Abstract
Historically, monkeypox (mpox) was a zoonotic disease endemic in Africa. However, in 2022, a global outbreak occurred following a substantial increase in cases in Africa, coupled with spread by international travellers to other continents. Between January 2022 and October 2023, about 91,000 confirmed cases from 115 countries were reported, leading the World Health Organization to declare a public health emergency. The basic biology of monkeypox virus (MPXV) can be inferred from other poxviruses, such as vaccinia virus, and confirmed by genome sequencing. Here the biology of MPXV is reviewed, together with a discussion of adaptive changes during MPXV evolution and implications for transmission. Studying MPXV biology is important to inform specific host interactions, to aid in ongoing outbreaks and to predict those in the future.
Similar content being viewed by others
Main
Monkeypox virus (MPXV) causes the zoonotic disease mpox (formally known as monkeypox) and is a member of the poxvirus family. It belongs to the Orthopoxvirus genus of the Chordopoxvirinae subfamily, which also includes VARV (variola virus, the agent of smallpox), VACV (vaccinia virus, basis of the smallpox vaccine), CPXV (cowpox virus, circulates in rodents of Northern Europe) and ECTV (ectromelia virus, an infectious agent in mice)1. MPXV was isolated and identified in pox-like skin lesions from captive cynomolgus monkeys in 19582 (Fig. 1). Infections in humans were first reported in Central and West Africa in the 1970s (Fig. 1). However, MPXV might have occurred earlier in humans, as cases could have been misdiagnosed as smallpox before its eradication. Human mpox has an incubation period of 5–21 days, with symptoms that include fever, a vesicular–pustular eruption and lymphadenopathy1. Although smallpox and mpox cause similar symptoms, the latter is milder and less transmissible, with estimated mortality rates of 10.6% in Central Africa and <3.6% in West Africa3 (Table 1).
Historically, mpox in Africa was thought to be a zoonosis, with some cases of limited transmission between family members reported (Fig. 1). The incidence of mpox has increased over the past 50 years in both Central and West Africa. Typically, children become sick and develop rash over the whole body4. The incidence of mpox in Africa has increased rapidly, with over 18,000 new cases in the Democratic Republic of the Congo (DRC) in the last two years. Cases have also been detected in the UK, Israel and Singapore in 2018–2019 and the United States in 2021, thought to be the result of infected travellers. Between 1 January 2022 and 31 October 2023, more than 91,000 confirmed mpox cases and 167 deaths from 115 countries have been reported. The global outbreak of mpox (Fig. 2) led the World Health Organization (WHO) to declare a ‘public health emergency of international concern’. External Situation Report #30 of the WHO stated that the majority of cases were in the United States (~60,000) and Europe (~26,000)5. Since summer 2022, the incidences outside of Africa have declined, with 868 new cases reported in September 2023. The global outbreak mostly affected men who have sex with men (MSM). The lesions occurred predominantly in the anal and oral mucosa4, and sexual transmission was documented for the first time5.
Data as of 7 February 2023, obtained from WHO at https://map.monkeypox.global.health/country. Incidences of mpox in individual countries range from 0 to 29,933, as indicated in the bar by light to dark colour intensity. Asterisks indicate geographical regions of mpox before 2022. Map generated with Datawrapper.
Further research is needed to understand whether these altered clinical and epidemiological features arose mainly due to recent evolutionary changes of MPXV or whether there were other contributing factors. In this Perspective I argue that the biology of MPXV clades must be studied to answer this question, as this understanding may help to predict and manage potential future outbreaks. Reviews that focus on other or broader aspects of the current mpox outbreak are available6,7,8,9.
Biology and genetics of MPXV
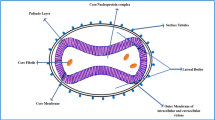
Poxviruses are large double-stranded DNA viruses that replicate in the host cell cytoplasm of many vertebrates and invertebrates10. Much of our understanding of poxvirus biology comes from studies with VACV, and this can be applied to MPXV. The replication cycle (Fig. 3) begins with viral binding to host cells, followed by fusion of viral and cellular membranes and entry of the virus core into the cytoplasm. Transcription activation within the viral core leads to synthesis of 118 VACV early messenger RNAs (mRNAs) that encode enzymes for genome replication in the cytoplasm, intermediate-stage transcription and proteins for immune defence. Following genome replication, 93 additional mRNAs are transcribed from intermediate and late genes that encode structural proteins and early transcription factors, which are assembled into mature virions (MVs) that contain a membrane derived from the endoplasmic reticulum (ER). The latter are wrapped in a double membrane derived from the trans-Golgi network (TGN), transported to the periphery of the cell on microtubules, and released following exocytosis and loss of the outermost membrane. Thus the liberated virions, known as enveloped virions (EVs) contain two membranes—one captured from the ER and the other from the TGN.
Virions, containing a double-stranded DNA genome, enzymes and transcription factors, attach to cells and fuse with the cell membrane, releasing cores into the cytoplasm. The cores synthesize early mRNAs that are translated into a variety of proteins, including growth factors, immune defence molecules, and factors for DNA replication and intermediate transcription. Uncoating occurs, and the DNA is replicated to form concatemeric molecules. Intermediate genes in the progeny DNA are transcribed, and the mRNAs are translated to form late transcription factors. The late genes are transcribed and the mRNAs are translated to form virion structural proteins, enzymes and early transcription factors. Assembly begins with the formation of discrete membrane structures. The concatemeric DNA intermediates are resolved into unit genomes and packaged in immature virions (IVs). Maturation proceeds to the formation of infectious intracellular MVs. The virions are wrapped by modified TGN and endosomal cisternae, and the wrapped virions (WVs) are transported to the periphery of the cell via microtubules. Fusion of the wrapped virions with the plasma membrane results in release of extracellular EVs. Figure adapted with permission from ref. 10, Wolters Kluwer Health, Inc.
Research on MPXV has been limited because of the stringent conditions required for safe experimentation, particularly for clade I. Nevertheless, it is very likely that the MPXV replication cycle is similar to that described in Fig. 3, because the 100 VACV genes essential for replication are highly conserved in MPXV11. Furthermore, VACV and MPXV have been shown to have similar gene expression profiles12, and MPXV replication was hindered when individual VACV protein homologues (involved in attachment, replication and host immune modulation) were silenced in RNA-interference experiments13.
Although essential genes for replication are highly conserved in orthopoxviruses, they harbour a variable number of accessory genes that are involved in host interactions and immune evasion14. The number of host interaction genes rank from 105 in CPXV, ~67 in MPXV, VACV and ECTV, to 53–55 in VARV15. It is likely that the high number of accessory genes in CPXV contributes to its wide host range. However, it can be beneficial for viral fitness to reduce the number of encoded proteins that are dispensable and thus save energy resources. On the other hand, losing genes could be a tradeoff and irreversibly narrow the host range. For example, gene loss by VARV may have contributed to its specificity for humans and the absence of an animal reservoir, helping with its eradication. Additionally, missense mutations can enhance the interactions of viral proteins with cellular proteins of one host while decreasing interactions with cellular proteins of another host. For example, the orthopoxviral gene (OPG) 41 protein has homology with the α-subunit of eukaryotic translation factor 2 (eIF2α) and acts as a competitive inhibitor of the antiviral protein kinase R (PKR). Putative functional orthologues are present in all orthopoxviruses except for MPXV and ectromelia (mouse pox virus), which have inactivating mutations. The deletion of OPG 41 in VACV leads to enhanced sensitivity to interferon and the inability to replicate in BHK cells, but not HeLa cells16. A detailed study of OPG 41 orthologues revealed that minor sequence differences can lead to differential inhibition of PKR in a mammalian species-specific manner17,18. The resistance of MPXV to loss of a functional OPG 41 is attributed to decreased production of double-stranded RNA, a potent stimulator of PKR19. Rarely, the absence of a gene exemplified by OPG 201, an inhibitor of interleukin-1β (IL-1β) that is present in MPXV and pathogenic VACV strains but not in attenuated vaccine strains, can enhance virulence20.
The rate of viral evolution is determined mostly by the frequency of mutations. The poxvirus-encoded proofreading DNA polymerase has a low error rate, and analyses of VARV in humans and MPXV in chimpanzees indicate 1 × 10−5 and 2 × 10−6 nucleotide substitutions per site per year, respectively21,22. This rate is considerably lower than the 0.8–2.38 × 10–3 and 2 × 10−3 nucleotide substitutions per site per year estimated for SARS-CoV-223 and influenza virus24, respectively. In vitro studies suggest that transient gene duplications (known as the accordion model) may precede further orthopoxviral mutational events, allowing accelerated adaptation to host antiviral defences25.
Genome sequencing indicates the existence of three MPXV clades that were not fully recognized until recently26,27,28. Clade I is found in Central African countries, particularly the DRC, but also the Republic of the Congo, Central African Republic, Cameroon, Gabon and South Sudan (Fig. 4). Clades IIa and IIb are found in West Africa: IIa is west of the Dahomi Gap in Sierra Leone, Liberia, Ivory Coast and Ghana, and IIb is east of the Gap in Nigeria (Fig. 4). The nucleotide difference between clade I and clade IIa genomes is 4–5%, whereas the difference between IIa and IIb is ~2%. Most clade differences are non-synonymous nucleotide polymorphisms and could potentially affect replication or host interactions29. However, nearly all genes in clades I, IIa and IIb seem to be intact, as exemplified by the conserved lengths of the host interactions genes (Supplementary Table 1). The absence of OPG 32 from clade IIa and IIb genomes and fragmentation of OPG 195 in clade IIa are exceptions (Supplementary Table 1). In VACV, OPG 32 encodes a complement control protein that contributes to virulence30,31,32, and OPG 195 encodes a homologue of the CPXV PIE (poxvirus immune evasion) domain protein that reduces antigen presentation33. OPG 197, the function of which is unknown, is present in MPXV clades IIa and IIb but missing from clade I. Individual clade I isolates exhibit less nucleotide diversity compared to the diversity of individual clade II isolates, suggesting a bottleneck during the evolution of clade I28. Estimates place the separations of clades I and II ~560 years ago and IIa and IIb ~238 years ago, which corresponds to climate changes that may have led to alterations in the geography of the rainforest28. The genomes of MPXV clade IIb, isolated from infected individuals in Nigeria in 2017 and later, are similar, but show numerous polymorphisms compared to the 1971 reference genome sequence of MPXV34. They show more GA-to-AA and CT-to-TT changes compared to clades I and IIa, suggesting that mutations stem from apolipoprotein B editing complex 3 (APOBEC3) cytosine deaminase activity, which can be a signature of sustained human transmission35. Humans show high-level expression of seven APOBEC3 genes in skin and mucosal tissues36,37. In contrast, rodents, in which poxviruses often circulate, show limited expression of only one APOBEC3 gene. These mutations are also enriched in human-specific VARV, though to a lesser degree than in MPXV clade IIb, but not in other rodent-associated orthopoxviruses such as VACV and CPXV, consistent with mutations occurring during human-to-human transmission38. Whether clade I isolates from recent outbreaks in the DRC show increased APOBEC3 type mutations would therefore be interesting to investigate. Although some such mutations may be adaptive, APOBEC3 editing is generally antiviral, as random changes are likely to decrease rather than increase viral fitness39. Some viruses, including HIV-1, encode proteins that hinder APOBEC3 editing40. However, there is no evidence that orthopoxviruses encode an APOBEC3 defence protein41.
Central African countries in which clade I MPXV is found are coloured orange. West African countries in which clade IIa and IIb are found are coloured red and yellow, respectively. Created with Geocurrents.com.
Vaccines and therapeutics that control orthopoxviral infections are also effective against MPXV because of the conservation of essential genes. Modern vaccines developed to prevent smallpox include Jynneos and ACAM2000, which are highly and moderately attenuated strains of VACV, respectively. Jynneos provided substantial protection against mpox during the 2022 outbreak42, and newer vaccines are under investigation43. Currently, no treatments have been approved for mpox. However, drugs developed to treat smallpox, including tecovirimat (which inhibits virus spread), brincidofovir and cidofovir (which inhibit genome replication; Fig. 3), as well as vaccinia immunoglobulin (VIG) have been used to treat mpox. Tecovirimat has been shown to inhibit the current outbreak strain of MPXV in vitro and in vivo44,45,46 and was used in the 2022 outbreak but without control groups47,48. Efficacy will be quantified in two ongoing clinical trials to treat mpox patients with tecovirimat in the United States (NCT02080767) and the DRC (NCT05559099).
In summary, the basic biology of MPXV is like that of other orthopoxviruses, but genome sequencing alone is inadequate to predict the clade differences in host interactions.
Epidemiology
Before the most recent global outbreak, the incidence of mpox had been increasing in Africa. However, accurate estimates are lacking due to the misdiagnosis of chickenpox, short transmission chains, occurrence in remote villages, lack of robust reporting systems, serologic cross-reactivity with smallpox and the smallpox vaccine, limited genetic confirmation, and civil unrest in endemic areas. The severity of mpox is highest in Central Africa where clade I MPXV occurs (Table 1). Case reporting, which is required in the DRC but without verification, has indicated an upward trend of cases49 from 38 in 1970–1979 to 18,788 in 2010–2019 and 6,216 in 20203. A further 12,569 cases were reported between 1 January and 12 November 2023. Lower numbers have been reported in other Central African countries, including the Central African Republic, Cameroon, Congo, Gabon and South Sudan, where reporting is not mandatory3. Primary zoonotic infection is thought to occur by hunting, handling or consumption of wild animals in tropical rainforests. Based mainly on serology, squirrels and other rodents are suspected to be the source of most infections. However, secondary transmission within a household is common, and up to seven serial transmissions have been reported in the DRC50,51. Children are more likely to be infected than adults, and factors associated with increased risk of transmission include sleeping in the same bed, sharing food and drinking out of the same cup. These risk factors suggest that close contact rather than aerosol spread is the main transmission route. Although the increased incidence of mpox may also be due to a combination of encroachment of humans into areas with infected animals, increased human population density and an expanded susceptible population following the termination of smallpox vaccination, infections have increased in urban areas, with evidence of human-to-human sexual transmission52.
Mpox disease is less severe in West Africa than in Central Africa, and fewer cases have been reported in Sierra Leone, Liberia, Côte d’Ivoire and Nigeria50. However, 189 mpox cases associated with clade IIb occurred in 2017 in Nigeria, where it had not been reported previously53. An epidemiology study linked 30% of 122 Nigerian cases (not all confirmed) in 2017–2018 to people who had lesions, particularly to members of the same household and prison inmates, suggesting human-to-human transmission. However, zoonotic transmissions were also suspected53. By 2022, there were 2,635 suspected and 988 confirmed cases, with a preponderance of males (66%) and a median age of 30 years in rural and urban areas of Nigeria54. In summary, there have been steady increases of mpox in Central Africa, possibly due to increased zoonoses, an abrupt increase in 2017 in Nigeria, as well as in 2022–2023 in the DRC, which could be due to increased human-to-human transmission.
So far, all reported human mpox cases outside Africa have been due to MPXV clades IIa and IIb55,56. In 2003, clade IIa caused a self-limited outbreak in the United States following the importation of rodents from Ghana (Fig. 1). However, few human infections in Africa and no human-to-human transmissions of clade IIa have been reported so far. Genome sequencing indicates that the global outbreak was caused by a clade IIb MPXV, similar to those exported in 2018 and 2019 and isolated in Nigeria between 2017 and 202034,56. Analysis of those MPXV genomes indicated that multiple clade IIb variants were circulating at that time. One group of variants, the B.1 variants, emerged in Europe between April and May 2022 and became predominant globally. Genome analysis suggests that dominance of B.1 could be due to a founder effect after chance introduction to the global community34. The sequences show high similarities to an MPXV isolated in 2021 from a person travelling from Nigeria to the United States35,57, as well as APOBEC3 signatures35,56,58. In fact, 93% of the 42 single nucleotide changes from the 2018 reference genome show the APOBEC3 signature, supporting the suggestion of ongoing human-to human transmission59. Furthermore, specific enrichments of APOBEC3 motif mutations have increased since 2017 in clade IIb viruses (approximately six per year)59, which is higher than the previously determined rate of orthopoxviral nucleotide substitutions. With the assumption that APOBEC3 mutations represent sustained human transmission, it was estimated that clade IIb entered the human population in 2015–2016, possibly by a single zoonotic event59. An outstanding question remains as to whether the global outbreak was caused by recent adaptive changes in MPXV in Nigeria, or whether the spread from Nigeria reflects its higher number of international travellers compared to other Central and West African countries60.
Animal reservoirs
The identities of the animals that transmit MPXV to humans in Africa are uncertain. The Asian monkeys from which MPXV was originally isolated were infected in captivity, and sera from wild Asian monkeys are negative for orthopoxviral antibodies61. By contrast, serological surveys in the lowland tropical forests of Central and West Africa determined that arboreal rodents, monkeys and bats were positive for orthopoxviral-specific antibodies62,63. The high prevalence of orthopoxviral antibodies in arboreal squirrels of the genera Funisciuris and Heliosciuris in four widely separated parts of the DRC suggests that they are important reservoir hosts62. Because infections are short in duration, it is rare to capture rodents that harbour the virus. So far, MPXV has been isolated in the DRC from only one rope squirrel (Funisciuris)61, but MPXV DNA was recovered in 9% of rope squirrel skins from museums across Central Africa, with the earliest dating back to 1899. Additionally, MPXV DNA has been found in other rodents and a tree shrew in the DRC64. In 2003, a human mpox outbreak in the United States (Fig. 1) was caused by handling prairie dogs that were exposed to MPXV clade IIa-infected African pouched rats, dormice and rope squirrels in a shipment from Ghana65. Although no human mpox cases had been reported in Ghana, a serological survey confirmed orthopoxviral infection in rodents and other wild animal species. Live MPXV was isolated in the republic of Côte d’Ivoire from a sooty mangabey primate in 201066, and an outbreak in chimpanzees occurred in 2017 in the same area22. Thus, many animal species may harbour MPXV and can potentially transmit to humans and non-human primates. However, the paucity of DNA sequencing from wild animals has severely limited our understanding of MPXV epidemiology and the extent to which specific adaptations have occurred in humans or animals.
Animal models
Animal models have been critical for studying the virulence and host range of orthopoxviruses, as well as the effectiveness of vaccines and therapeutics. Strict biosafety rules need to be followed for experimentation with MPXV. The Centers for Disease Control and Prevention (CDC) and National Institutes of Health (NIH) Biosafety in Microbiological and Biomedical Practices (sixth edition) recommends that all researchers in contact with Central and West African clades of MPXV receive a smallpox vaccination every three years. Central African strains of MPXV are classified as ‘select agents’ in the United States, and research requires registration with the CDC, as well as secure Biosafety Level-3 (BSL-3) and Animal Biosafety Level-3 (ABSL-3) facilities and trained investigators. The minimal precaution for working with West African strains of MPXV is BSL-2 with BSL-3 practices. Chimeric viruses containing Central African genes are also handled as select agents.
Several animals have been used as experimental models, but most of them are not naturally infected by MPXV. They can be divided into non-human primates (NHPs), wild North American rodents, wild African rodents, and inbred mice (Table 2). Most NHP studies have been carried out by infecting Asian cynomolgus monkeys with a clade I strain by the respiratory or intravenous route as a model for smallpox to study vaccines and therapeutics67,68,69. A clade I strain induced greater morbidity and fatalities than a clade IIa strain in NHPs infected by aerosol, subcutaneous and intranasal routes70,71. A systematic analysis of protein kinases suggested differences in the modulation of host cell signalling responses by clade I compared to clade IIa72.
Rodents have several advantages over NHPs as models for investigating viral diseases, including their small size, cost and availability. The susceptibility of North American rodents to MPXV is in the following order: ground squirrels > prairie dogs > deer mice (Table 2). In addition to virulence studies, prairie dogs have been used to determine transmission through contaminated bedding, housing and nasal discharge73, as well as efficacy of the smallpox vaccine74. Several African rodents—the rope squirrel, Gambian pouched rat and dormouse—were investigated partly because they may provide insights into potential reservoir animals (Table 2). An experimental advantage of the African dormouse is that they can be bred and maintained in captivity75,76. The multimammate rat is present in parts of sub-Saharan Africa, and although not naturally infected with MPXV, it has served as a model for non-lethal mucosal infection, which can occur by both rectal and vaginal routes with clade IIb77. Rectal and genital infection led to higher transmission rates in co-housed animals than transdermal infection.
The house mouse (Mus musculus) is the main experimental model for most viruses because of the availability of immunological reagents, inbred strains, easy breeding and commercial availability. However, adult immunocompetent BALB/c and C57BL/6 strains are relatively resistant to MPXV, in contrast to VACV78,79. This resistance motivated the screening of 38 inbred mouse strains, of which Castaneous (CAST/EiJ) and two other wild-derived strains were found to be highly susceptible to a clade I MPXV, whereas classically inbred mice were resistant80,81. The susceptibility of the CAST/EiJ mouse correlates with a relatively low basal level of natural killer cells82,83. Statistically significant differences in virulence of clades (I > IIa > IIb) were found in this model76. The predominant 2022 outbreak strain did not cause weight loss or lethality in CAST/EiJ mice compared with the lethality of similar doses of clade I and IIa viruses, suggesting that the transmission of clade IIb in humans correlates with diminished virulence for rodents76. CAST/EiJ mice elicit strong immunoglobulin-G and T-cell responses and so are useful for assessing vaccine and drug efficacy in addition to virulence studies44,80.
In summary, clade I MPXV is more virulent than clade II in humans, NHPs and rodents, but virulence varies with the animal and infection route. NHP models may mimic human infection and are useful for preclinical testing of vaccines and therapeutics. North American and African wild rodents can serve as models for potential and existing reservoir species, respectively. CAST/EiJ mice exhibited statistically significant differences in morbidity and lethality between different MPXV clades, and thus could be used for detailed genetic studies comparing those clades.
Functional genetics of MPXV
Functional genetic studies have been critical in identifying orthopoxviral host interactions and VACV, CPXV and ectromelia virus genes that contribute to virulence and host range14. The same is anticipated for MPXV. Although a study of large deletions introduced into the clade I MPXV genome found decreased replication in monkey and human cell lines, as well as attenuation in CAST/EiJ mice84, more refined genetic studies are needed to assess the contributions of individual genes. OPG 32 is present in clade I MPXV but absent from clades IIa and IIb. Deletion of OPG 32 from clade I reduced morbidity and mortality in prairie dogs85, but not in rhesus macaques86. Moreover, adding the OPG 32 gene to clade IIa MPXV resulted in modest effects in prairie dogs, suggesting that it is not a major determinant of the virulence difference between clade I and IIa MPXV. The conclusions of these studies are provisional, as the relatively small numbers of outbred prairie dogs and monkeys were insufficient to achieve statistical power and significance. Screening the numerous genes that have point mutations in MPXVs would be challenging using these animal models.
Determining the genetic basis for MPXV clade differences in terms of virulence and transmission are a high priority. This information could be used to help develop treatments and predict the outcomes of future outbreaks. However, safety concerns and the value of specific experiments need to be weighed up and addressed to comply with ‘dual-use research of concern’ and ‘potential pandemic pathogen care and oversight’. Although in vitro studies provide some insight87, differences between MPXV clades have not been demonstrated in primary cells or organoids, indicating the need for animal models. At present, the inbred CAST/EiJ mouse seems the most appropriate for screening the contributions of individual genes for virulence, as clade differences are profound and statistically significant compared to other animal models. An experimental approach that could potentially ensure safety involves replacing genes of clade I MPXV with genes of the less virulent and less transmissible clade IIa. The expectation is that some replacements will decrease the virulence of clade I, presenting a loss rather than a gain of function. However, redundant virulence enhancing genes (common in poxviruses) could confound the effects of single gene replacements. In addition, genes that confer virulence in mice may not be the ones most relevant for humans, and confirmation in other animals, including NHPs, is important.
Outlook
Mpox has been endemic in Africa for at least 50 years, and the 2022 outbreak of mpox highlights the overdue action needed by global health and research communities88. We need to take steps to control infections in Africa, where the disease has long been a burden, and to prevent MPXV from becoming endemic outside Africa. One immediate action is a more equitable global distribution of vaccines and therapeutics to reduce disease and human-to-human transmission. Combined epidemiological and genome sequencing studies in Nigeria and the DRC could help determine how variants are spreading and whether adaptation to humans is ongoing. Intensive efforts to isolate and sequence the genomes of MPXV from wild animals or index human zoonotic infections in Nigeria might help to ascertain whether APOBEC3 signature mutations are a result of sustained human transmission. Additionally, surveillance is needed in Central and West African countries where clades I and IIa MPXV are endemic, as they have the potential to cause future global outbreaks. Clade I is of particular concern because of its virulence and the evidence of human-to-human transmission among family members, and more recently by sexual transmission. Currently, clade IIa seems less concerning because of its low virulence, few documented human infections, and no reported human-to-human transmission. Although MPXV clades I, IIa and IIb have been geographically segregated, climate change might alter the distribution of animal hosts. Together with increased human travel, this could lead to mixing of clades and opportunities for rare recombination events. Ghana reported its first human cases of mpox in 2022–2023, despite the long-known presence of clade IIa MPXV in wild animals there. Genome sequencing is needed to evaluate whether human MPXV infections in Ghana are due to zoonotic infection with clade IIa MPXV, the spread of clade IIb from Nigeria or the spread of clade I from the DRC. If the former, this could mean that clade IIa is adapting to humans.
Analysing viral gene expression and host responses in vitro could provide insights into the differences in virulence and transmission between different MPXV clades, but these must be validated in animal models. Clade I MPXV is more virulent than clade IIa using aerosol challenge in cynomolgus monkeys70, and similar studies are needed for clade IIb. Evaluation of the relative abilities of clade I, IIa and IIb strains to infect NHPs by various routes, including the rectal mucosa, could be informative. Studies with CAST/EiJ mice have shown that a prototype 2022 clade IIb B.1 isolate is considerably more attenuated than either the clade I or IIa strains. Further experiments with other clade IIb strains from the 2017–2018 outbreak and current strains from Nigeria could be used to correlate genetic and phenotypic changes. The ability of clade I and IIa MPXV to infect North American rodents suggested that MPXV could become a zoonotic infection in North and South America, and similar studies with clade IIb strains are needed. The close connection between humans, animals and the environment emphasizes the importance of the ‘one health’ approach to studies of MPXV89.
In conclusion, we need to better understand the biology of MPXV clades, including differences in immune defence and host interactions, to tackle the burden of mpox, particularly in Africa. A more equitable distribution of vaccines and therapeutics, a greater understanding of mpox epidemiology, identification of MPXV animal reservoirs that can transmit MPXV to humans, and a better understanding of human-to-human transmission are all needed if we are to better manage or indeed prevent future mpox outbreaks.
References
Satheshkumar, P. S. & Damon, I. in Fields Virology: DNA Viruses Vol. 4 (eds Howley, P. M. et al.) Ch. 17 (Wolters Kluwer, 2021).
von Magnus, P., Andersen, E. K., Petersen, K. B. & Birch-Andersen, A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microb. Scand. 46, 156–176 (1959).
Bunge, E. M. et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 16, e0010141 (2022).
Thornhill, J. P. et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 387, 679–691 (2022).
Multi-Country Outbreak of mpox External Situation Report #30—25 November 2023 (World Health Organization, 2023); https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-30---25-november-2023
Forni, D., Cagliani, R., Molteni, C., Clerici, M. & Sironi, M. Monkeypox virus: the changing facets of a zoonotic pathogen. Infect. Genet. Evol. 105, 105372 (2022).
Lum, F. M. et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 22, 597–613 (2022).
Falendysz, E. A., Lopera, J. G., Rocke, T. E. & Osorio, J. E. Monkeypox virus in animals: current knowledge of viral transmission and pathogenesis in wild animal reservoirs and captive animal models. Viruses 15, 905 (2023).
Roper, R. L. et al. Monkeypox (Mpox) requires continued surveillance, vaccines, therapeutics and mitigating strategies. Vaccine 41, 3171–3177 (2023).
Moss, B. & Smith, G. L. in Fields Virology Vol. 2 (eds Howley, P. M. & Knipe, D. M.) Ch. 16 (Wolters Kluwer, 2021).
Shchelkunov, S. N. et al. Analysis of the Monkeypox virus genome. Virology 297, 172–194 (2002).
Rubins, K. H. et al. Comparative analysis of viral gene expression programs during poxvirus infection: a transcriptional map of the vaccinia and monkeypox genomes. PLoS ONE 3, e2628 (2008).
Alkhalil, A. et al. Inhibition of Monkeypox virus replication by RNA interference. Virol. J. 6, 188 (2009).
Yu, H. B., Bruneau, R. C., Brennan, G. & Rothenburg, S. Battle Royale: innate recognition of poxviruses and viral immune evasion. Biomedicines 9, 765 (2021).
Senkevich, T. G., Yutin, N., Wolf, Y. I., Koonin, E. V. & Moss, B. Ancient gene capture and recent gene loss shape the evolution of orthopoxvirus-host interaction genes. mBio 12, e0149521 (2021).
Langland, J. O. & Jacobs, B. L. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299, 133–141 (2002).
Cao, J., Varga, J. & Deschambault, Y. Poxvirus encoded eIF2α homolog, K3 family proteins, is a key determinant of poxvirus host species specificity. Virology 541, 101–112 (2020).
Park, C. et al. Orthopoxvirus K3 orthologs show virus- and host-specific inhibition of the antiviral protein kinase PKR. PLoS Pathog. 17, e1009183 (2021).
Arndt, W. D. et al. Monkeypox virus induces the synthesis of less dsRNA than vaccinia virus, and is more resistant to the anti-poxvirus drug, IBT, than vaccinia virus. Virology 497, 125–135 (2016).
Alcami, A. & Smith, G. L. A mechanism for inhibition of fever by a virus. Proc. Natl Acad. Sci. USA 93, 11029–11034 (1996).
Firth, C. et al. Using time-structured data to estimate evolutionary rates of double-stranded DNA viruses. Mol. Biol. Evol. 27, 2038–2051 (2010).
Patrono, L. V. et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat. Microbiol. 5, 955–965 (2020).
De Maio, N. et al. Mutation rates and selection on synonymous mutations in SARS-CoV-2. Genome Biol. Evol. 13, evab087 (2021).
Shu, L. L., Bean, W. J. & Webster, R. G. Analysis of the evolution and variation of the human influenza A virus nucleoprotein gene from 1933 to 1990. J. Virol. 67, 2723–2729 (1993).
Elde, N. C. et al. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 150, 831–841 (2012).
Likos, A. M. et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 86, 2661–2672 (2005).
Happi, C. et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for Monkeypox virus. PLoS Biol. 20, e3001769 (2022).
Forni, D., Molteni, C., Cagliani, R. & Sironi, M. Geographic structuring and divergence time frame of Monkeypox virus in the endemic region. J. Infect. Dis. 227, 742–751 (2023).
Li, H. et al. The evolving epidemiology of Monkeypox virus. Cytokine Growth Factor Rev. 68, 1–12 (2022).
Kotwal, G. J., Isaacs, S. N., Mckenzie, R., Frank, M. M. & Moss, B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250, 827–830 (1990).
Isaacs, S. N., Kotwal, G. J. & Moss, B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl Acad. Sci. USA 89, 628–632 (1992).
Girgis, N. M. et al. The vaccinia virus complement control protein modulates adaptive immune responses during infection. J. Virol. 85, 2547–2556 (2011).
McCoy, W., Wang, X. L., Yokoyama, W., Hansen, T. & Fremont, D. Structural basis of MHCI antigen presentation sabotage by cowpox encoded protein CPXV203. J. Immunol. 188, 168.18 (2012).
Ndodo, N. et al. Distinct monkeypox virus lineages co-circulating in humans before 2022. Nat. Med. 29, 2317–2324 (2023).
Gigante, C. M. et al. Multiple lineages of Monkeypox virus detected in the United States, 2021–2022. Science 378, 560–564 (2022).
Vartanian, J. P., Guetard, D., Henry, M. & Wain-Hobson, S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science 320, 230–233 (2008).
Warren, C. J. et al. APOBEC3A functions as a restriction factor of human papillomavirus. J. Virol. 89, 688–702 (2015).
Forni, D., Cagliani, R., Pozzoli, U. & Sironi, M. An APOBEC3 mutational signature in the genomes of human-infecting orthopoxviruses. mSphere 8, e0006223 (2023).
Harris, R. S. & Dudley, J. P. APOBECs and virus restriction. Virology 479-480, 131–145 (2015).
Li, Y. L. et al. The structural basis for HIV-1 Vif antagonism of human APOBEC3G. Nature 615, 728–733 (2023).
Kremer, M. et al. Vaccinia virus replication is not affected by APOBEC3 family members. Virol. J. 3, 86 (2006).
Deputy, N. P. et al. Vaccine effectiveness of JYNNEOS against Mpox disease in the United States. N. Engl. J. Med. 388, 2434–2443 (2023).
Freyn, A. W. et al. An mpox virus mRNA-lipid nanoparticle vaccine confers protection against lethal orthopoxviral challenge. Sci. Transl. Med. 15, eadg3540 (2023).
Warner, B. M. et al. In vitro and in vivo efficacy of tecovirimat against a recently emerged 2022 Monkeypox virus isolate. Sci. Transl. Med. 14, eade7646 (2022).
Frenois-Veyrat, G. et al. Tecovirimat is effective against Human Monkeypox virus in vitro at nanomolar concentrations. Nat. Microbiol. 7, 1951–1955 (2022).
Nguyen, B. T. et al. Early administration of tecovirimat shortens the time to mpox clearance in a model of human infection. PLoS Biol. 21, e3002249 (2023).
Desai, A. N. et al. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA 328, 1348–1350 (2022).
Matias, W. R. et al. Tecovirimat for the treatment of human monkeypox: an initial series from Massachusetts, United States. Open Forum Infect. Dis. 9, ofac377 (2022).
Mpox (Monkeypox)—Democratic Republic of the Congo (World Health Organization, 2023); https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON493
Heymann, D. L., Szczeniowski, M. & Esteves, K. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 54, 693–702 (1998).
Nolen, L. D. et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 22, 1014–1021 (2016).
Kibungu, E. M. et al. Clade I-associated mpox cases associated with sexual contact, the Democratic Republic of the Congo. Emerg. Infect. Dis. 30, 172–176 (2024).
Yinka-Ogunleye, A. et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect. Dis. 19, 872–879 (2019).
Update on monkeypox (MPX) in Nigeria, epi-week: 52 (December 26, 2022–January 1, 2023). reliefweb https://reliefweb.int/report/nigeria/update-monkeypox-mpx-nigeria-epi-week-52-december-26-2022-january-1-2023 (2023).
Mauldin, M. R. et al. Exportation of Monkeypox virus from the African continent. J. Infect. Dis. 225, 1367–1376 (2022).
Isidro, J. et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of Monkeypox virus. Nat. Med. 28, 1569–1572 (2022).
Luna, N. et al. Monkeypox virus (MPXV) genomics: a mutational and phylogenomic analyses of B.1 lineages. Travel Med. Infect. Dis. 52, 102551 (2023).
Kohli, R. M. & Isaacs, S. N. Mpox evolution: has the current outbreak revealed a pox on ‘U’? J. Infect. Dis. 227, 828–830 (2023).
O'Toole, Á. et al. APOBEC3 deaminase editing in mpox virus as evidence for sustained human transmission since at least 2016. Science 382, 595–600 (2023).
Marwah, A. et al. Estimating the size of the Monkeypox virus outbreak in Nigeria and implications for global control. J. Travel Med. 29, taac149 (2022).
Khodakevich, L., Jezek, Z. & Kinzanzka, K. Isolation of Monkeypox virus from wild squirrel infected in Nature. Lancet 1, 98–99 (1986).
Jezek, Z. & Fenner, F. Human monkeypox. Monogr. Virol. 17, 1–140 (1988).
Doty, J. B. et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses 9, 283 (2017).
Mariën, J. et al. Monkeypox viruses circulate in distantly-related small mammal species in the Democratic Republic of the Congo. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-414280/v1 (2021).
Reynolds, M. G. et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: evidence for multi-species involvement in the absence of widespread human disease. Am. J. Trop. Med. Hyg. 82, 746–754 (2010).
Radonic, A. et al. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg. Infect. Dis. 20, 1009–1011 (2014).
Johnson, R. F. et al. Comparative analysis of monkeypox virus infection of cynomolgus macaques by the intravenous or intrabronchial inoculation route. J. Virol. 85, 2112–2125 (2011).
Earl, P. L. et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428, 182–185 (2004).
Huggins, J. et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob. Agents Chemother. 53, 2620–2625 (2009).
Chen, N. H. et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340, 46–63 (2005).
Saijo, M. et al. Virulence and pathophysiology of the Congo Basin and West African strains of Monkeypox virus in non-human primates. J. Gen. Virol. 90, 2266–2271 (2009).
Kindrachuk, J. et al. Systems kinomics demonstrates Congo Basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol. Cell. Proteom. 11, M111.015701 (2012).
Hutson, C. L. et al. Transmissibility of the monkeypox virus clades via respiratory transmission: investigation using the prairie dog-monkeypox virus challenge system. PLoS ONE 8, e55488 (2013).
Keckler, M. S. et al. Establishment of the black-tailed prairie dog (Cynomys ludovicianus) as a novel animal model for comparing smallpox vaccines administered preexposure in both high- and low-dose monkeypox virus challenges. J. Virol. 85, 7683–7698 (2011).
Schultz, D. A., Sagartz, J. E., Huso, D. L. & Buller, R. M. L. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology 383, 86–92 (2009).
Americo, J. L., Earl, P. L. & Moss, B. Virulence differences of mpox (monkeypox) virus clades I, IIa and IIb.1 in a small animal model. Proc. Natl Acad. Sci. USA 120, e2220415120 (2023).
Port, J. R. et al. Rectal and vaginal challenge with mpox virus increases virus dissemination and contact transmission compared to skin challenge in the multimammate rat (Mastomys natalensis). Preprint at bioRxiv https://doi.org/10.1101/2023.05.07.539622 (2023).
Osorio, J. E., Iams, K. P., Meteyer, C. U. & Rocke, T. E. Comparison of Monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS ONE 4, e6592 (2009).
Hutson, C. L. et al. Comparison of West African and Congo Basin monkeypox viruses in BALB/c and C57BL/6 mice. PLoS ONE 5, e8912 (2010).
Americo, J. L., Moss, B. & Earl, P. L. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 84, 8172–8180 (2010).
Earl, P. L., Americo, J. L. & Moss, B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology 481, 161–165 (2015).
Earl, P. L., Americo, J. L. & Moss, B. Insufficient innate immunity contributes to the susceptibility of the castaneous mouse to orthopoxvirus infection. J. Virol. 91, e01042-17 (2017).
Earl, P. L., Americo, J. L. & Moss, B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 16, e1008505 (2020).
Lopera, J. G., Falendysz, E. A., Rocke, T. E. & Osorio, J. E. Attenuation of monkeypox virus by deletion of genomic regions. Virology 475, 129–138 (2015).
Hudson, P. N. et al. Elucidating the role of the complement control protein in monkeypox pathogenicity. PLoS ONE 7, e35086 (2012).
Estep, R. D. et al. Deletion of the monkeypox inhibitor of complement enzymes locus impacts the adaptive immune response to Monkeypox virus in a non human primate model of infection. J. Virol. 85, 9527–9542 (2011).
Li, P. et al. Mpox virus infection and drug treatment modelled in human skin organoids. Nat. Microbiol. 8, 2067–2079 (2023).
Tomori, O. & Ogoina, D. Monkeypox: the consequences of neglecting a disease, anywhere. Science 377, 1261–1263 (2022).
Reynolds, M. G., Doty, J. B., McCollum, A. M., Olson, V. A. & Nakazawa, Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One Health. Expert Rev. Anti Infect. Ther. 17, 129–139 (2019).
Tesh, R. B. et al. Experimental infection of ground squirrels (Spermophilius tridecemlineatus) with monkeypox virus. Emerg. Infect. Dis. 10, 1563–1567 (2004).
Sbrana, E., Xiao, S. Y., Newman, P. C. & Tesh, R. B. Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med. Hyg. 76, 155–164 (2007).
Hutson, C. L. et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 90, 323–333 (2009).
Hutson, C. L. et al. Dosage comparison of Congo Basin and West African strains of monkeypox virus using a prairie dog animal model of systemic orthopoxvirus disease. Virology 402, 72–82 (2010).
Hutson, C. L. et al. Comparison of monkeypox virus clade kinetics and pathology within the prairie dog animal model using a serial sacrifice study design. BioMed. Res. Int. https://doi.org/10.1155/2015/965710 (2015).
Deschambault, Y. et al. Experimental infection of North American deer mice with clade I and II Monkeypox virus isolates. Emerg. Infect. Dis. 29, 858–860 (2023).
Falendysz, E. A. et al. Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.). PLoS Negl. Trop. Dis. 11, e0005809 (2017).
Falendysz, E. A. et al. Further assessment of monkeypox virus infection in Gambian pouched rats (Cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Negl. Trop. Dis. 9, e0004130 (2015).
Acknowledgements
Support was provided by the Division of Intramural Research, NIAID, NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Data 1
Host interaction proteins of CPXV and MPXV clades.
Rights and permissions
About this article
Cite this article
Moss, B. Understanding the biology of monkeypox virus to prevent future outbreaks. Nat Microbiol 9, 1408–1416 (2024). https://doi.org/10.1038/s41564-024-01690-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01690-1
- Springer Nature Limited
This article is cited by
-
We must learn from past outbreaks
Nature Microbiology (2024)