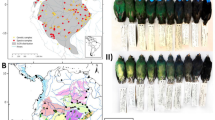
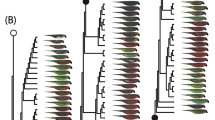
Abstract
Several ecogeographical ‘rules’ have been proposed to explain colour variation at broad spatial and phylogenetic scales but these rarely consider whether colours are based on pigments or structural colours. However, mechanism can have profound effects on the function and evolution of colours. Here, we combine geographic information, climate data and colour mechanism at broad phylogenetic (9,409 species) and spatial scales (global) to determine how transitions between pigmentary and structural colours influence speciation dynamics and range distributions in birds. Among structurally coloured species, we find that rapid dispersal into tropical regions drove the accumulation of iridescent species, whereas the build-up of non-iridescent species in the tropics was driven by a combination of dispersal and faster in situ evolution in the tropics. These results could be explained by pleiotropic links between colouration and dispersal behaviour or ecological factors influencing colonization success. These data elucidate geographic patterns of colouration at a global scale and provide testable hypotheses for future work on birds and other animals with structural colours.




Similar content being viewed by others
Data availability
All data needed to replicate these analyses are available via Dryad at https://doi.org/10.5061/dryad.02v6wwqc0 (ref. 85).
Code availability
All code needed to perform analyses is available via Zenodo at https://doi.org/10.5281/zenodo.11491149 (ref. 67).
References
Currie, D. J. et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (2004).
Willig, M. R., Kaufman, D. M. & Stevens, R. D. Latitudinal gradients of biodiversity: pattern, process, scale and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (2003).
Lawson, A. M. & Weir, J. T. Latitudinal gradients in climatic-niche evolution accelerate trait evolution at high latitudes. Ecol. Lett. 17, 1427–1436 (2014).
Weir, J. T. & Wheatcroft, D. A latitudinal gradient in rates of evolution of avian syllable diversity and song length. Proc. R. Soc. Lond. B 278, 1713–1720 (2011).
Quintero, I., Landis, M., Jetz, W. & Morlon, H. The build-up of the present-day tropical diversity of tetrapods. Proc. Natl Acad. Sci. USA 120, e2220672120 (2023).
Doutrelant, C. et al. Worldwide patterns of bird colouration on islands. Ecol. Lett. 19, 537–545 (2016).
Cooney, C. R. et al. Latitudinal gradients in avian colourfulness. Nat. Ecol. Evol. 6, 622–629 (2022).
Dalrymple, R. L. et al. Birds, butterflies and flowers in the tropics are not more colourful than those at higher latitudes. Glob. Ecol. Biogeogr. 24, 1424–1432 (2015).
Stournaras, K. E. et al. How colorful are fruits? Limited color diversity in fleshy fruits on local and global scales. New Phytol. 198, 617–629 (2013).
Dale, J., Dey, C. J., Delhey, K., Kempenaers, B. & Valcu, M. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370 (2015).
Dunn, P. O., Armenta, J. K. & Whittingham, L. A. Natural and sexual selection act on different axes of variation in avian plumage color. Sci. Adv. 1, e1400155–e1400155 (2015).
Bailey, S. Latitudinal gradients in colors and patterns of passerine birds. Condor 80, 372–381 (1978).
Shawkey, M. D. & D’Alba, L. Interactions between colour-producing mechanisms and their effects on the integumentary colour palette. Philos. Trans. R. Soc. B 372, 20160536 (2017).
Stoddard, M. C. & Prum, R. O. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052 (2011).
Newton, I. Opticks (Dover, 1704).
Prum, R. O. in Bird Coloration Vol. I (eds Mcgraw, K. J. & Hill, G. E.) 295–353 (Harvard Univ. Press, 2006).
Noh, H. et al. How noniridescent colors are generated by quasi-ordered structures of bird feathers. Adv. Mater. 22, 2871–2880 (2010).
Maia, R., Rubenstein, D. R. & Shawkey, M. D. Key ornamental innovations facilitate diversification in an avian radiation. Proc. Natl Acad. Sci. USA 110, 10687–10692 (2013).
Gomez, D. & Théry, M. Simultaneous crypsis and conspicuousness in color patterns: comparative analysis of a neotropical rainforest bird community. Am. Nat. 169, S42–S61 (2007).
Eliason, C. M. & Shawkey, M. D. Rapid, reversible response of iridescent feather color to ambient humidity. Opt. Express 18, 21284–21292 (2010).
Shawkey, M. D. et al. Structural color change following hydration and dehydration of iridescent mourning dove (Zenaida macroura) feathers. Zoology 114, 59–68 (2011).
Eliason, C. M. & Shawkey, M. D. Decreased hydrophobicity of iridescent feathers: a potential cost of shiny plumage. J. Exp. Biol. 214, 2157–2163 (2011).
Rogalla, S., Patil, A., Dhinojwala, A., Shawkey, M. D. & D’Alba, L. Enhanced photothermal absorption in iridescent feathers. J. R. Soc. Interface 18, 20210252 (2021).
Thomas, D. B. et al. Ancient origins and multiple appearances of carotenoid-pigmented feathers in birds. Proc. R. Soc. Lond. B 281, 20140806 (2014).
Friedman, N. R., Hofmann, C. M., Kondo, B. & Omland, K. E. Correlated evolution of migration and sexual dichromatism in the New World orioles (icterus). Evolution 63, 3269–3274 (2009).
Durrer, H. Schillerfarben der vogelfeder als evolutionsproblem. Denkschr. Schweiz. Nat. Forsch. Ges. 91, 1–127 (1977).
Ducrest, A.-L., Keller, L. & Roulin, A. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol. Evol. 23, 502–510 (2008).
Camacho, C., Pérez-Rodríguez, L., Abril-Colón, I., Canal, D. & Potti, J. Plumage colour predicts dispersal propensity in male pied flycatchers. Behav. Ecol. Sociobiol. 72, 2 (2018).
Mittelbach, G. G. et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (2007).
Dalrymple, R. L. et al. Abiotic and biotic predictors of macroecological patterns in bird and butterfly coloration. Ecol. Monogr. 88, 204–224 (2018).
Griggio, M., Serra, L., Licheri, D., Campomori, C. & Pilastro, A. Moult speed affects structural feather ornaments in the blue tit. J. Evol. Biol. 22, 782–792 (2009).
Stevens, G. C. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256 (1989).
Winger, B. M. & Bates, J. M. The tempo of trait divergence in geographic isolation: avian speciation across the Marañon Valley of Peru. Evolution 69, 772–787 (2015).
Lucas, A. M. & Stettenheim, P. R. in Avian Anatomy: Integument 341–419 (US Government Printing Office, 1972).
Claramunt, S. & Cracraft, J. A new time tree reveals Earth historys imprint on the evolution of modern birds. Sci. Adv. 1, e1501005 (2015).
Rogalla, S. et al. The evolution of darker wings in seabirds in relation to temperature-dependent flight efficiency. J. R. Soc. Interface 18, 20210236 (2021).
Shawkey, M. D., Hauber, M. E., Estep, L. K. & Hill, G. E. Evolutionary transitions and mechanisms of matte and iridescent plumage coloration in grackles and allies (Icteridae). J. R. Soc. Interface 3, 777–786 (2006).
Maia, R., D’Alba, L. & Shawkey, M. D. What makes a feather shine? A nanostructural basis for glossy black colours in feathers. Proc. R. Soc. Lond. B 278, 1973–1980 (2011).
Xiao, M., Dhinojwala, A. & Shawkey, M. Nanostructural basis of rainbow-like iridescence in common bronzewing Phaps chalcoptera feathers. Opt. Express 22, 14625–14636 (2014).
Rogalla, S., Shawkey, M. D. & D’Alba, L. Thermal effects of plumage coloration. Ibis 164, 933–948 (2022).
Maia, R., Caetano, J. V. O., Bao, S. N. & Macedo, R. H. Iridescent structural colour production in male blue-black grassquit feather barbules: the role of keratin and melanin. J. R. Soc. Interface 6, S203–S211 (2009).
Prum, R. O., Torres, R., Williamson, S. & Dyck, J. Coherent light scattering by blue feather barbs. Nature 396, 28–29 (1998).
Londoño, G. A., Chappell, M. A., Castañeda, M. D. R., Jankowski, J. E. & Robinson, S. K. Basal metabolism in tropical birds: latitude, altitude and the ‘pace of life’. Funct. Ecol. 29, 338–346 (2014).
Sinnott-Armstrong, M. A. et al. Global geographic patterns in the colours and sizes of animal‐dispersed fruits. Glob. Ecol. Biogeogr. 27, 1339–1351 (2018).
Hu, D. et al. A bony-crested Jurassic dinosaur with evidence of iridescent plumage highlights complexity in early paravian evolution. Nat. Commun. 9, 217 (2018).
Vinther, J., Briggs, D. E. G., Clarke, J., Mayr, G. & Prum, R. O. Structural coloration in a fossil feather. Biol. Lett. 6, 128–131 (2010).
Eliason, C. M. & Clarke, J. A. Cassowary gloss and a novel form of structural color in birds. Sci. Adv. 6, eaba0187 (2020).
Eliason, C. M., Maia, R., Parra, J. L. & Shawkey, M. D. Signal evolution and morphological complexity in hummingbirds (Aves: Trochilidae). Evolution 74, 447–458 (2020).
Nordén, K. K., Eliason, C. M. & Stoddard, M. C. Evolution of brilliant iridescent feather nanostructures. eLife 10, e71179 (2021).
Eliason, C. M., Maia, R. & Shawkey, M. D. Modular color evolution facilitated by a complex nanostructure in birds. Evolution 69, 357–367 (2015).
Jetz, W. W., Thomas, G. H. G. H., Joy, J. B. J. B., Hartmann, K. K. & Mooers, A. O. A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012).
Eliason, C. M. andersen, M. J. & Hackett, S. J. Using historical biogeography models to study color pattern evolution. Syst. Biol. 68, 755–766 (2019).
Billerman, S. M., Keeney, B. K., Rodewald, P. G. & Schulenberg, T. S. Birds of the World (Cornell Laboratory of Ornithology, 2022).
Durrer, H. in Biology of the Integument Vol. 2 Vertebrates (eds Bereiter-Hahn, J. et al.) 239–247 (Springer, 1986).
Auber, L. The distribution of structural colours and unusual pigments in the class Aves. Ibis 99, 463–476 (1957).
Mcgraw, K. J. in Bird Coloration Vol. 1 (eds Hill, G. E. & Mcgraw, K. J.) 354–398 (Harvard Univ. Press, 2006).
Dyck, J. Structure and light reflection of green feathers of fruit doves (Ptilinopus spp.) and an imperial pigeon (Ducula concinna). Biol. Skr. K. Dan. Vidensk. Selsk. 30, 2–43 (1987).
Dyck, J. Reflectance spectra of plumage areas colored by green feather pigments. Auk 109, 293–301 (1992).
Prum, R. O. R. O., Lafountain, A. M. A. M., Berro, J. J., Stoddard, M. C. M. C. & Frank, H. A. H. A. Molecular diversity, metabolic transformation and evolution of carotenoid feather pigments in cotingas (Aves: Cotingidae). J. Comp. Physiol. B 182, 1095–1116 (2012).
Dyck, J. Winter plumage of the rock ptarmigan: structure of the air-filled barbules and function of the white colour. Dan. Orn. Foren. Tidsskr. 73, 41–58 (1979).
Barrera-Guzmán, A. O., Aleixo, A., Shawkey, M. D. & Weir, J. T. Hybrid speciation leads to novel male secondary sexual ornamentation of an Amazonian bird. Proc. Natl Acad. Sci. USA 115, E218–E225 (2018).
Dunning, J. et al. How woodcocks produce the most brilliant white plumage patches among the birds. J. R. Soc. Interface 20, 20220920 (2023).
Lex, A., Gehlenborg, N., Strobelt, H., Vuillemot, R. & Pfister, H. UpSet: visualization of intersecting sets. IEEE Trans. Vis. Comput. Graph. 20, 1983–1992 (2014).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26 (2008).
Species Distribution Data Request (BirdLife International; accessed 21 March 2018); http://datazone.birdlife.org/species/requestdis
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Eliason, C. et al. Data from: Why there are so many structurally coloured bird species in the tropics? Zenodo 10.5281/zenodo.11491148 (2024).
Vilela, B. & Villalobos, F. letsR: a new R package for data handling and analysis in macroecology. Methods Ecol. Evol. 6, 1229–1234 (2015).
Read, Q. D. et al. Tropical bird species have less variable body sizes. Biol. Lett. 14, 20170453 (2018).
Alves, D. M. C. C., Diniz-Filho, J. A. F. & Villalobos, F. Geographical diversification and the effect of model and data inadequacies: the bat diversity gradient as a case study. Biol. J. Linn. Soc. Lond. 121, 894–906 (2017).
FitzJohn, R. G. Diversitree: comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 3, 1084–1092 (2012).
Goldberg, E. E., Lancaster, L. T. & Ree, R. H. Phylogenetic inference of reciprocal effects between geographic range evolution and diversification. Syst. Biol. 60, 451–465 (2011).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Burleigh, J. G., Kimball, R. T. & Braun, E. L. Building the avian tree of life using a large-scale, sparse supermatrix. Mol. Phylogenet. Evol. 84, 53–63 (2015).
Beaulieu, J. M. & O’meara, B. C. Detecting hidden diversification shifts in models of trait-dependent speciation and extinction. Syst. Biol. 65, 583–601 (2016).
Höhna, S. et al. RevBayes: Bayesian phylogenetic inference using graphical models and an interactive model-specification language. Syst. Biol. 65, 726–736 (2016).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2011).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Jackson, D. A. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204–2214 (1993).
Tobias, J. A. et al. AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett. 25, 581–597 (2022).
Ho, L. S. T. & Ané, C. A linear-time algorithm for gaussian and non-gaussian trait evolution models. Syst. Biol. 63, 397–408 (2014).
Shawkey, M. D. & Hill, G. E. Significance of a basal melanin layer to production of non-iridescent structural plumage color: evidence from an amelanotic Steller’s jay (Cyanocitta stelleri). J. Exp. Biol. 209, 1245–1250 (2006).
Igic, B., D’Alba, L. & Shawkey, M. D. Manakins can produce iridescent and bright feather colours without melanosomes. J. Exp. Biol. 219, 1851–1859 (2016).
Ives, A. R. R2s for correlated data: phylogenetic models, LMMs and GLMMs. Syst. Biol. 68, 234–251 (2019).
Eliason, C. et al. Transitions between colour mechanisms affect speciation dynamics and range distributions of birds [Dataset]. Dryad https://doi.org/10.5061/dryad.02v6wwqc0 (2024).
Acknowledgements
We thank J. Bates for fruitful discussions early in the project. M. Nelson and R. Ree provided technical comments that were immensely helpful in conducting the final analyses. We also thank O. Pauwels, P. Kamminga and A. Nackaerts for access to the collections of the RBINS, Naturalis and RMMA, respectively. We thank G. Debruyn with help in collecting data. This work was partially supported by the National Science Foundation (NSF EP-2112468 to C.M.E.), EOARD (FA98655-23-1-7041 to M.D.S. and L.D.), AFOSR (FA9550-18-0-0447 to M.D.S.) and FWO (G007117N and G0E8322N both to M.D.S.).
Author information
Authors and Affiliations
Contributions
C.M.E. designed the study. C.M.E., M.P.J.N., C.B., E.B., L.D. and M.D.S. collected data. C.M.E. analysed the data and produced figures. C.M.E. wrote the initial manuscript. C.M.E., M.P.J.N., C.B., E.B., L.D. and M.D.S. revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Christopher Cooney and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Diversification dynamics are distinct for different forms of structural colouration.
Panels show support for the “structurally coloured dispersers” hypothesis H1 (a), the “tropical transitions” hypothesis H2 (b) and the “structurally coloured speciators” hypothesis H3 (c) for different range overlap cut-offs ordered by decreasing stringency (see Methods for details). Point colours indicate different colouration mechanisms: iridescent structural colour (ISC; purple) and non-iridescent structural colour (NISC; gold). Significant effects indicated with filled circles. See Fig. 4a for model details and Extended Data Fig. 5 for results for alternate phylogenies and species range map grid cell resolutions. Note different y-axis scales for each panel.
Extended Data Fig. 2 Climatic variation explains prevalence of species with structural colour.
Panels show proportion/probability of being ISC (a–c) or NISC (d–f) as a function of climate PC1 (a,d), climate PC2 (b,e), and natural log-transformed body mass in grams (c,f). Lines are drawn from parameters estimated with phylogenetic logistic regression in phyloglm, with non-significant relationships indicated as dashed lines. Climate PC1 (β = 0.05), associated with cooler, drier, more seasonally variable climates, and climate PC2 (β = −0.05), associated with less seasonally variable and hotter climates, were both significant predictors of ISC (a,b), but body size was not (c, pseudo R2 = 0.09). Only climate PC1 (β = −0.03) was a significant predictor of NISC (d, pseudo R2 = 0.07). See Table 2 for statistical details and Supplementary Fig. 3 for results under alternative phylogenies and grid cell resolutions.
Extended Data Fig. 3 Different habitats support different numbers of structurally coloured species.
Bars show number of species with pigment-based colouration (dark blue), iridescent structural colouration (pink), and non-iridescent structural colouration (gold) in different habitats. Numbers to right of bars are proportions of species in that habitat with either form of structural colouration (that is, ISC or NISC). Habitat bars sharing similar superscript letters are not significantly different in proportions of overall numbers of structurally coloured species (two-sided phylogenetic GLM, P < 0.05, phylogenetic signal = 0.026).
Extended Data Fig. 4 Effects of recoding areas on ClaSSE parameter estimates.
Mean parameter estimates (points) and 95% credible intervals (vertical lines) for temperate (a–e) and tropical regions (f–j) under a 33-parameter ClaSSE model (see Fig. 4a for details). Parameters are dispersal rates (a,f), rates of structural colour gain (b,g), rates of structural colour loss (c,h), speciation rates (d,i), and extinction rates (e,j). Colours correspond to pigment-based colours (PBC; dark blue), iridescent structural colours (ISC; purple) and non-iridescent structural colours (NISC; gold). Similar to recent work studying dispersal and speciation rates across tetrapods5, less stringent cut-offs result in fewer widespread species and, subsequently, higher dispersal rates into tropical regions (Extended Data Fig. 1a). Results are shown for the phylogeny from ref. 51 and a 2º map grid cell resolution.
Supplementary information
Supplementary Information
Supplementary Text 1–3, Figs. 1–5, Tables 1–3 and References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eliason, C.M., Nicolaï, M.P.J., Bom, C. et al. Transitions between colour mechanisms affect speciation dynamics and range distributions of birds. Nat Ecol Evol 8, 1723–1734 (2024). https://doi.org/10.1038/s41559-024-02487-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-024-02487-5
- Springer Nature Limited