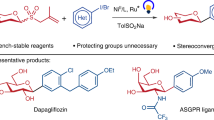
Abstract
Carbohydrates play important roles in medicinal chemistry and biochemistry. However, their synthesis relies on specially designed glycosyl donors, which are often unstable and require multi-step synthesis. Furthermore, the catalytic and stereoselective installation of arylated quaternary stereocentres on sugar rings remains a formidable challenge. Here we report a facile and versatile method for the synthesis of diverse C–R (where R is an aryl, heteroaryl, alkenyl, alkynyl or alkyl) glycosides from readily available and bench-stable 1-deoxyglycosides. The reaction proceeds under mild conditions and exhibits high stereoselectivity across a broad range of glycosyl units. This protocol can be used to synthesize challenging 2-deoxyglycosides, unprotected glycosides, non-classical glycosides and deuterated glycosides. We further developed the catalyst-controlled site-divergent functionalization of carbohydrates for the synthesis of various unexplored carbohydrates containing arylated quaternary stereocentres that are inaccessible by existing methods. The synthetic utility of this strategy is further demonstrated in the synthesis of pharmaceutically relevant molecules and carbohydrates.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information files. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2262193 (14), 2292208 (23), 2251328 (60), 2235883 (72), 2292209 (79) and 2251329 (107). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Elshahawi, S. I., Shaaban, K. A., Kharel, M. K. & Thorson, J. S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 44, 7591–7697 (2015).
Bokor, É. et al. C-glycopyranosyl arenes and hetarenes: synthetic methods and bioactivity focused on antidiabetic potential. Chem. Rev. 117, 1687–1764 (2017).
Cao, X. et al. Carbohydrate-based drugs launched during 2000–2021. Acta Pharm. Sin. B 12, 3783–3821 (2022).
Bera, S., Chatterjee, B. & Mondal, D. Construction of quaternary stereocenters on carbohydrate scaffolds. RSC Adv. 6, 77212–77242 (2016).
Štambaský, J., Hocek, M. & Kočovský, P. C-nucleosides: synthetic strategies and biological applications. Chem. Rev. 109, 6729–6764 (2009).
Yang, Y. & Yu, B. Recent advances in the chemical synthesis of C-glycosides. Chem. Rev. 117, 12281–12356 (2017).
Kitamura, K., Ando, Y., Matsumoto, T. & Suzuki, K. Total synthesis of aryl C‑glycoside natural products: strategies and tactics. Chem. Rev. 118, 1495–1598 (2018).
Nielsen, M. M. & Pedersen, C. M. Catalytic glycosylations in oligosaccharide synthesis. Chem. Rev. 118, 8285–8358 (2018).
McKay, M. J. & Nguyen, H. M. Recent advances in transition metal-catalyzed glycosylation. ACS Catal. 2, 1563–1595 (2012).
Chen, A., Yang, B., Zhou, Z. & Zhu, F. Recent advances in transition-metal-catalyzed glycosyl cross-coupling reactions. Chem Catal. 2, 3430–3470 (2022).
Xu, L., Fan, N. & Hu, X. Recent development in the synthesis of C-glycosides involving glycosyl radicals. Org. Biomol. Chem. 18, 5095–5109 (2020).
Gong, H. & Gagné, M. R. Diastereoselective Ni-catalyzed Negishi cross-coupling approach to saturated, fully oxygenated C-Alkyl and C-Aryl glycosides. J. Am. Chem. Soc. 130, 12177–12183 (2008).
Zhao, C., Jia, X., Wang, X. & Gong, H. Ni-catalyzed reductive coupling of alkyl acids with unactivated tertiary alkyl and glycosyl halides. J. Am. Chem. Soc. 136, 17645–17651 (2014).
Liu, J., Lei, C. & Gong, H. Nickel-catalyzed reductive coupling of glucosyl halides with aryl/vinyl halides enabling β-selective preparation of C-aryl/vinyl glucosides. Sci. China Chem. 62, 1492–1496 (2019).
Li, Y. et al. Chemoselective and diastereoselective synthesis of C-Aryl nucleoside analogues by nickel-catalyzed cross-coupling of furanosyl acetates with aryl iodides. Angew. Chem. Int. Ed. 61, e202110391 (2022).
Wang, Q. et al. Palladium-catalysed C–H glycosylation for synthesis of C-aryl glycosides. Nat. Catal. 2, 793–800 (2019).
Sun, Q. et al. Stereoselective synthesis of C-vinyl glycosides via palladium catalyzed C–H glycosylation of alkenes. Angew. Chem. Int. Ed. 60, 19620–19625 (2021).
Lv, W., Chen, Y., Wen, S., Ba, D. & Cheng, G. Modular and stereoselective synthesis of C-aryl glycosides via Catellani reaction. J. Am. Chem. Soc. 142, 14864–14870 (2020).
Wang, Q., Duan, J., Tang, P., Chen, G. & He, G. Synthesis of non-classical heteroaryl C-glycosides via Minisci-type alkylation of N-heteroarenes with 4-glycosyl-dihydropyridines. Sci. China Chem. 63, 1613–1618 (2020).
Ghouilem, J. et al. Diastereoselective Pd-catalyzed anomeric C(sp3)–H activation: synthesis of α-(hetero)aryl C-glycosides. ACS Catal. 11, 1818–1826 (2021).
Nicolas, L. et al. Diastereoselective metal-catalyzed synthesis of C-aryl and C-vinyl glycosides. Angew. Chem. Int. Ed. 51, 11101–11104 (2012).
Adak, L. et al. Synthesis of aryl C-glycosides via iron-catalyzed cross coupling of halosugars: stereoselective anomeric arylation of glycosyl radicals. J. Am. Chem. Soc. 139, 10693–10701 (2017).
Wang, Q. et al. Iron-catalysed reductive cross-coupling of glycosyl radicals for the stereoselective synthesis of C-glycosides. Nat. Synth. 1, 235–244 (2022).
Zhu, F., Rourke, M. J., Yang, T., Rodriguez, J. & Walczak, M. A. Highly stereospecific cross-coupling reactions of anomeric stannanes for the synthesis of C‑aryl glycosides. J. Am. Chem. Soc. 138, 12049–12052 (2016).
Zhu, F., Rodriguez, J., O’Neill, S. & Walczak, M. A. Acyl glycosides through stereospecific glycosyl cross-coupling: rapid access to C(sp3)-linked glycomimetics. ACS Cent. Sci. 4, 1652–1662 (2018).
Ma, Y. et al. Highly stereoselective synthesis of aryl/heteroaryl-C-nucleosides via the merger of photoredox and nickel catalysis. Chem. Commun. 55, 14657–14660 (2019).
Zhu, M. & Messaoudi, S. Diastereoselective decarboxylative alkynylation of anomeric carboxylic acids using Cu/photoredox dual catalysis. ACS Catal. 11, 6334–6342 (2021).
Ji, P. et al. Visible-light-mediated, chemo- and stereoselective radical process for the synthesis of C-glycoamino acids. Org. Lett. 21, 3086–3092 (2019).
Qi, R. et al. Visible-light-promoted stereoselective C(sp3)–H glycosylation for the synthesis of C-glycoamino acids and C-glycopeptides. Angew. Chem. Int. Ed. 61, e202200822 (2022).
Miquel, N., Doisneau, G. & Beau, J. M. Reductive samariation of anomeric 2-pyridylsulfones with catalytic nickel: an unexpected improvement in the synthesis of 1,2-trans-diequatorial C-glycosyl compounds. Angew. Chem. Int. Ed. 39, 4111–4114 (2000).
Mazkas, D., Skrydstrup, T., Doumeix, O. & Beau, J. M. Samarium iodide induced intramolecular C-glycoside formation: efficient radical formation in the absence of an additive. Angew. Chem. Int. Ed. 33, 1383–1386 (1994).
Shang, W. et al. Generation of glycosyl radicals from glycosyl sulfoxides and its use in the synthesis of C‐linked glycoconjugates. Angew. Chem. Int. Ed. 60, 385–390 (2021).
Wang, Q. et al. Visible light activation enables desulfonylative cross-coupling of glycosyl sulfones. Nat. Synth. 1, 967–974 (2022).
Xie, D., Wang, Y., Zhang, X., Fu, Z. & Niu, D. Alkyl/glycosyl sulfoxides as radical precursors and their use in the synthesis of pyridine derivatives. Angew. Chem. Int. Ed. 61, e202204922 (2022).
Zhang, C. et al. Direct synthesis of unprotected aryl C-glycosides by photoredox Ni-catalysed cross-coupling. Nat. Synth. 2, 251–260 (2023).
Masuda, K., Nagatomo, M. & Inoue, M. Direct assembly of multiply oxygenated carbon chains by decarbonylative radical-radical coupling reactions. Nat. Chem. 9, 207–212 (2017).
Wei, Y., Ben-ȥvi, B. & Diao, T. Diastereoselective synthesis of aryl C-glycosides from glycosyl esters via C–O bond homolysis. Angew. Chem. Int. Ed. 60, 9433–9438 (2021).
Jiang, Y. et al. Catalytic multicomponent synthesis of C-acyl glycosides by consecutive cross-electrophile couplings. Angew. Chem. Int. Ed. 61, e202211043 (2022).
Wei, Y., Wang, Q. & Koh, M. J. A photoinduced, nickel-catalyzed reaction for the stereoselective assembly of C-linked glycosides and glycopeptides. Angew. Chem. Int. Ed. 61, e202214247 (2022).
Chen, A. et al. Recent advances in glycosylation involving novel anomeric radical precursors. J. Carbohydr. Chem. 40, 361–400 (2021).
Shatskiy, A., Stepanova, E. V. & Kärkäs, M. D. Exploiting photoredox catalysis for carbohydrate modification through C–H and C–C bond activation. Nat. Rev. Chem. 6, 782–805 (2022).
Ghouilem, J., de Robichon, M., Le Bideau, F., Ferry, A. & Messaoudi, S. Emerging organometallic methods for the synthesis of C-branched (hetero)aryl, alkenyl, and alkyl glycosides: C–H functionalization and dual photoredox approaches. Chemistry 27, 491–511 (2021).
Probst, N. et al. Palladium(II)-catalyzed diastereoselective 2,3-trans C(sp3)–H arylation of glycosides. ACS Catal. 8, 7781–7786 (2018).
Wu, J., Kopp, A. & Ackermann, L. Synthesis of C-oligosaccharides through versatile C(sp3)–H glycosylation of glycosides. Angew. Chem. Int. Ed. 61, e202114993 (2022).
Dumoulin, A., Matsui, J. K., Gutiérrez-Bonet, Á. & Molander, G. A. Synthesis of non-classical arylated C-saccharides through nickel/photoredox dual catalysis. Angew. Chem. Int. Ed. 57, 6614–6618 (2018).
Zhao, G. et al. Nickel-catalyzed radical migratory coupling enables C‑2 arylation of carbohydrates. J. Am. Chem. Soc. 143, 8590–8596 (2021).
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem Catal. 1, 523–598 (2021).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 138, 12715–12718 (2016).
Shaw, M. H., Shurtleff, V. W., Terrett, J. A., Cuthbertson, J. D. & MacMillan, D. W. C. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 352, 1304–1308 (2016).
Shields, B. J. & Doyle, A. G. Direct C(sp3)–H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc. 138, 12719–12722 (2016).
Shen, Y., Gu, Y. & Martin, R. sp3 C–H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc. 140, 12200–12209 (2018).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Wang, C., Dixneuf, P. H. & Soulé, J. F. Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. Chem. Rev. 118, 7532–7585 (2018).
Tellis, J. C. et al. Single-electron transmetalation via photoredox/nickel dual catalysis: unlocking a new paradigm for sp3–sp2 cross-coupling. Acc. Chem. Res. 49, 1429–1439 (2016).
Cheung, K. P. S., Sarkar, S. & Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 122, 1543–1625 (2022).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 16, 10035–10074 (2016).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C–H functionalization reactions. Chem. Rev. 122, 1925–2016 (2022).
Jeffrey, J. L., Terrett, J. A. & MacMillan, D. W. C. O–H hydrogen bonding promotes H-atom transfer from a C–H bonds for C-alkylation of alcohols. Science 349, 1532–1536 (2015).
Dimakos, V., Su, H. Y., Garrett, G. E. & Taylor, M. S. Site-selective and stereoselective C–H alkylations of carbohydrates via combined diarylborinic acid and photoredox catalysis. J. Am. Chem. Soc. 141, 5149–5153 (2019).
Gorelik, D. J., Turner, J. A., Virk, T. S., Foucher, D. A. & Taylor, M. S. Site- and stereoselective C–H alkylations of carbohydrates enabled by cooperative photoredox, hydrogen atom transfer, and organotin catalysis. Org. Lett. 23, 5180–5518 (2021).
Matsumoto, A., Yamamoto, M. & Maruoka, K. Cationic DABCO-based catalyst for site-selective C–H alkylation via photoinduced hydrogen-atom transfer. ACS Catal. 12, 2045–2051 (2022).
Wan, L. C., Witte, M. D. & Minnaard, A. J. Site-selective carbon–carbon bond formation in unprotected monosaccharides using photoredox catalysis. Chem. Commun. 53, 4926–4929 (2017).
Chen, D., Chu, J. C. K. & Rovis, T. Directed γ-C(sp3)–H alkylation of carboxylic acid derivatives through visible light photoredox catalysis. J. Am. Chem. Soc. 139, 14897–14900 (2017).
Li, Y., Miyamoto, S., Torigoe, T. & Kuninobu, Y. Regioselective C(sp3)–H alkylation of a fructopyranose derivative by 1,6-HAT. Org. Biomol. Chem. 19, 3124–3127 (2021).
Jeffery, A. & Nair, V. An improved method for the synthesis of anhydroalditols. Tetrahedron Lett. 36, 3627–3630 (1995).
Ye, Y., Chen, H., Yao, K. & Gong, H. Iron-catalyzed reductive vinylation of tertiary alkyl oxalates with activated vinyl halides. Org. Lett. 22, 2070–2075 (2020).
Salamone, M. & Bietti, M. Tuning reactivity and selectivity in hydrogen atom transfer from aliphatic C–H bonds to alkoxyl radicals: role of structural and medium effects. Acc. Chem. Res. 48, 2895–2903 (2015).
Dondi, D. et al. Regio- and stereoselectivity in the decatungstate photocatalyzed alkylation of alkenes by alkylcyclohexanes. Chemistry 15, 7949–7957 (2009).
Perry, I. B. et al. Direct arylation of strong aliphatic C–H bonds. Nature 560, 70–75 (2018).
Ravelli, D., Fagnoni, M., Fukuyama, T., Nishikawa, T. & Ryu, I. Site-selective C–H functionalization by decatungstate anion photocatalysis: synergistic control by polar and steric effects expands the reaction scope. ACS Catal. 8, 701–713 (2018).
Ke, Y., Li, W., Liu, W. & Kong, W. Ni-catalyzed ligand-controlled divergent and selective synthesis. Sci. China Chem. 66, 2951–2976 (2023).
Chen, K. et al. Functional-group translocation of cyano groups by reversible C–H sampling. Nature 620, 1007–1012 (2023).
Ping, Y. et al. Switchable 1,2-rearrangement enables expedient synthesis of structurally diverse fluorine-containing scaffolds. J. Am. Chem. Soc. 144, 11626–11637 (2022).
van Rijssel, E. R. et al. Furanosyl oxocarbenium ion stability and stereoselectivity. Angew. Chem. Int. Ed. 53, 10381–10385 (2014).
Xu, G. et al. Design, synthesis, and biological evaluation of deuterated C-aryl glycoside as a potent and long-acting renal sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 57, 1236–1251 (2014).
Fernandes, R. A., Chavan, V. P., Mulay, S. V. & Manchoju, A. Chiron approach to the total synthesis of (−)-juglomycin A, (+)-kalafungin, (+)-frenolicin B, and (+)-deoxyfrenolicin. J. Org. Chem. 77, 10455–10460 (2012).
Hrdlicka, P. J. et al. Synthesis and biological evaluation of nucleobase-modified analogs of the anticancer compounds 3′-C-ethynyluridine (EUrd) and 3′-C-ethynylcytidine (ECyd). Bioorg. Med. Chem. 13, 1249–1260 (2005).
Acknowledgements
This project was supported by the National Natural Science Foundation of China (22171215 to W.K., 22301225 to Y.P. and 22201222 to Q.X.), the Cultivation Program of Wuhan Institute of Photochemistry and Technology (GHY2023KF007 to W.K.), the National Key R&D Program of China (2022YFA1505100 and 2023YFA1508600 to Q.X.), Hubei Provincial Outstanding Youth Fund (2022CFA092 to W.K.), Hubei Provincial Natural Science Foundation (2023AFB034 to Y.P.) and GuangDong Basic and Applied Basic Research Foundation (2022A1515010246 to W.K. and 2022A1515110113 to Y.P.). We acknowledge the Core Facility of Wuhan University for X-ray single crystal diffraction analysis. X.Q. acknowledges the supercomputing system in the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
W.K. conceived and directed this project. S.X., Y.P., Y.K. and R.M. conducted the experimental investigations. M.X., G.W. and X.Q. performed the DFT calculations. S.X. and Y.P. analysed and interpreted the experimental data. W.K. and X.Q. wrote the manuscript with feedback from other authors. All authors contributed to discussions. S.X. and Y.P. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Markus Kärkäs and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Computational studies of the ligand-controlled site-selective sugar arylation with aryl bromide.
a, Free energy profile of Ni(0)-catalyzed sugar arylation with ligand DTBPy (L1). b, Free energy profile of Ni(0)-catalyzed sugar arylation with ligand TBTPy (L2). Black path denotes the reaction of 2º radical 56 and the gray path denotes the reaction of 3º radical 57. See Supplementary Fig. 51 for full free energy profile of Ni(0)/L2-catalyzed sugar arylation with 3º radical 57. All energies were calculated at M06/6-311+G(d,p)–SDD/SMD(acetonitrile)//B3LYP-D3(BJ)/6-31G(d)–LANL2DZ level of theory.
Supplementary information
Supplementary Information
Supplementary Tables 1–44, Figs. 1–131, optimization studies, experimental procedures, product characterization and X-ray crystallographic analysis.
Supplementary Data 1
Nuclear magnetic resonance spectra.
Supplementary Data 2
Crystallographic data for compound 14, CCDC reference 2262193.
Supplementary Data 3
Crystallographic data for compound 23, CCDC reference 2292208.
Supplementary Data 4
Crystallographic data for compound 60, CCDC reference 2251328.
Supplementary Data 5
Crystallographic data for compound 72, CCDC reference 2235883.
Supplementary Data 6
Crystallographic data for compound 79, CCDC reference 2292209.
Supplementary Data 7
Crystallographic data for compound 107, CCDC reference 2251329.
Supplementary Data 8
Computational details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, S., Ping, Y., Xu, M. et al. Stereoselective and site-divergent synthesis of C-glycosides. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01629-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01629-3
- Springer Nature Limited