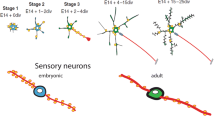
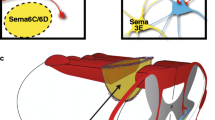
Abstract
Mammalian neurons lose the ability to regenerate their central nervous system axons as they mature during embryonic or early postnatal development. Neuronal maturation requires a transformation from a situation in which neuronal components grow and assemble to one in which these components are fixed and involved in the machinery for effective information transmission and computation. To regenerate after injury, neurons need to overcome this fixed state to reactivate their growth programme. A variety of intracellular processes involved in initiating or sustaining neuronal maturation, including the regulation of gene expression, cytoskeletal restructuring and shifts in intracellular trafficking, have been shown to prevent axon regeneration. Understanding these processes will contribute to the identification of targets to promote repair after injury or disease.





Similar content being viewed by others
References
He, Z. & Jin, Y. Intrinsic control of axon regeneration. Neuron 90, 437–451 (2016).
Zheng, B. & Tuszynski, M. H. Regulation of axonal regeneration after mammalian spinal cord injury. Nat. Rev. Mol. Cell Biol. 24, 396–413 (2023).
Björklund, A. Long distance axonal growth in the adult central nervous system. J. Neurol. 242, S33–S35 (1994).
Lu, P. et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 (2012).
Wang, X., Terman, J. & Martin, G. Regeneration of supraspinal axons after transection of the thoracic spinal cord in the developing opossum, Didelphis virginiana. J. Comp. Neurol. 398, 83–97 (1998).
Ruven, C. et al. Long-distance axon growth ability of corticospinal neurons is lost in a segmentally-distinct manner. Preprint in bioRxiv https://doi.org/10.1101/2022.03.20.484375 (2022). Using a novel microsurgical approach to lesion axons in the developing mouse, this preprint reports that neurons lose the ability to regenerate as they transition from elongating to arborizing axons during early postnatal development.
Mahar, M. & Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 19, 323–337 (2018).
Palmisano, I. & Di Giovanni, S. Advances and limitations of current epigenetic studies investigating mammalian axonal regeneration. Neurotherapeutics 15, 529–540 (2018).
Blanquie, O. & Bradke, F. Cytoskeleton dynamics in axon regeneration. Curr. Opin. Neurobiol. 51, 60–69 (2018).
Bradke, F., Di Giovanni, S. & Fawcett, J. Neuronal maturation: challenges and opportunities in a nascent field. Trends Neurosci. 43, 360–362 (2020).
Fawcett, J. W. The struggle to make CNS axons regenerate: why has it been so difficult? Neurochem. Res. 45, 144–158 (2020).
Hilton, B. J. et al. An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron 110, 51–69.e7 (2022). Core molecular components of the presynaptic active zone with a limited role in axon growth during neuronal development play a major role in preventing axon growth and regeneration in mature neurons.
Hollville, E., Romero, S. E. & Deshmukh, M. Apoptotic cell death regulation in neurons. FEBS J. 286, 3276–3298 (2019).
Schelski, M. & Bradke, F. Neuronal polarization: from spatiotemporal signaling to cytoskeletal dynamics. Mol. Cell. Neurosci. 84, 11–28 (2017).
Coles, C. H. & Bradke, F. Coordinating neuronal actin–microtubule dynamics. Curr. Biol. 25, R677–R691 (2015).
Wallace, J. L. & Pollen, A. A. Human neuronal maturation comes of age: cellular mechanisms and species differences. Nat. Rev. Neurosci. 25, 7–29 (2023).
Bareyre, F. M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269 (2004).
Fouad, K., Pedersen, V., Schwab, M. E. & Brösamle, C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol. 11, 1766–1770 (2001).
Li, Y. et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 587, 613–618 (2020). Neonatal microglia resolve inflammation by secreting peptidase inhibitors to prevent fibrotic scarring and enable robust repair following spinal cord injury.
Schwab, M. E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 11, 799–811 (2010).
Vinopal, S. et al. Centrosomal microtubule nucleation regulates radial migration of projection neurons independently of polarization in the developing brain. Neuron 111, 1241–1263.e16 (2023). This study shows how the two interwoven dynamic processes — radial migration and axon growth — are separately controlled: by selective dependence of centrosomal and acentrosomal microtubule nucleation.
Luo, L. & O’Leary, D. D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 (2005).
O’Leary, D. D. & Terashima, T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and “waiting periods”. Neuron 1, 901–910 (1988).
Stanfield, B. B., O’Leary, D. D. & Fricks, C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature 298, 371–373 (1982).
Südhof, T. C. Towards an understanding of synapse formation. Neuron 100, 276–293 (2018).
Südhof, T. C. The presynaptic active zone. Neuron 75, 11–25 (2012).
Washbourne, P. et al. Cell adhesion molecules in synapse formation. J. Neurosci. 24, 9244–9249 (2004).
Petanjek, Z. et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl Acad. Sci. USA 108, 13281–13286 (2011).
Kano, M. & Hashimoto, K. Synapse elimination in the central nervous system. Curr. Opin. Neurobiol. 19, 154–161 (2009).
Kano, M. et al. Persistent multiple climbing fiber innervation of cerebellar purkinje cells in mice lacking mGluR1. Neuron 18, 71–79 (1997).
Caceres, A., Ye, B. & Dotti, C. G. Neuronal polarity: demarcation, growth and commitment. Curr. Opin. Cell Biol. 24, 547–553 (2012).
Haas, K. in Molecular Mechanisms of Synaptogenesis (eds Dityatev, A. & El-Husseini, A.) 297–309 (Springer, 2006).
Ambrogini, P. et al. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 1017, 21–31 (2004).
Bean, B. P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007).
Atwood, H. L. & Karunanithi, S. Diversification of synaptic strength: presynaptic elements. Nat. Rev. Neurosci. 3, 497–516 (2002).
Canty, A. & Murphy, M. Molecular mechanisms of axon guidance in the developing corticospinal tract. Prog. Neurobiol. 85, 214–235 (2008).
Goldberg, J. L., Klassen, M. P., Hua, Y. & Barres, B. A. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296, 1860–1864 (2002).
Tedeschi, A. et al. The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92, 419–434 (2016).
Kitao, Y., Robertson, B., Kudo, M. & Grant, G. Neurogenesis of subpopulations of rat lumbar dorsal root ganglion neurons including neurons projecting to the dorsal column nuclei. J. Comp. Neurol. 371, 249–257 (1996).
Sharma, N. et al. The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398 (2020). This paper presents a transcriptomic atlas of the developing mouse dorsal root ganglion and shows that primary sensory neurons mature owing to the expression of subtype-restricted transcription factors in response to extrinsic cues.
Prasad, T. & Weiner, J. A. Direct and indirect regulation of spinal cord Ia afferent terminal formation by the γ-protocadherins. Front. Mol. Neurosci. 4, 54 (2011).
Ramón y Cajal, S. Asociación método del nitrato de plata con el embrionario para el estudio de los focos motores y sensitivos. Trab. Lab. Invest. Biol. Univ. Madr. 3, 65–96 (1904).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Ylera, B. et al. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr. Biol. 19, 930–936 (2009).
Richardson, P. & Issa, V. Peripheral injury enhances central regeneration of primary sensory neurones. Nature 309, 791–793 (1984).
Karimi-Abdolrezaee, S., Verge, V. M. & Schreyer, D. J. Developmental down-regulation of GAP-43 expression and timing of target contact in rat corticospinal neurons. Exp. Neurol. 176, 390–401 (2002).
Koseki, H. et al. Selective rab11 transport and the intrinsic regenerative ability of CNS axons. eLife 6, e26956 (2017).
Lorenzana, A. O., Lee, J. K., Mui, M., Chang, A. & Zheng, B. A surviving intact branch stabilizes remaining axon architecture after injury as revealed by in vivo imaging in the mouse spinal cord. Neuron 86, 947–954 (2015).
Kim, H. et al. Oligodendrocyte precursor cells stop sensory axons regenerating into the spinal cord. Cell Rep. 42, 113068 (2023).
Filous, A. R. et al. Entrapment via synaptic-like connections between NG2 proteoglycan + cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J. Neurosci. 34, 16369–16384 (2014).
Liu, K. et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 13, 1075–1081 (2010).
Enes, J. et al. Electrical activity suppresses axon growth through Cav1.2 channels in adult primary sensory neurons. Curr. Biol. 20, 1154–1164 (2010).
Eggermann, E., Bucurenciu, I., Goswami, S. P. & Jonas, P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci. 13, 7–21 (2012).
Calloway, N., Gouzer, G., Xue, M. & Ryan, T. A. The active-zone protein Munc13 controls the use-dependence of presynaptic voltage-gated calcium channels. eLife 4, e07728 (2015).
Augustin, I., Rosenmund, C., Südhof, T. C. & Brose, N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400, 457–461 (1999).
van de Bospoort, R. et al. Munc13 controls the location and efficiency of dense-core vesicle release in neurons. J. Cell Biol. 199, 883–891 (2012).
Panayotis, N., Karpova, A., Kreutz, M. R. & Fainzilber, M. Macromolecular transport in synapse to nucleus communication. Trends Neurosci. 38, 108–116 (2015).
Hardingham, G. E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Herold, S., Jagasia, R., Merz, K., Wassmer, K. & Lie, D. C. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol. Cell Neurosci. 46, 79–88 (2011).
Bloom, O. E. & Morgan, J. R. Membrane trafficking events underlying axon repair, growth, and regeneration. Mol. Cell. Neurosci. 48, 339–348 (2011).
Broeke, J. H. et al. Munc18 and Munc13 regulate early neurite outgrowth. Biol. Cell 102, 479–488 (2010).
Zhu, X.-H. et al. Quantitative imaging of energy expenditure in human brain. Neuroimage 60, 2107–2117 (2012).
Zhou, B. et al. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J. Cell Biol. 214, 103–119 (2016).
Aprea, J. et al. Transcriptome sequencing during mouse brain development identifies long non‐coding RNAs functionally involved in neurogenic commitment. EMBO J. 32, 3145–3160 (2013).
Gallegos, D. A., Chan, U., Chen, L.-F. & West, A. E. Chromatin regulation of neuronal maturation and plasticity. Trends Neurosci. 41, 311–324 (2018).
Allshire, R. C. & Madhani, H. D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 19, 229–244 (2018).
Zhang, Y. et al. Overview of histone modification. Adv. Exp. Med. Biol. 1283, 1–16 (2021).
Guibert, S. & Weber, M. Functions of DNA methylation and hydroxymethylation in mammalian development. Curr. Top. Dev. Biol. 104, 47–83 (2013).
Yoo, A. S. & Crabtree, G. R. ATP-dependent chromatin remodeling in neural development. Curr. Opin. Neurobiol. 19, 120–126 (2009).
Venkatesh, I., Simpson, M. T., Coley, D. M. & Blackmore, M. G. Epigenetic profiling reveals a developmental decrease in promoter accessibility during cortical maturation in vivo. Neuroepigenetics 8, 19–26 (2016).
Wang, X.-W. et al. Histone methyltransferase Ezh2 coordinates mammalian axon regeneration via regulation of key regenerative pathways. J. Clin. Invest. 134, e163145 (2023). This paper demonstrates the importance of histone methylation in axon regeneration.
Renthal, W. et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 108, 128–144.e9 (2020). Single-nucleus RNA sequencing demonstrates that peripheral axotomy of primary sensory neurons triggers reversible transcriptional reprogramming to enable axon regeneration.
Tetzlaff, W., Alexander, S. W., Miller, F. D. & Bisby, M. A. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J. Neurosci. 11, 2528–2544 (1991).
Poplawski, G. H. et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature 581, 77–82 (2020). Gene expression profiling of corticospinal neurons following spinal cord injury reveals that mature neurons can upregulate the expression of regeneration-associated genes following axotomy but fail to sustain their expression.
Fernandes, K. J., Fan, D. P., Tsui, B., Cassar, S. & Tetzlaff, W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: differential regulation of GAP‐43, tubulins, and neurofilament‐M. J. Comp. Neurol. 414, 495–510 (1999).
Wang, Z. et al. Injury distance limits the transcriptional response to spinal injury. Preprint at bioRxiv https://doi.org/10.1101/2024.05.27.596075 (2024).
Kim, H. J. et al. Deep scRNA sequencing reveals a broadly applicable Regeneration Classifier and implicates antioxidant response in corticospinal axon regeneration. Neuron 111, 3953–3969.e5 (2023). Single-cell sequencing (at high depth yet with a low throughput) of corticospinal neurons that regenerate due to genetic deletion of the tumour suppressor genes PTEN and SOCS3 establishes a role for antioxidant response in axon regeneration.
Moore, D. L. et al. KLF family members regulate intrinsic axon regeneration ability. Science 326, 298–301 (2009).
Norsworthy, M. W. et al. Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron 94, 1112–1120.e4 (2017).
Venkatesh, I., Mehra, V., Wang, Z., Califf, B. & Blackmore, M. G. Developmental chromatin restriction of ro‐growth gene networks acts as an epigenetic barrier to axon regeneration in cortical neurons. Dev. Neurobiol. 78, 960–977 (2018).
Deaton, A. M. & Bird, A. CpG islands and the regulation of transcription. Genes Dev. 25, 1010–1022 (2011).
Weng, Y.-L. et al. An intrinsic epigenetic barrier for functional axon regeneration. Neuron 94, 337–346.e6 (2017).
Loh, Y.-H. E. et al. Comprehensive mapping of 5-hydroxymethylcytosine epigenetic dynamics in axon regeneration. Epigenetics 12, 77–92 (2017).
Lu, Y. et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020). Overexpression of Yamanaka factors in mature retinal ganglion cell neurons elicits axon regeneration by reverting these neurons to a more youthful DNA methylation pattern.
Oh, Y. M. et al. Epigenetic regulator UHRF1 inactivates REST and growth suppressor gene expression via DNA methylation to promote axon regeneration. Proc. Natl Acad. Sci. USA 115, E12417–E12426 (2018).
Smith, J., Sen, S., Weeks, R. J., Eccles, M. R. & Chatterjee, A. Promoter DNA hypermethylation and paradoxical gene activation. Trends cancer 6, 392–406 (2020).
Reverdatto, S. et al. Developmental and injury-induced changes in DNA methylation in regenerative versus non-regenerative regions of the vertebrate central nervous system. BMC Genomics 23, 2 (2022).
Lindner, R., Puttagunta, R., Nguyen, T. & Di Giovanni, S. DNA methylation temporal profiling following peripheral versus central nervous system axotomy. Sci. Data 1, 140038 (2014).
Margueron, R. & Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349 (2011).
Pereira, J. D. et al. Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc. Natl Acad. Sci. USA 107, 15957–15962 (2010).
Zhang, M. et al. Neuronal histone methyltransferase EZH2 regulates neuronal morphogenesis, synaptic plasticity, and cognitive behavior in mice. Neurosci. Bull. 39, 1512–1532 (2023).
Henriquez, B. et al. Ezh1 and Ezh2 differentially regulate PSD-95 gene transcription in developing hippocampal neurons. Mol. Cell. Neurosci. 57, 130–143 (2013).
Scimemi, A. Structure, function, and plasticity of GABA transporters. Front. Cell. Neurosci. 8, 161 (2014).
Habib, A. A. et al. Expression of the oligodendrocyte‐myelin glycoprotein by neurons in the mouse central nervous system. J. Neurochem. 70, 1704–1711 (1998).
Becker, T. et al. Tenascin‐R inhibits regrowth of optic fibers in vitro and persists in the optic nerve of mice after injury. Glia 29, 330–346 (2000).
Hollis, E. R. II Axon guidance molecules and neural circuit remodeling after spinal cord injury. Neurotherapeutics 13, 360–369 (2016).
Kim, J. et al. Polycomb-and methylation-independent roles of EZH2 as a transcription activator. Cell Rep. 25, 2808–2820.e4 (2018).
Laugesen, A., Højfeldt, J. W. & Helin, K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol. Cell 74, 8–18 (2019).
Jambhekar, A., Dhall, A. & Shi, Y. Roles and regulation of histone methylation in animal development. Nat. Rev. Mol. Cell Biol. 20, 625–641 (2019).
Shvedunova, M. & Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 23, 329–349 (2022).
Gräff, J. & Tsai, L.-H. Histone acetylation: molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 14, 97–111 (2013).
Gaub, P. et al. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 17, 1392–1408 (2010).
Cho, Y., Sloutsky, R., Naegle, K. M. & Cavalli, V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell 155, 894–908 (2013).
Puttagunta, R. et al. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat. Commun. 5, 3527 (2014).
Jiang, J. et al. MicroRNA-26a supports mammalian axon regeneration in vivo by suppressing GSK3β expression. Cell Death Dis. 6, e1865 (2015).
Wu, D. & Murashov, A. K. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front. Mol. Neurosci. 6, 35 (2013).
Liu, C.-M., Wang, R.-Y., Jiao, Z.-X., Zhang, B.-Y. & Zhou, F.-Q. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev. 27, 1473–1483 (2013).
Kebede, A. F., Schneider, R. & Daujat, S. Novel types and sites of histone modifications emerge as players in the transcriptional regulation contest. FEBS J. 282, 1658–1674 (2015).
Richmond, S. et al. Localization of the glutamate receptor subunit GluR1 on the surface of living and within cultured hippocampal neurons. Neuroscience 75, 69–82 (1996).
Bradke, F. & Dotti, C. G. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19, 1175–1186 (1997).
Eva, R., Koseki, H., Kanamarlapudi, V. & Fawcett, J. W. EFA6 regulates selective polarised transport and axon regeneration from the axon initial segment. J. Cell Sci. 130, 3663–3675 (2017).
Rasband, M. N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11, 552–562 (2010).
Yoshimura, T. & Rasband, M. N. Axon initial segments: diverse and dynamic neuronal compartments. Curr. Opin. Neurobiol. 27, 96–102 (2014).
Eichel, K. & Shen, K. The function of the axon initial segment in neuronal polarity. Dev. Biol. 489, 47–54 (2022).
Maeder, C. I., Shen, K. & Hoogenraad, C. C. Axon and dendritic trafficking. Curr. Opin. Neurobiol. 27, 165–170 (2014).
Villarroel‐Campos, D., Bronfman, F. C. & Gonzalez‐Billault, C. Rab GTPase signaling in neurite outgrowth and axon specification. Cytoskeleton 73, 498–507 (2016).
Britt, D. J., Farias, G. G., Guardia, C. M. & Bonifacino, J. S. Mechanisms of polarized organelle distribution in neurons. Front. Cell Neurosci. 10, 88 (2016).
Guedes-Dias, P. & Holzbaur, E. L. F. Axonal transport: driving synaptic function. Science 366, eaaw9997 (2019).
Gerges, N. Z., Backos, D. S. & Esteban, J. A. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J. Biol. Chem. 279, 43870–43878 (2004).
Gonzalez-Gutierrez, A., Lazo, O. M. & Bronfman, F. C. The Rab5-Rab11 endosomal pathway is required for BDNF-induced CREB transcriptional regulation in hippocampal neurons. J. Neurosci. 40, 8042–8054 (2020).
Yap, C. C., Digilio, L., McMahon, L. P., Garcia, A. D. R. & Winckler, B. Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. J. Cell Biol. 217, 3141–3159 (2018).
Petrova, V., Nieuwenhuis, B., Fawcett, J. W. & Eva, R. Axonal organelles as molecular platforms for axon growth and regeneration after injury. Int. J. Mol. Sci. 22, 1798 (2021).
Cheah, M. et al. Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J. Neurosci. 36, 7283–7297 (2016).
Yap, C. C. et al. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J. Cell Biol. 180, 827–842 (2008).
Hollis, E. R., Jamshidi, P., Low, K., Blesch, A. & Tuszynski, M. H. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl Acad. Sci. USA 106, 7215–7220 (2009).
Duan, X. et al. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85, 1244–1256 (2015).
Maday, S. & Holzbaur, E. L. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev. Cell 30, 71–85 (2014).
Nieuwenhuis, B. et al. PI 3-kinase delta enhances axonal PIP3 to support axon regeneration in the adult CNS. EMBO Mol. Med. 12, e11674 (2020).
Petrova, V. et al. Protrudin functions from the endoplasmic reticulum to support axon regeneration in the adult CNS. Nat. Commun. 11, 5614 (2020). This paper demonstrates the importance of endoplasmic reticulum and associated proteins in axon regeneration.
Ferguson, S. M. Axonal transport and maturation of lysosomes. Curr. Opin. Neurobiol. 51, 45–51 (2018).
Devine, M. J. & Kittler, J. T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 19, 63–80 (2018).
Cheng, X. T. & Sheng, Z. H. Developmental regulation of microtubule-based trafficking and anchoring of axonal mitochondria in health and diseases. Dev. Neurobiol. 81, 284–299 (2021).
Andrews, M. R. et al. Axonal localization of integrins in the CNS is neuronal type and age dependent. eNeuro 3, ENEURO.0029-16.2016 (2016).
Franssen, E. H. et al. Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS. J. Neurosci. 35, 8359–8375 (2015).
Montagnac, G. et al. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr. Biol. 19, 184–195 (2009).
Werner, A. et al. Impaired axonal regeneration in α7 integrin-deficient mice. J. Neurosci. 20, 1822–1830 (2000).
Hight-Warburton, W. & Parsons, M. Regulation of cell migration by α4 and α9 integrins. Biochem. J. 476, 705–718 (2019).
Cheah, M. et al. Integrin-driven axon regeneration in the spinal cord activates a distinctive CNS regeneration program. J. Neurosci. 43, 4775–4794 (2023).
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
Santos, T. E. et al. Axon growth of CNS neurons in three dimensions is amoeboid and independent of adhesions. Cell Rep. 32, 107907 (2020). This paper challenges the classical concept of how CNS axons grow: not pulling with their growth cones on the substrate to move themselves forward but rather through an amoeboid movement, where microtubules protrude further distally.
Witte, H., Neukirchen, D. & Bradke, F. Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619–632 (2008).
Kamiguchi, H. The role of cell adhesion molecules in axon growth and guidance. Adv. Exp. Med. Biol. 621, 95–103 (2007).
Haspel, J. et al. Critical and optimal Ig domains for promotion of neurite outgrowth by L1/Ng-CAM. J. Neurobiol. 42, 287–302 (2000).
Maness, P. F. & Schachner, M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 (2007).
Chen, J. et al. Adeno-associated virus-mediated L1 expression promotes functional recovery after spinal cord injury. Brain 130, 954–969 (2007).
Verma, P. et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J. Neurosci. 25, 331–342 (2005).
Hanz, S. et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40, 1095–1104 (2003).
Holt, C. E. & Schuman, E. M. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80, 648–657 (2013).
Bourke, A. M., Schwarz, A. & Schuman, E. M. De-centralizing the central dogma: mRNA translation in space and time. Mol. Cell 83, 452–468 (2023).
Costa, R. O. et al. Synaptogenesis stimulates a proteasome-mediated ribosome reduction in axons. Cell Rep. 28, 864–876.e6 (2019).
Kim, E. & Jung, H. Local mRNA translation in long-term maintenance of axon health and function. Curr. Opin. Neurobiol. 63, 15–22 (2020).
Dalla Costa, I. et al. The functional organization of axonal mRNA transport and translation. Nat. Rev. Neurosci. 22, 77–91 (2021).
Luarte, A., Cornejo, V. H., Bertin, F., Gallardo, J. & Couve, A. The axonal endoplasmic reticulum: one organelle-many functions in development, maintenance, and plasticity. Dev. Neurobiol. 78, 181–208 (2018).
Yalcin, B. et al. Modeling of axonal endoplasmic reticulum network by spastic paraplegia proteins. eLife 6, e23882 (2017).
Rao, K. et al. Spastin, atlastin, and ER relocalization are involved in axon but not dendrite regeneration. Mol. Biol. Cell 27, 3245–3256 (2016).
Farias, G. G. et al. Feedback-driven mechanisms between microtubules and the endoplasmic reticulum instruct neuronal polarity. Neuron 102, 184–201.e8 (2019).
Kurowska, Z., Brundin, P., Schwab, M. E. & Li, J. Y. Intracellular Nogo-A facilitates initiation of neurite formation in mouse midbrain neurons in vitro. Neuroscience 256, 456–466 (2014).
Cartoni, R., Pekkurnaz, G., Wang, C., Schwarz, T. L. & He, Z. A high mitochondrial transport rate characterizes CNS neurons with high axonal regeneration capacity. PLoS One 12, e0184672 (2017).
Cartoni, R. et al. The mammalian-specific protein Armcx1 regulates mitochondrial transport during axon regeneration. Neuron 92, 1294–1307 (2016).
Han, Q. et al. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 31, 623–641.e8 (2020). Removing an anchor on mitochondria that becomes active as neurons mature enhances mitochondrial transport and enables corticospinal regeneration following CNS injury.
Govek, E.-E., Newey, S. E. & Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 19, 1–49 (2005).
Rosenberg, S. S. & Spitzer, N. C. Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 3, a004259 (2011).
Sánchez-Alegría, K., Flores-León, M., Avila-Muñoz, E., Rodríguez-Corona, N. & Arias, C. PI3K signaling in neurons: a central node for the control of multiple functions. Int. J. Mol. Sci. 19, 3725 (2018).
Garvalov, B. K. et al. Cdc42 regulates cofilin during the establishment of neuronal polarity. J. Neurosci. 27, 13117–13129 (2007).
Tahirovic, S. et al. Rac1 regulates neuronal polarization through the WAVE complex. J. Neurosci. 30, 6930–6943 (2010).
Dupraz, S. et al. RhoA controls axon extension independent of specification in the developing brain. Curr. Biol. 29, 3874–3886.e9 (2019).
Ng, J. & Luo, L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 44, 779–793 (2004).
West, A. E., Griffith, E. C. & Greenberg, M. E. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 3, 921–931 (2002).
Hausser, M., Spruston, N. & Stuart, G. J. Diversity and dynamics of dendritic signaling. Science 290, 739–744 (2000).
Thomas, G. M. & Huganir, R. L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183 (2004).
Kennedy, M. B. Synaptic signaling in learning and memory. Cold Spring Harb. Perspect. Biol. 8, a016824 (2016).
Czech, M. P. PIP2 and PIP3: complex roles at the cell surface. Cell 100, 603–606 (2000).
Park, K. K. et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966 (2008).
Geoffroy, C. G., Hilton, B. J., Tetzlaff, W. & Zheng, B. Evidence for an age-dependent decline in axon regeneration in the adult mammalian central nervous system. Cell Rep. 15, 238–246 (2016).
Du, K. et al. Pten deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J. Neurosci. 35, 9754–9763 (2015).
Lewandowski, G. & Steward, O. AAVshRNA-mediated suppression of PTEN in adult rats in combination with salmon fibrin administration enables regenerative growth of corticospinal axons and enhances recovery of voluntary motor function after cervical spinal cord injury. J. Neurosci. 34, 9951–9962 (2014).
Hammarlund, M., Nix, P., Hauth, L., Jorgensen, E. M. & Bastiani, M. Axon regeneration requires a conserved MAP kinase pathway. Science 323, 802–806 (2009).
Chen, L. et al. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron 71, 1043–1057 (2011).
Shin, J. E. et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74, 1015–1022 (2012).
Watkins, T. A. et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl Acad. Sci. USA 110, 4039–4044 (2013).
Saikia, J. M. et al. A critical role for DLK and LZK in axonal repair in the mammalian spinal cord. J. Neurosci. 42, 3716–3732 (2022). Two kinases implicated in retrograde injury signalling, DLK and LZK, have redundant roles in promoting axon regeneration and compensatory sprouting following axonal injury in the mature mammalian CNS.
Hannila, S. S. & Filbin, M. T. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 209, 321–332 (2008).
Qiu, J. et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 (2002).
Neumann, S., Bradke, F., Tessier-Lavigne, M. & Basbaum, A. I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34, 885–893 (2002).
Gao, Y. et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44, 609–621 (2004).
Chierzi, S., Ratto, G. M., Verma, P. & Fawcett, J. W. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur. J. Neurosci. 21, 2051–2062 (2005).
Flynn, K. C. et al. ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron 76, 1091–1107 (2012).
Tomasek, J. J., Haaksma, C. J., Eddy, R. J. & Vaughan, M. B. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat. Rec. 232, 359–368 (1992).
Bradke, F. & Dotti, C. G. The role of local actin instability in axon formation. Science 283, 1931–1934 (1999).
Schelski, M. & Bradke, F. Microtubule retrograde flow retains neuronal polarization in a fluctuating state. Sci. Adv. 8, eabo2336 (2022). This paper and Burute et al.191 showed that the microtubule array of neurites of developing neurons is not a steady structure: they flow constantly in a retrograde direction back to the cell body.
Burute, M., Jansen, K. I., Mihajlovic, M., Vermonden, T. & Kapitein, L. C. Local changes in microtubule network mobility instruct neuronal polarization and axon specification. Sci. Adv. 8, eabo2343 (2022).
Stiess, M. et al. Axon extension occurs independently of centrosomal microtubule nucleation. Science 327, 704–707 (2010).
Erez, H. et al. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J. Cell Biol. 176, 497–507 (2007).
Ertürk, A., Hellal, F., Enes, J. & Bradke, F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J. Neurosci. 27, 9169–9180 (2007).
Ruschel, J. et al. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348, 347–352 (2015).
Hellal, F. et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931 (2011).
Griffin, J. M. et al. Rehabilitation enhances epothilone-induced locomotor recovery after spinal cord injury. Brain Commun. 5, fcad005 (2023).
Ruschel, J. & Bradke, F. Systemic administration of epothilone D improves functional recovery of walking after rat spinal cord contusion injury. Exp. Neurol. 306, 243–249 (2018).
O’Shea, T. M., Burda, J. E. & Sofroniew, M. V. Cell biology of spinal cord injury and repair. J. Clin. Invest. 127, 3259–3270 (2017).
Dent, E. W. Dynamic microtubules at the synapse. Curr. Opin. Neurobiol. 63, 9–14 (2020).
Guedes-Dias, P. et al. Kinesin-3 responds to local microtubule dynamics to target synaptic cargo delivery to the presynapse. Curr. Biol. 29, 268–282.e8 (2019).
Bharat, V. et al. Capture of dense core vesicles at synapses by JNK-dependent phosphorylation of synaptotagmin-4. Cell Rep. 21, 2118–2133 (2017).
Zhang, W. & Benson, D. L. Stages of synapse development defined by dependence on F-actin. J. Neurosci. 21, 5169–5181 (2001).
Chia, P. H., Patel, M. R. & Shen, K. NAB-1 instructs synapse assembly by linking adhesion molecules and F-actin to active zone proteins. Nat. Neurosci. 15, 234–242 (2012).
Chia, P. H., Chen, B., Li, P., Rosen, M. K. & Shen, K. Local F-actin network links synapse formation and axon branching. Cell 156, 208–220 (2014).
Rust, M. B. ADF/cofilin: a crucial regulator of synapse physiology and behavior. Cell. Mol. Life Sci. 72, 3521–3529 (2015).
Tedeschi, A. et al. ADF/cofilin-mediated actin turnover promotes axon regeneration in the adult CNS. Neuron 103, 1073–1085.e6 (2019). Rejuvenating actin dynamics at the growth cone — a major intracellular process enabling rapid axon growth during embryonic development — promotes regeneration of mature axons following CNS injury.
Pinto-Costa, R. et al. Profilin 1 delivery tunes cytoskeletal dynamics toward CNS axon regeneration. J. Clin. Invest. 130, 2024–2040 (2020).
Stern, S. et al. RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron 109, 3436–3455.e9 (2021). The small GTPase RhoA has opposing roles in neurons and reactive astrocytes following CNS injury: neuronal RhoA prevents axon regeneration but astrocytic RhoA is beneficial for regenerating axons.
Shekhtmeyster, P. et al. Trans-segmental imaging in the spinal cord of behaving mice. Nat. Biotechnol. 41, 1729–1733 (2023).
Schafer, S. T. et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 186, 2111–2126.e20 (2023).
Skinnider, M. A. et al. Single-cell and spatial atlases of spinal cord injury in the Tabulae Paralytica. Nature 631, 150–163 (2024).
Matson, K. J. et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat. Commun. 13, 5628 (2022). This paper generated an atlas using single-nucleus sequencing to profile how different cell types respond to spinal cord injury and showed that a specific subpopulation (spinocerebellar neurons) has a higher capacity to sprout and form new circuits in the injured spinal cord.
Squair, J. W. et al. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Science 381, 1338–1345 (2023). This paper demonstrates the importance of neuronal identity in functional axon regeneration following CNS injury.
Yang, S.-G., Wang, X.-W., Qian, C. & Zhou, F.-Q. Reprogramming neurons for regeneration: the fountain of youth. Prog. Neurobiol. 214, 102284 (2022).
Lundberg, E. & Borner, G. H. Spatial proteomics: a powerful discovery tool for cell biology. Nat. Rev. Mol. Cell Biol. 20, 285–302 (2019).
Banker, G. The development of neuronal polarity: a retrospective view. J. Neurosci. 38, 1867–1873 (2018).
Hilton, B. J. & Bradke, F. Can injured adult CNS axons regenerate by recapitulating development? Development 144, 3417–3429 (2017).
Shen, Y. et al. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596 (2009).
Smith, G. M. & Gallo, G. The role of mitochondria in axon development and regeneration. Dev. Neurobiol. 78, 221–237 (2018).
Kapitein, L. C. & Hoogenraad, C. C. Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol. Cell Neurosci. 46, 9–20 (2011).
Farias, G. G., Guardia, C. M., Britt, D. J., Guo, X. & Bonifacino, J. S. Sorting of dendritic and axonal vesicles at the pre-axonal exclusion zone. Cell Rep. 13, 1221–1232 (2015).
Huber, L. A. et al. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. J. Cell Biol. 123, 47–55 (1993).
Mignogna, M. L. & D’Adamo, P. Critical importance of RAB proteins for synaptic function. Small GTPases 9, 145–157 (2018).
Zhou, F.-Q. & Snider, W. D. Intracellular control of developmental and regenerative axon growth. Philos. Trans. R. Soc. B Biol. Sci. 361, 1575–1592 (2006).
Park, K. K., Liu, K., Hu, Y., Kanter, J. L. & He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 223, 45–50 (2010).
Gentile, J. E., Carrizales, M. G. & Koleske, A. J. Control of synapse structure and function by actin and its regulators. Cells 11, 603 (2022).
Acknowledgements
B.J.H. is supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2024-03986), the Canadian Foundation for Innovation, and the Michael Smith Foundation for Health Research BC. This work was supported by Deutsche Forschungsgesellschaft (DFG), the International Foundation for Research in Paraplegia (IRP) and Wings for Life (to F.B). F.B. is a member of the excellence cluster ImmunoSensation2, the SFBs 1089 and 1158, and is a recipient of the Roger De Spoelberch Prize. We also thank P. Scheiffele for discussions.
Author information
Authors and Affiliations
Contributions
B.J.H., J.W.F., J.M.G. and F.B. researched data for the article. B.J.H., J.W.F and F.B. wrote the article. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Vibhu Sahni, who co-reviewed with Julia Kaiser; Binhai Zheng, who co-reviewed with Carmine Chavez-Martinez; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Active zone
-
The section of the presynaptic plasma membrane within which synaptic vesicle exocytosis takes place.
- Autophagosome
-
A double-membraned vesicle that is formed during autophagy and engulfs and degrades intracellular material.
- Axon initial segment
-
(AIS). The region of the axon close to the soma. The AIS is responsible for the generation of action potentials and demarcates the boundary between axonal and somatodendritic compartments of the neuron.
- Cell adhesion molecules
-
Proteins located on the surface of the cell that mediate interactions between cells or between cells and the extracellular matrix.
- Chromatin
-
The combination of DNA and histone proteins that together comprise chromosomes.
- Cytoskeletal dynamics
-
The interactions between cytoskeletal filaments and accessory proteins that dictate cytoskeletal assembly, disassembly and function.
- Differentiation
-
The process through which immature and less specialized cells acquire structural and functional specificity.
- Endosomes
-
Membrane-bound vesicles that have a role in intracellular sorting in eukaryotic cells.
- Enhancers
-
Sequences of DNA that are located in proximity to a gene and that can be bound by proteins to enhance the likelihood of that gene’s transcription.
- Epigenetic mechanisms
-
Processes that regulate gene expression without changing the DNA sequence.
- Extracellular matrix
-
The network of extracellular molecules that provide structural and biochemical support to cells.
- Growth cone
-
A specialized and motile structure found at the distal tip of a growing neurite.
- Local translation
-
Synthesis of proteins at a specialized site within the cell, such as the axon, dendrite, or synapse.
- Microtubule
-
A polymer of tubulin that is a major part of the cytoskeleton.
- Nucleosomes
-
Segments of DNA wound around histone proteins, constituting the fundamental organization of DNA packaging and resembling ‘beads on a string’.
- Promoters
-
Sequences of DNA to which proteins can bind in order to initiate the transcription of RNA.
- Regeneration-associated genes
-
(RAGs). A historical term in the axon regeneration field referring to genes with expression profiles that positively correlate with axon growth competence, such as those upregulated after peripheral nerve injury.
- Retrograde injury signalling
-
The process by which the neuron signals from its injured axon back to its nucleus to orchestrate the cell body’s response to injury.
- Transcytosis
-
A type of specialized transport in which molecules and/or cargo are taken into a vesicle, transported to a different area of the cell and then secreted.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hilton, B.J., Griffin, J.M., Fawcett, J.W. et al. Neuronal maturation and axon regeneration: unfixing circuitry to enable repair. Nat. Rev. Neurosci. 25, 649–667 (2024). https://doi.org/10.1038/s41583-024-00849-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-024-00849-3
- Springer Nature Limited