Abstract
There is an increasing amount of evidence that biomolecular condensates are linked to neurodegenerative diseases associated with protein aggregation, such as Alzheimer’s disease and amyotrophic lateral sclerosis, although the mechanisms underlying this link remain elusive. In this Review, we summarize the possible connections between condensates and protein aggregation. We consider both liquid-to-solid transitions of phase-separated proteins and the partitioning of proteins into host condensates. We distinguish five key factors by which the physical and chemical environment of a condensate can influence protein aggregation, and we discuss their relevance in studies of protein aggregation in the presence of biomolecular condensates: increasing the local concentration of proteins, providing a distinct chemical microenvironment, introducing an interface wherein proteins can localize, changing the energy landscape of aggregation pathways, and the presence of chaperones in condensates. Analysing the role of biomolecular condensates in protein aggregation may be essential for a full understanding of amyloid formation and offers a new perspective that can help in developing new therapeutic strategies for the prevention and treatment of neurodegenerative diseases.







Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Ross, C. A. & Poirier, M. A. Protein aggregation and neurodegenerative disease. Nat. Med. 10, S10–S17 (2004).
Willbold, D., Strodel, B., Schröder, G. F., Hoyer, W. & Heise, H. Amyloid-type protein aggregation and prion-like properties of amyloids. Chem. Rev. 121, 8285–8307 (2021).
Dobson, C. M. The amyloid phenomenon and its links with human disease. Cold Spring Harb. Perspect. Biol. 9, a023648 (2017).
Emin, D. et al. Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease. Nat. Commun. 13, 5512 (2022).
Cascella, R. et al. Probing the origin of the toxicity of oligomeric aggregates of α-synuclein with antibodies. ACS Chem. Biol. 14, 1352–1362 (2019).
Meisl, G. et al. Uncovering the universality of self-replication in protein aggregation and its link to disease. Sci. Adv. 8, 6831 (2022).
Alberti, S. & Hyman, A. A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 (2021).
Alberti, S. & Hyman, A. A. Are aberrant phase transitions a driver of cellular aging? BioEssays 38, 959–968 (2016).
Vazquez, D. S., Toledo, P. L., Gianotti, A. R. & Ermácora, M. R. Protein conformation and biomolecular condensates. Curr. Res. Struct. Biol. 4, 285–307 (2022).
Nakashima, K. K., Vibhute, M. A. & Spruijt, E. Biomolecular chemistry in liquid phase separated compartments. Front. Mol. Biosci. 6, 21 (2019).
Bhattacharya, A. et al. Lipid sponge droplets as programmable synthetic organelles. Proc. Natl Acad. Sci. USA 117, 18206–18215 (2020).
de Jong, B. Coacervation. Proc. R. Acad. Amst. 32, 849–856 (1929).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Peeples, W. & Rosen, M. K. Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat. Chem. Biol. 17, 693–702 (2021).
Zhang, Y., Narlikar, G. J. & Kutateladze, T. G. Enzymatic reactions inside biological condensates. J. Mol. Biol. 433, 166624 (2021).
Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 (2015).
Nedelsky, N. B. & Taylor, J. P. Pathological phase transitions in ALS-FTD impair dynamic RNA–protein granules. RNA 28, 97–113 (2022).
Dewey, C. M. et al. TDP-43 aggregation in neurodegeneration: are stress granules the key? Brain Res. 1462, 16–25 (2012).
Törnquist, M. et al. Secondary nucleation in amyloid formation. Chem. Commun. 54, 8667–8684 (2018).
Michaels, T. C. T. et al. Chemical kinetics for bridging molecular mechanisms and macroscopic measurements of amyloid fibril formation. Annu. Rev. Phys. Chem. 69, 273–298 (2018).
Sinnige, T. et al. Kinetic analysis reveals that independent nucleation events determine the progression of polyglutamine aggregation in C. elegans. Proc. Natl Acad. Sci. USA 118, e2021888118 (2021).
Ignatova, Z. & Gierasch, L. M. Monitoring protein stability and aggregation in vivo by real-time fluorescent labeling. Proc. Natl Acad. Sci. USA 101, 523–528 (2004).
Lipiński, W. P. et al. Biomolecular condensates can both accelerate and suppress aggregation of α-synuclein. Sci. Adv. 8, eabq6495 (2022).
Knowles, T. P. J., Vendruscolo, M. & Dobson, C. M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 15, 384–396 (2014).
Farzadfard, A. et al. Thermodynamic characterization of amyloid polymorphism by microfluidic transient incomplete separation. Chem. Sci. 15, 2528–2544 (2024).
Weber, C., Michaels, T. & Mahadevan, L. Spatial control of irreversible protein aggregation. eLife 8, 42315 (2019).
Khurana, R. et al. Mechanism of thioflavin T binding to amyloid fibrils. J. Struct. Biol. 151, 229–238 (2005).
Wetzel, R. Amyloids, prions & other aggregates. Methods Enzymol. 309, 3–820 (1999).
Hellstrand, E., Boland, B., Walsh, D. M. & Linse, S. Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem. Neurosci. 1, 13–18 (2010).
Zurlo, E. et al. In situ kinetic measurements of α-synuclein aggregation reveal large population of short-lived oligomers. PLoS ONE 16, e0245548 (2021).
Fakhree, M. A. A., Nolten, I. S., Blum, C. & Claessens, M. M. A. E. Different conformational subensembles of the intrinsically disordered protein α-synuclein in cells. J. Phys. Chem. Lett. 9, 1249–1253 (2018).
Veldhuis, G., Segers-Nolten, I., Ferlemann, E. & Subramaniam, V. Single-molecule FRET reveals structural heterogeneity of SDS-bound α-synuclein. ChemBioChem 10, 436–439 (2009).
Iljina, M. et al. Quantitative analysis of co-oligomer formation by amyloid-beta peptide isoforms. Sci. Rep. 6, 28658 (2016).
Tittelmeier, J., Druffel-Augustin, S., Alik, A., Melki, R. & Nussbaum-Krammer, C. Dissecting aggregation and seeding dynamics of α-Syn polymorphs using the phasor approach to FLIM. Commun. Biol. 5, 1345 (2022).
Ray, S. et al. Mass photometric detection and quantification of nanoscale α-synuclein phase separation. Nat. Chem. 15, 1306–1316 (2023).
Murakami, T. et al. ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88, 678–690 (2015).
Lin, Y., Protter, D. S. W., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Mathieu, C., Pappu, R. V. & Taylor, J. P. Beyond aggregation: pathological phase transitions in neurodegenerative disease. Science 370, 55–60 (2020).
Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 (2015).
Kim, H. J. et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 (2013).
Conicella, A. E., Zerze, G. H., Mittal, J. & Fawzi, N. L. ALS mutations disrupt phase separation mediated by α-helical structure in the TDP-43 low-complexity C-terminal domain. Structure 24, 1537–1549 (2016).
Kang, H. et al. PARIS undergoes liquid–liquid phase separation and poly(ADP‐ribose)‐mediated solidification. EMBO Rep. 24, e56166 (2023).
Gruijs da Silva, L. A. et al. Disease‐linked TDP‐43 hyperphosphorylation suppresses TDP‐43 condensation and aggregation. EMBO J. 41, e108443 (2022).
Tomaszewski, A. et al. Solid-to-liquid phase transition in the dissolution of cytosolic misfolded-protein aggregates. iScience 26, 108334 (2023).
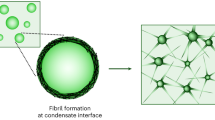
Linsenmeier, M. et al. The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils. Nat. Chem. 15, 1340–1349 (2023).
Wegmann, S. et al. Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J. 37, e98049 (2018).
Boyko, S. et al. Liquid-liquid phase separation of tau protein: the crucial role of electrostatic interactions. J. Biol. Chem. 294, 11054–11059 (2019).
Wen, J. et al. Conformational expansion of tau in condensates promotes irreversible aggregation. J. Am. Chem. Soc. 143, 13056–13064 (2021).
Boyko, S., Surewicz, K. & Surewicz, W. K. Regulatory mechanisms of tau protein fibrillation under the conditions of liquid–liquid phase separation. Proc. Natl Acad. Sci. USA 117, 31882–31890 (2020).
Ray, S. et al. Synuclein aggregation nucleates through liquid–liquid phase separation. Nat. Chem. 12, 705–716 (2020).
Ray, S. et al. Spatiotemporal solidification of α-synuclein inside the liquid droplets. Preprint at https://doi.org/10.1101/2021.10.20.465113 (2021).
Sawner, A. S. et al. Modulating α-synuclein liquid-liquid phase separation. Biochem 60, 3676–3696 (2021).
Hardenberg, M. C. et al. Observation of an α-synuclein liquid droplet state and its maturation into Lewy body-like assemblies. J. Mol. Cell Biol. 13, 282–294 (2021).
Küffner, A. M. et al. Sequestration within biomolecular condensates inhibits Aβ-42 amyloid formation. Chem. Sci. 12, 4373–4382 (2021).
Choi, C. H., Lee, D. S. W., Sanders, D. W. & Brangwynne, C. P. Condensate interfaces can accelerate protein aggregation. Biophys. J. 123, 1404–1413 (2023).
Shen, Y. et al. Biomolecular condensates undergo a generic shear-mediated liquid-to-solid transition. Nat. Nanotechnol. 15, 841–847 (2020).
Riback, J. A. et al. Composition-dependent thermodynamics of intracellular phase separation. Nature 581, 209–214 (2020).
Elbaum-Garfinkle, S. et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl Acad. Sci. USA 112, 7189–7194 (2015).
Onuchic, P. L., Milin, A. N., Alshareedah, I., Deniz, A. A. & Banerjee, P. R. Divalent cations can control a switch-like behavior in heterotypic and homotypic RNA coacervates. Sci. Rep. 9, 12161 (2019).
McCall, P. M. et al. Partitioning and enhanced self-assembly of actin in polypeptide coacervates. Biophys. J. 114, 1636–1645 (2018).
Samanta, N. et al. Sequestration of proteins in stress granules relies on the in-cell but not the in vitro folding stability. J. Am. Chem. Soc. 143, 19909–19918 (2021).
Frottin, F. et al. The nucleolus functions as a phase-separated protein quality control compartment. Science 365, 342–347 (2019).
Bauermann, J., Laha, S., McCall, P. M., Jülicher, F. & Weber, C. A. Chemical kinetics and mass action in coexisting phases. J. Am. Chem. Soc. 144, 19294–19304 (2022).
Michaels, T. C. T., Mahadevan, L. & Weber, C. A. Enhanced potency of aggregation inhibitors mediated by liquid condensates. Phys. Rev. Res. 4, 043173 (2022).
Stender, E. G. P. et al. Capillary flow experiments for thermodynamic and kinetic characterization of protein liquid-liquid phase separation. Nat. Commun. 12, 7289 (2021).
Taylor, N. O., Wei, M. T., Stone, H. A. & Brangwynne, C. P. Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys. J. 117, 1285–1300 (2019).
Yewdall, N. A., André, A. A. M., Lu, T. & Spruijt, E. Coacervates as models of membraneless organelles. Curr. Opin. Colloid Interface Sci. 52, 101416 (2021).
Pönisch, W., Michaels, T. C. T. & Weber, C. A. Aggregation controlled by condensate rheology. Biophys. J. 122, 197–214 (2023).
Ahmad, B., Chen, Y. & Lapidus, L. J. Aggregation of α-synuclein is kinetically controlled by intramolecular diffusion. Proc. Natl Acad. Sci. USA 109, 2336–2341 (2012).
Wei, M. T. et al. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 9, 1118–1125 (2017).
Wang, H., Kelley, F. M., Milovanovic, D., Schuster, B. S. & Shi, Z. Surface tension and viscosity of protein condensates quantified by micropipette aspiration. Biophys. Rep. 1, 100011 (2021).
Li, J., Uversky, V. N. & Fink, A. L. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochem 40, 11604–11613 (2001).
Murthy, A. C. et al. Molecular interactions underlying liquid–liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol. 26, 637–648 (2019).
Smisdom, N. et al. Fluorescence recovery after photobleaching on the confocal laser-scanning microscope: generalized model without restriction on the size of the photobleached disk. J. Biomed. Opt. 16, 046021 (2011).
Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. & Webb, W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Jawerth, L. M. et al. Salt-dependent rheology and surface tension of protein condensates using optical traps. Phys. Rev. Lett. 121, 258101 (2018).
Zhou, H. X. Determination of condensate material properties from droplet deformation. J. Phys. Chem. B 124, 8372–8379 (2020).
Kalwarczyk, T. et al. Motion of nanoprobes in complex liquids within the framework of the length-scale dependent viscosity model. Adv. Colloid Interface Sci. 223, 55–63 (2015).
Bubak, G. et al. Quantifying nanoscale viscosity and structures of living cells nucleus from mobility measurements. J. Phys. Chem. Lett. 12, 294–301 (2021).
Munishkina, L. A., Cooper, E. M., Uversky, V. N. & Fink, A. L. The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J. Mol. Recognit. 17, 456–464 (2004).
Vagenende, V., Yap, M. G. S. & Trout, B. L. Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochem 48, 11084–11096 (2009).
Roussel, M. R. Foundations of Chemical Kinetics (IOP Publishing, 2023).
Abyzov, A., Blackledge, M. & Zweckstetter, M. Conformational dynamics of intrinsically disordered proteins regulate biomolecular condensate chemistry. Chem. Rev. 122, 6719–6748 (2022).
Rubinstein, M. & Colby, R. H. Polymer Physics (Oxford Univ. Press, 2003).
Garaizar, A. et al. Aging can transform single-component protein condensates into multiphase architectures. Proc. Natl Acad. Sci. USA 119, e2119800119 (2022).
Breydo, L. et al. The crowd you’re in with: effects of different types of crowding agents on protein aggregation. Biochim. Biophys. Acta Proteins Proteom. 1844, 346–357 (2014).
Schreck, J. S., Bridstrup, J. & Yuan, J. M. Investigating the effects of molecular crowding on the kinetics of protein aggregation. J. Phys. Chem. B 124, 9829–9839 (2020).
Grigolato, F. & Arosio, P. The role of surfaces on amyloid formation. Biophys. Chem. 270, 106533 (2021).
Galvagnion, C. et al. Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 11, 229–234 (2015).
Marie, G. et al. Acceleration of α-synuclein aggregation by exosomes. J. Biol. Chem. 290, 2969–2982 (2015).
Morinaga, A. et al. Critical role of interfaces and agitation on the nucleation of Aβ amyloid fibrils at low concentrations of Aβ monomers. Biochim. Biophys. Acta Proteins Proteom. 1804, 986–995 (2010).
Gray, J. J. The interaction of proteins with solid surfaces. Curr. Opin. Struct. Biol. 14, 110–115 (2004).
Zapadka, K. L., Becher, F. J., Gomes dos Santos, A. L. & Jackson, S. E. Factors affecting the physical stability (aggregation) of peptide therapeutics. Interface Focus 7, 20170030 (2017).
Camino, J. D., Gracia, P. & Cremades, N. The role of water in the primary nucleation of protein amyloid aggregation. Biophys. Chem. 269, 106520 (2021).
Folkmann, A. W., Putnam, A., Lee, C. F. & Seydoux, G. Regulation of biomolecular condensates by interfacial protein clusters. Science 373, 1218–1224 (2021).
Garcia-Jove Navarro, M. et al. RNA is a critical element for the sizing and the composition of phase-separated RNA–protein condensates. Nat. Commun. 10, 3230 (2019).
Welsh, T. J. et al. Surface electrostatics govern the emulsion stability of biomolecular condensates. Nano Lett. 22, 612–621 (2022).
Vabulas, R. M., Raychaudhuri, S., Hayer-Hartl, M. & Hartl, F. U. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2, a004390 (2010).
Chirita, C. N., Congdon, E. E., Yin, H. & Kuret, J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry 44, 5862–5872 (2005).
Menon, S. & Mondal, J. Conformational plasticity in α-synuclein and how crowded environment modulates it. J. Phys. Chem. B 127, 4032–4049 (2023).
Farag, M. et al. Condensates formed by prion-like low-complexity domains have small-world network structures and interfaces defined by expanded conformations. Nat. Commun. 13, 7722 (2022).
Ohgita, T. et al. Intramolecular interaction kinetically regulates fibril formation by human and mouse α-synuclein. Sci. Rep. 13, 10885 (2023).
Kumari, P. et al. Structural insights into α-synuclein monomer–fibril interactions. Proc. Natl Acad. Sci. USA 118, e2012171118 (2021).
Guseva, S. et al. Liquid-liquid phase separation modifies the dynamic properties of intrinsically disordered proteins. J. Am. Chem. Soc. 145, 10548–10563 (2023).
Zhao, M. et al. Partitioning of small molecules in hydrogen-bonding complex coacervates of poly(acrylic acid) and poly(ethylene glycol) or pluronic block copolymer. Macromolecules 50, 3818–3830 (2017).
Huang, S. et al. Effect of small molecules on the phase behavior and coacervation of aqueous solutions of poly(diallyldimethylammonium chloride) and poly(sodium 4-styrene sulfonate). J. Colloid Interface Sci. 518, 216–224 (2018).
Lipiński, W. P. et al. Fibrils e merging from droplets: molecular guiding principles behind phase transitions of a short peptide-based condensate studied by solid-state NMR. Chem. Eur. J. 29, e202301159 (2023).
Leblanc, S. J., Kulkarni, P. & Weninger, K. R. Single molecule FRET: a powerful tool to study intrinsically disordered proteins. Biomolecules 8, 140 (2018).
Holmstrom, E. D. et al. Accurate transfer efficiencies, distance distributions, and ensembles of unfolded and intrinsically disordered proteins from single-molecule FRET. Methods Enzymol. 611, 287–325 (2018).
Bordignon, E. & Polyhach, Y. EPR techniques to probe insertion and conformation of spin-labeled proteins in lipid bilayers. Meth. Mol. Biol. 974, 329–355 (2013).
Maltseva, D. et al. Fibril formation and ordering of disordered FUS LC driven by hydrophobic interactions. Nat. Chem. 15, 1146–1154 (2023).
Dyson, H. J. & Wright, P. E. Insights into the structure and dynamics of unfolded proteins from nuclear magnetic resonance. Adv. Protein Chem. 62, 311–340 (2002).
Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14, 630–642 (2013).
Hatters, D. M., Lindner, R. A., Carver, J. A. & Howlett, G. J. The molecular chaperone, α-crystallin, inhibits amyloid formation by apolipoprotein C-II. J. Biol. Chem. 276, 33755–33761 (2001).
Webster, J. M., Darling, A. L., Uversky, V. N. & Blair, L. J. Small heat shock proteins, big impact on protein aggregation in neurodegenerative disease. Front. Pharmacol. 10, 1047 (2019).
Bruinsma, I. B. et al. Inhibition of α-synuclein aggregation by small heat shock proteins. Proteins 79, 2956–2967 (2011).
Wentink, A. S. et al. Molecular dissection of amyloid disaggregation by human HSP70. Nature 587, 483–488 (2020).
Li, Y. et al. Hsp70 exhibits a liquid-liquid phase separation ability and chaperones condensed FUS against amyloid aggregation. iScience 25, 104356 (2022).
Shammas, S. L. et al. Binding of the molecular chaperone αb-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys. J. 101, 1681–1689 (2011).
Shorter, J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE 6, e26319 (2011).
Daturpalli, S., Waudby, C. A., Meehan, S. & Jackson, S. E. Hsp90 inhibits α-synuclein aggregation by interacting with soluble oligomers. J. Mol. Biol. 425, 4614–4628 (2013).
Zhang, Z. Y. et al. TRIM11 protects against tauopathies and is down-regulated in Alzheimer’s disease. Science 381, eadd6696 (2023).
Liu, Z. et al. Hsp27 chaperones FUS phase separation under the modulation of stress-induced phosphorylation. Nat. Struct. Mol. Biol. 27, 363–372 (2020).
Gu, J. et al. Hsp40 proteins phase separate to chaperone the assembly and maintenance of membraneless organelles. Proc. Natl Acad. Sci. USA 117, 31123–31133 (2020).
Hiller, S. Chaperone-bound clients: the importance of being dynamic. Trends Biochem. Sci. 44, 517–527 (2019).
Zbinden, A., Pérez-Berlanga, M., De Rossi, P. & Polymenidou, M. Phase separation and neurodegenerative diseases: a disturbance in the force. Dev. Cell 55, 45–68 (2020).
Mateju, D. et al. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 36, 1669–1687 (2017).
Acknowledgements
This work was supported financially by a Vidi grant from the Netherlands Organization for Scientific Research (NWO).
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and editing of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Alexander Buell, Sara Linse and Soumik Ray, and the other anonymous referee(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Visser, B.S., Lipiński, W.P. & Spruijt, E. The role of biomolecular condensates in protein aggregation. Nat Rev Chem 8, 686–700 (2024). https://doi.org/10.1038/s41570-024-00635-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-024-00635-w
- Springer Nature Limited