methods that have poor stability at high temperatures (>573 K)8,35. Such Y, P co-doped WS2 exhibits a linear current–voltage (I–V) characteristic with a low resistance of 387 kΩ per □, close to that reported for 1T′ WS2 (430 kΩ per □)6, three orders of magnitude lower than those of 2H Y-doped WS2 (413 MΩ per □) and exfoliated WS2 (124 MΩ per □) (Fig. 4g, Supplementary Fig. 63 and Supplementary Table 3).
The accordion-like structure, highly exposed surfaces and abundant 1T phase (Fig. 4d and Supplementary Fig. 64) of the resultant transition-metal dichalcogenides mean that they could be directly used as electrocatalysts for the hydrogen evolution reaction (Supplementary Figs. 65 and 66). We believe that our synthetic protocol has the potential to convert a series of non-vdW solids to 2D vdW nanocrystals with selected phases, achieving high-throughput monolayers, specific dopants and tailored electronic features, as well as broad applications in fields such as electronics, catalysis and energy storage.
Methods
Synthesis of MAX phases
Some MAX phases were synthesized by ball-milling of commercially available powders and subsequent calcination treatments36,37,38,39,40. Taking Mo2GeC as an example, commercial Mo, Ge and graphite in a molar ratio of 2:1.05:1 were sealed in an agate container with agate balls and milled at 600 rpm for 20 h. The mixture was then heated at a rate of 3 K min−1 until it reached 1,673 K and was maintained at this high temperature for 4 h. After cooling to room temperature, the bulk was ground to produce MAX-Mo2GeC. For other MAX phases, the details are listed in Materials and Methods in the Supplementary Information.
Synthesis of 2D transition-metal chalcogenides
Transition-metal sulfides were prepared by the reaction of MAX phases or MoB with H2S gas at temperatures of 1,073–1,373 K. Taking a TMD-MoS2 as an example, 300 mg of MAX-Mo2GeC was heated at a heating rate of 10 K min−1 under Ar flow, and an H2S/Ar (10 vol.% H2S) mixture was injected when the temperature reached 1,073 K. MAX-Mo2GeC was maintained at this temperature for 4 h, generating TMD-MoS2. Transition metal selenides were prepared by the reaction of MAX phases, MoB and MoSi2 with Se vapours at temperatures of 1,073–1,373 K. Specifically, 2 g of Se powder and 300 mg of MAX-Ti3SiC2 were placed in low- (973 K) and high- (1,173–1,273 K) temperature zones, respectively.
Synthesis of 2D heteroatom-doped transition-metal chalcogenides
Heteroatom-doped transition-metal chalcogenides were synthesized by the reaction of quaternary MAX phases with chalcogen-containing gases. Specifically, 300 mg of MAX-(W2/3Y1/3)2AlC was heated at a heating rate of 10 K min−1 under Ar flow, and then H2S/Ar mixture was injected when the temperature reached 1,273 K. MAX-(W2/3Y1/3)2AlC was maintained there for 4 h to produce Y-doped WS2. Nb-doped TiSe2 was derived from MAX-(Ti1/2Nb1/2)2AlC using the same procedures as for making TMD-TiSe2.
Synthesis of 2D heteroatoms (Y and P) co-doped WS2
Y, P co-doped WS2 was synthesized by the reaction of MAX-(W2/3Y1/3)2AlC with H2S gas and P vapour at a high temperature of 1,273 K. Specifically, 300 mg of (W2/3Y1/3)2AlC and 1 g of P were placed into two separate crucibles, where P powder was placed in a low-temperature upstream zone maintained at 873 K. (W2/3Y1/3)2AlC was heated at a heating rate of 10 K min−1 under Ar flow, and then the H2S/Ar mixture was injected when the temperature reached 1,273 K. (W2/3Y1/3)2AlC was maintained at 1,273 K for 4 h to produce Y, P co-doped WS2. Similarly, P-doped MoS2 was prepared by the reaction of MAX-Mo2GeC with H2S gas and P vapour at a high temperature of 1,073 K.
Synthesis of P-doped WS2
P-doped WS2 was synthesized by the reaction of bulk WS2 with P vapour at high temperature. Specifically, 300 mg of WS2 powders were put in a porcelain boat with 1 g of P at the upstream zone.Then the boat was heated to 1,273 K at a heating rate of 10 K min−1 under Ar flow and kept there for 30 min to generate P-doped WS2.
Fabrication of thin films of Y, P co-doped WS2, Y-doped WS2 and exfoliated WS2
Y, P co-doped WS2 thin film was fabricated by vacuum filtration of Y, P co-doped WS2 nanosheets on nylon membrane filters, which were acquired by a facile liquid exfoliation of accordion-like Y, P co-doped WS2 in an isopropyl alcohol solvent1. Other thin films of Y-doped WS2, WS2, Nb-doped TiSe2 and TiSe2 were similarly obtained.
Characterization
The morphology and microstructure of materials were characterized by scanning electron microscopy (Zeiss MERLIN Compact), transmission electron microscopy (JEOL 2100F), spherical aberration-corrected transmission electron microscopy (FEI Titan G2) and X-ray diffraction (Rigaku D/MAX2200pc). Raman spectra were recorded on a Renishaw inVia Microscopic confocal Raman spectrometer using a 532-nm laser beam. X-ray photoelectron spectroscopy was recorded by a Thermo Electron ESCALAB 250 XPS spectrometer. Atomic force microscopy measurements were carried out on a Dimension ICON scanning probe microscope (Veeco/Bruker). X-ray absorption near-edge fine structure (XANES) and extended X-ray absorption fine structure (EXAFS) data for the Y K-edge were collected on BL14W1 and BL1W1B at the Shanghai Synchrotron Radiation Facility and the Beijing Synchrotron Radiation Facility, respectively. XANES data for the P K-edge were collected on BL4B7B at the Beijing Synchrotron Radiation Facility. Current-versus-voltage measurements were conducted using the two-electrode method on an electrochemical workstation (CHI760E, CH Instruments) in a voltage range of −1 V to 1 V at a scan rate of 10 mV s−1. The electrical conductivities of powder samples were investigated on a four-probe powder resistivity tester (ST2722-SZ, Suzhou Jingge Electronic Co., Ltd).
Vapour pressure calculations
With the aid of log Kf values for two-phase equilibria solid–gas or liquid–gas (AZsol,liq–AZgas), we calculated the vapour pressures of AZ gases according to the following equation23:
where p is the vapour pressure, a is the activity of AZ in the condensed phase, Kf is the equilibrium constant of formation reaction and log Kf values taken from the literature23 are partially listed in Supplementary Tables 4–7. In general, a = 1 for pure substances in a condensed phase.
For the evaporation equation of pure substances at a given temperature T:
and
where p is in units of bar (1 bar = 105 Pa). Taking 1/T and ln p as the horizontal and vertical axis respectively, linear curves were made as shown in Supplementary Fig. 6. According to the Clausius–Clapeyron equation25:
where p is vapour pressure, ΔHm is molar enthalpy of evaporation, R is molar gas constant and C is a constant, these linear curves confirm that the Clausius–Clapeyron equation can be applied to evaluate the relationship between vapour pressure and temperature.
In the case of AZ substances that lack log Kf values in the literature, the boiling point and the corresponding vapour pressure (1 atm = 101,325 Pa) are selected to evaluate the relationship between vapour pressure and temperature (Supplementary Table 8)24.
Data availability
The data that support the findings of this study are available from the corresponding authors on reasonable request.
References
Coleman, J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011).
Lin, Z. et al. Solution-processable 2D semiconductors for high-performance large-area electronics. Nature 562, 254–258 (2018).
Huang, Y. et al. Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano 9, 10612–10620 (2015).
Zhou, J. et al. A library of atomically thin metal chalcogenides. Nature 556, 355–359 (2018).
Chen, X. et al. CVD-grown monolayer MoS2 in bioabsorbable electronics and biosensors. Nat. Commun. 9, 1690–1701 (2018).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013).
Zhu, J. et al. Argon plasma induced phase transition in monolayer MoS2. J. Am. Chem. Soc. 139, 10216–10219 (2017).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011).
Zhang, J. et al. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 1, 985–992 (2018).
Kundu, D., Adams, B. D., Ort, V. D., Vajargah, S. H. & Nazar, L. F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 1, 16119–16126 (2016).
Zhang, C. et al. High capacity silicon anodes enabled by MXene viscous aqueous ink. Nat. Commun. 10, 849–857 (2019).
Yan, M. et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 30, 1703725–1703730 (2018).
Wang, Y. et al. In situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction. Adv. Funct. Mater. 28, 1703363–1703368 (2018).
Liu, J., Qian, X. & Fu, L. Crystal field effect induced topological crystalline insulators in monolayer IV–VI semiconductors. Nano Lett. 15, 2657–2661 (2015).
Balan, A. P. et al. Exfoliation of a non-van der Waals material from iron ore hematite. Nat. Nanotechnol. 13, 602–609 (2018).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Barsoum, M. W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides (Wiley, 2013).
Sokol, M., Natu, V., Kota, S. & Barsoum, M. W. On the chemical diversity of the MAX phases. Trends Chem. 1, 210–223 (2019).
Xuan, J. et al. Organic-base-driven intercalation and delamination for the production of functionalized titanium carbide nanosheets with superior photothermal therapeutic performance. Angew. Chem. Int. Ed. 55, 14569–14574 (2016).
Ghidiu, M., Lukatskaya, M. R., Zhao, M. Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Li, M. et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 141, 4730–4737 (2019).
Barin, I. Thermochemical Data of Pure Substances 3rd edn (VCH, 1995).
Yaws, C. L. The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals 2nd edn (Elsevier, 2015).
Winterbone, D. E. Advanced Thermodynamics for Engineers (Butterworth-Heinemann, 1997).
Naguib, M., Mochalin, V. N., Barsoum, M. W. & Gogotsi, Y. 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv. Mater. 26, 992–1005 (2014).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098–16114 (2017).
Anasori, B. et al. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 9, 9507–9516 (2015).
Meshkian, R. et al. W-based atomic laminates and their 2D derivative W1.33C MXene with vacancy ordering. Adv. Mater. 30, 1706409–1706416 (2018).
Naguib, M. et al. Two-dimensional transition metal carbides. ACS Nano 6, 1322–1331 (2012).
Deepak, F. L. et al. Fullerene-like (IF) NbxMo1-xS2 nanoparticles. J. Am. Chem. Soc. 129, 12549–12562 (2007).
Yang, S. Z. et al. Rhenium-doped and stabilized MoS2 atomic layers with basal-plane catalytic activity. Adv. Mater. 30, 1803477–1803483 (2018).
Liu, Q. et al. Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions: the correlation between structure and electrical/optical properties. Adv. Mater. 27, 4837–4844 (2015).
Radhakrishnan, S. et al. An insight into the phase transformation of WS2 upon fluorination. Adv. Mater. 30, 1803366–1803375 (2018).
Lin, Y. C., Dumcencon, D. O., Huang, Y. S. & Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotechnol. 9, 391–396 (2014).
Sun, Z. M. Progress in research and development on MAX phases: a family of layered ternary compounds. Int. Mater. Rev. 56, 143–166 (2011).
Barsoum, M. W. The MN+1AXN phases: a new class of solids: thermodynamically stable nanolaminates. Prog. Solid State Chem. 28, 201–281 (2000).
Eklund, P., Beckers, M., Jansson, U., Hogberg, H. & Hultman, L. The Mn+1AXn phases: materials science and thin-film processing. Thin Solid Films 518, 1851–1878 (2010).
Magnuson, M. & Mattesini, M. Chemical bonding and electronic-structure in MAX phases as viewed by X-ray spectroscopy and density functional theory. Thin Solid Films 621, 108–130 (2017).
Naguib, M. et al. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 135, 15966–15969 (2013).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant numbers 51622203 and 51572007), the Youth 1000-Talent Program of China and the 111 Project (grant number B17002). X.Z. also thanks Shenzhen Basic Research Projects (grant number JCYJ20170407155608882), the China Postdoctoral Science Foundation (grant number 2018M631458) and the Development and Reform Commission of Shenzhen Municipality for the development of the “Low-Dimensional Materials and Devices” Discipline, Guangdong Innovative and Entrepreneurial Research Team Program (grant number 2017ZT07C341). We thank the Shanghai Synchrotron Radiation Facility and Beijing Synchrotron Radiation Facility for support. We thank X. Chen, Q. Zhang, W. Zhou and M. Li for help with the TEM analysis; S. Chen and Y. Lin for help with the EXAFS analysis; and L. Ma for suggestions.
Author information
Authors and Affiliations
Contributions
S.Y., S.L. and P.M.A. supervised the project. S.Y. and Z.D. designed and carried out all of the experiments. S.Z. and X.Z. carried out the density functional theory calculations. S.W. and B.L. performed the scanning electron microscope, TEM and X-ray diffraction measurements. B.L. carried out the X-ray photoelectron spectroscopy and Raman analysis. L.S. contributed to the XANES and EXAFS measurements. Z.D. performed the atomic force microscopy. Z.D. and Y.G. designed the film electrodes and carried out the electrical and electrocatalytic measurements. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Per Eklund, Wei Sun Leong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains the Supplementary Information (sections 1-6), which includes Supplementary Materials and Methods, Supplementary Figures 1-73, Supplementary Tables 1-9, Supplementary Text and Supplementary References–see contents page for details.
Rights and permissions
About this article
Cite this article
Du, Z., Yang, S., Li, S. et al. Conversion of non-van der Waals solids to 2D transition-metal chalcogenides. Nature 577, 492–496 (2020). https://doi.org/10.1038/s41586-019-1904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1904-x
- Springer Nature Limited
This article is cited by
-
Flash-within-flash synthesis of gram-scale solid-state materials
Nature Chemistry (2024)
-
Emerging chiral two-dimensional materials
Nature Chemistry (2024)
-
Carbon aerogel supported Ni–Fe catalysts for superior oxygen evolution reaction activity
Carbon Letters (2024)
-
Epitaxial substitution of metal iodides for low-temperature growth of two-dimensional metal chalcogenides
Nature Nanotechnology (2023)
-
Emergence of superconductivity in 2D Cu0.65NbS2 nanosheets via copper ion hopping
Science China Materials (2023)