Abstract
Since the birth of cognitive science, researchers have used reaction time and accuracy to measure cognitive ability. Although recognition of these two measures is often based on empirical observations, the underlying consensus is that most cognitive behaviors may be along two fundamental dimensions: cognitive processing speed (CPS) and cognitive processing accuracy (CPA). In this study, we used genomic-wide association studies (GWAS) data from 14 cognitive traits to show the presence of those two factors and revealed the specific neurobiological basis underlying them. We identified that CPS and CPA had distinct brain phenotypes (e.g. white matter microstructure), neurobiological bases (e.g. postsynaptic membrane), and developmental periods (i.e. late infancy). Moreover, those two factors showed differential associations with other health-related traits such as screen exposure and sleep status, and a significant causal relationship with psychiatric disorders such as major depressive disorder and schizophrenia. Utilizing an independent cohort from the Adolescent Brain Cognitive Development (ABCD) study, we also uncovered the distinct contributions of those two factors on the cognitive development of young adolescents. These findings reveal two fundamental factors underlying various cognitive abilities, elucidate the distinct brain structural fingerprint and genetic architecture of CPS and CPA, and hint at the complex interrelationship between cognitive ability, lifestyle, and mental health.
Similar content being viewed by others
Introduction
Cognitive performance is often assessed by how well an individual completes a task within a given time frame, typically measured using response time and accuracy. The universality of these two measures in evaluating different cognitive abilities reflects that there may be two fundamental dimensions of speed and accuracy underlying most human cognitive activities. Cognitive processing speed (CPS) represents a fundamental cognitive capability that gauges the swiftness of information processing, integration, and execution. It is typically assessed through reaction time or the duration of cognitive tasks1,2. In contrast, cognitive processing accuracy (CPA) necessitates individuals to seamlessly coordinate various fundamental cognitive processes such as attention, response inhibition, and working memory, in addition to specific knowledge relevant to the task at hand3,4. These functions reflect different aspects of cognitive ability and exhibit significant associations with a wide range of cognitive behaviors and psychiatric disorders5,6,7. As a result, comprehending the neuroimage signature and genetic architecture underlying these two abilities not only provides insights into the foundations of various cognitive functions but also sheds light on the neurobiological factors influencing behavior in the context of mental disorders.
Genome-wide association studies (GWAS) can identify single nucleotide polymorphisms (SNPs) contributing to specific phenotypes, revealing potential neurobiological processes associated with these significant SNPs8. Several GWAS studies have been conducted to understand the genetic basis of CPS5,6,7,9,10,11,12. One such study utilized multiple cognitive tasks to provide a comprehensive assessment of processing speed7. However, due to limited sample sizes (ranging from n = 1311–32,070), these studies might lack the statistical power to detect significant associations in GWAS analyses. Other studies chose to use the reaction time of a single task (e.g., the ‘snap’ game) to measure the processing speed5,6,11,12. Despite their larger sample sizes (ranging from n = 282,217–432,297; details available in Supplementary Data 1), these studies failed to yield consistent results. This inconsistency indicates that using a single cognitive measure to assess CPS may not be adequate. Previous research lacks clear criteria for assessing CPA, and as a result, no studies have directly investigated the genetic basis of this cognitive function.
In this work, we propose an approach using Genomic Structural Equation Modeling (GenomicSEM13) based on GWAS data of a range of cognitive measures to obtain reliable and comprehensive descriptor of CPS and CPA. We carry out the genetic correlation and annotation analyses and find the underlying neurobiology, encompassing aspects such as brain structures, and the neurobiological basis of these two cognitive functions. In addition, we find the significant genetic correlation between these two factors and health-related phenotypes, as well as the significant causal relationships between CPS/CPA and psychiatric disorders using Mendelian randomization (MR) analysis14,15. Finally, a polygenic scores (PGS) analysis16 reveals the distinct contributions of those two factors on the cognitive development of young adolescents in the Adolescent Brain Cognitive Development (ABCD) study17,18.
Results
Genomic structural equation modeling revealed two latent factors corresponding to cognitive processing speed and accuracy
The primary GWAS summary statistics for cognitive measurements utilized in this study were originally presented in a prior study, which compiled a GWAS dataset featuring 2173 traits from 455,422 individuals in the UK Biobank19. From this extensive dataset, we specifically identified 14 cognition-related phenotypes from 7 tasks (See detailed information on those phenotypes in Supplementary Data 2). Utilizing linkage disequilibrium score regression (LDSC20), the genetic correlation analysis showed 73 significant correlations among all 91 pairs of traits (absolute values of the significant genetic correlations ranged from 0.18 to 0.99, p < 5.5e−4, Supplementary Fig. 1, Supplementary Data 3), suggesting high intercorrelation among those cognitive phenotypes.
Initially, exploratory factor analysis was performed to determine the number of latent factors from these traits. The analysis revealed that a two-factor model (58%) could explain more variation compared to a model with a common factor (49%). Models with factors beyond two failed to converge. The two factors were robustly loaded [abs(loading) > 0.4] on traits reflecting cognitive processing speed (CPS) and cognitive processing accuracy (CPA), respectively (Fig. 1a and Supplementary Data 4). It is clear that speed-related tasks were all loaded to the first factor while most of the accuracy-related tasks were clustered to the second factor. It is worth noting that fluid intelligence might contain both speed and accuracy components. Our analysis revealed that the fluid intelligence score loaded more heavily on CPA rather than CPS, possibly due to the way it was measured in UK Biobank, i.e., the 13 fluid intelligence tasks mainly assessed the accuracy.
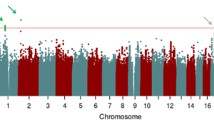
a The results of a confirmatory factor analysis with two latent factors from 14 cognitive traits. Arrows indicate the factor loading and the standard error was in the brackets. b Manhattan plot of the two factors, the color of the dot suggests the significance value of 2.5 e-8 (red, Bonferroni correction for two phenotypes).
Subsequently, a confirmatory factor analysis was employed to estimate SNP effects for the identified CPS and CPA factors (Fig. 1b). The SNP heritability was estimated as z = 26.64 for CPS and z = 25.35 for CPA using the LDSC method. We identified 118 and 55 leading SNPs for CPS and CPA, respectively, with p-values < 2.5e-8, window size <250 kb, and r2 < 0.1 (Supplementary Data 4–5). For the leading SNPs of CPS, 101 loci have been reported for certain traits such as cognitive ability (30 loci), intelligence (24), reaction time (20), and cognitive speed (2). Seventeen were novel based on the reference of the GWAS Catalog (Supplementary Data 5). Three of the Seventeen loci showed significant association (p < 5e-8) with at least one original trait. For the leading SNPs of CPA, 54 loci were reported for traits like cognitive ability (42 loci) and intelligence (39). Only one SNP (rs111959380) was new in the current analysis (Supplementary Data 6).
Genetic correlation between CPS/CPA and related cognitive measures in previous studies
Given the operational definition used here, the results of the above two factors may be associated with existing cognitive measures, such as common executive function (cEF11), general intelligence (g factor10,21,22), educational attainment23, cognitive and noncognitive skills24, and two principal components underlying multiple cognitive performance25. We compare the factors deciphered from the present analysis and those in the prior studies using genetic correlation. Strong correlations were observed between CPA and general intelligence10,21,22 (rg = 0.981–0.983), while moderate correlations were found between CPS and reaction time5 (rg = 0.55, se = 0.02). cEF11 exhibited a comparable genetic correlation with both CPA (rg = 0.86, se = 0.02) and CPS (rg = -0.85, se = 0.02), indicating it measures a mixture of speed and accuracy. Additionally, educational attainment23 displayed a moderate correlation with CPA (rg = 0.68, se = 0.02) and a weak correlation with CPS (rg = -0.26, se = 0.019). Noncognitive phenotypes24,25 showed weak correlations with both CPS and CPA (rg = -0.04 to 0.32; see Supplementary Data 7 for complete results of this analysis). Moreover, CPS and CPA showed a moderate correlation (rg = -0.69, se = 0.02). It is essential to highlight that CPS was measured in response time, and shorter response time meant higher cognitive processing speed, thus the two factors showed positive genetic correlation in terms of cognitive ability. These findings were consistent with the above loci results and suggested that the extracted CPS factor differed from other reported traits used in previous GWAS studies. The high correlation between the CPA component and the general cognitive factor might be because the general cognitive ability was a common factor extracted from the accuracy of various cognitive tasks10,21,22.
Cognitive processing speed and accuracy exhibited distinct associations with neuroimaging phenotypes
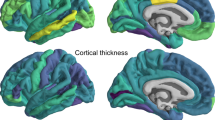
To explore potential brain structures associated with CPA and CPS, we calculated their genetic correlations with brain volume, cortical thickness, as well as fractional anisotropy (FA) and mean diffusivity (MD)-weighted white matter tract measurements (A total of 408 phenotypes26, Supplementary Data 8). Generally, CPA exhibited significant correlations with the brain volumes of anterior cingulate and insula areas (rg = 0.14 to 0.21, FDR corrected p < 0.05; Fig. 2a and Supplementary Data 8), and CPS showed more limited correlations with the brain volumes, primarily in the left lateral ventricle (Supplementary Fig. 2), left anterior occipital, and right medial orbital areas (rg = 0.11 to 0.27, FDR corrected p < 0.05; Fig. 2a and Supplementary Data 8). Only CPS had selected correlations with mean cortical thickness of bilateral superior transversal areas (rg = 0.12 and 0.13, FDR corrected p < 0.05; Fig. 2b, Supplementary Data 8).
a, b show the cortical volumes and cortical thickness that were significantly correlated with CPS/CPA in the Destrieux-a2009s parcellation74 (148 regions). c, d show the mean FA and MD in the ICBM-DTI white matter atlas75 (48 tracts) that were significantly correlated with CPS/CPA. The color indicates the standard z score of the genetic correlation (estimation/standard error). Non-significant tracts were grayed in the figure. CPS cognitive processing speed, CPA cognitive processing accuracy.
For white matter phenotypes, CPS demonstrated strong correlations with the FA in 11 tracts (rg = -0.15 to -0.11, FDR corrected p < 0.05; Fig. 2c, Supplementary Data 8), and the MD in 8 tracts (rg = 0.10 to 0.14, FDR corrected p < 0.05; Fig. 2d). Those tracts mainly included the bilateral anterior corona radiata, superior longitudinal fasciculus, superior frontal-occipital fasciculus, and corpus callosum. In contrast, none of the white matter tracts were significantly correlated with CPA. Moreover, genetic correlations of CPS with mean FA in bilateral corpus callosum, which is the major cross-hemisphere pathway, were significantly higher than those for CPA (z = 3.56 and 3.28, p < 0.0006; Supplementary Data 9). This may suggest that connections between the left and right hemispheres of the brain are particularly important for cognitive processing speed.
Genetic correlations between cognitive processing speed and accuracy and health-related traits
We tested the genetic correlations between the two factors and 40 traits related to mental health19,27,28,29,30,31,32,33,34,35,36, encompassing socioeconomic status (2), risk behaviors (15), psychiatric disorders (10), personality (5), and sleep (9). Among these, 11 traits exhibited significant genetic correlations with CPS (rg = -0.55 to 0.09, Bonferroni corrected p < 0.05), while 27 traits showed significance for CPA (rg = -0.38 to 0.60, corrected p < 0.05). Notably, part of these correlations (18 traits) differed significantly between the two factors (z = -9.71 to 8.39, corrected p < 0.05; Fig. 3 and Supplementary Data 10). Because a shorter response time means higher cognitive processing speed, the genetic correlation with CPS was negated when compared with those of CPS in Fig. 3, and the original values for these analyses can be found in Supplementary Data 10. For the majority of these traits, the absolute values of genetic correlation were higher with CPA than CPS, except for some, such as playing computer games (r = 0.55 vs. 0.30, z = 6.48, p < 5e−8) and bipolar disorders (r = -0.25 vs. -0.13, z = 3.54, p < 5e-4). These results underscored the significant associations between general cognitive functions and health-related phenotypes, revealing more prominent roles of CPA for various risk behaviors compared to CPS.
The traits were classed into five categories including risk behaviors (e.g. smoking, drinking, screen exposure, and driving, n = 32,614 to 455,838), personality (n = 20,669), socioeconomic status (SES, n = 392,422), psychiatric disorders (n = 5910 to 123,787), and sleep-related phenotypes (n = 57,215–455,848). See Supplementary Data 10 for the details of the sample size used in these analyses. The forest plot indicates the estimation (dot) and standard error (line) of the genetic correlations between each trait and the cognitive processing speed (CPS, green) or accuracy (CPA, blue). The green or blue sign (*) indicates a significant genetic correlation compared to zero, while the red sign indicates a significant difference in the genetic correlations between the two factors. The error band indicates the estimation ± 95%CI of the genetic correlation. Z-test was utilized to obtain the two-sided p-values. Significant results were defined as Bonferroni corrected p < 0.05. See Supplementary Data 10 for the exact p-values.
Mendelian randomization revealed a significant causal relationship between the two factors and psychiatric disorders
We investigated the causal relationship between the two latent factors and nine psychiatric disorders through a two-directional two-sample Mendelian randomization (MR14,15) analysis (Supplementary Data 11–12). In the forward MR analysis, we observed a significant causal influence of CPS on schizophrenia (b = 2.31, se = 0.32, p < 10-12), along with a significant causal influence of CPA on schizophrenia (b = -2.07, se = 0.55, p < 0.0002). In the reverse MR analysis, a significant causal influence of major depressive disorder (MDD) on CPS (b = 0.005, se = 0.0009, p < 10-7) was found, as well as schizophrenia exerting a causal influence on both CPS (b = 0.027, se = 0.003, p < 10-24) and CPA (b = -0.018, se = 0.003, p < 10-7). Sensitive analyses validated the above causal results including other four MR methods (Fig. 4), leave-one-out analyses (Supplementary Fig. 3), and assessment for pleiotropy (Supplementary Data 12).
The forest plot showed significant causalities in the solid square with different estimation methods. Effective sample size was 92983 and 57604 for MDD and Schizophrenia, respectively. CPS cognitive processing speed, CPA cognitive processing accuracy. The error band indicates ± 95% CI of the causal effect in the Mendelian randomization analyses. Significant results were defined as Bonferroni corrected p < 0.05. See Supplementary Data 12 for the exact p-values.
Annotation analysis revealed distinct neurobiological substrates underlying cognitive processing speed and accuracy
We performed annotation analyses on the GWAS summary statistics of CPS and CPA using the MAGMA software37 in the FUMA platform38. Notably, 513 and 445 significant genes were identified for CPS and CPA, respectively (Supplementary Data 13-14, Bonferroni-corrected p < 0.05). Enrichment analysis revealed one significant Gene Ontology (GO) term39 for CPS, namely, postsynaptic membrane (b = 0.30, se = 0.035, Bonferroni-corrected p < 0.05, Fig. 5a, Supplementary Data 15). Additionally, two significant terms were identified for CPA, namely, the generation of neurons and neurogenesis (b = 0.032 and 0.031, se = 0.028 and 0.026, Bonferroni-corrected p < 0.05, Fig. 5a, Supplementary Data 16).
a The top 5 results of the MAGMA gene-set analysis for the two factors separately (full results were shown in Supplementary Data 15–16). b The top 20 results of GTEx tissue-specific enrichment analysis (full results were shown in Supplementary Data 17). c BrainSpan general development stage enrichment analysis. Significant results are shown in brown. d Results of the cell-type-specific annotation analysis and different colors indicate different datasets. The dashed line in all the panels indicates the significance threshold after the Bonferroni corrected p < 0.05. CPS cognitive processing speed; CPA cognitive processing accuracy; LGN inh LAMP5 inhibitory lysosome-associated membrane protein 5 in the lateral geniculate nucleus; GABA Gamma-aminobutyric acid; Ex excitatory.
We further tested how the significant genes were expressed in GTEx 54 general tissues40 for CPS and CPA. Similar tissues were enriched in both factors, particularly brain-related tissues and the pituitary as expected (Fig. 5b and Supplementary Data 17). Moreover, we found the mRNA expression of CPS-related genes was significantly higher during late infancy stages, a key developmental stage for white matter myelination that may support the processing speed (b = 0.08, se = 0.026, p < 0.0006, Fig. 5c, Supplementary Data 18), while no significant results were observed for the mRNA expression of CPA-related genes across developmental periods.
In the cell-type-specific analysis40, both factors showed significant enrichment in Gamma-aminobutyric acid (GABA)-related cell types in the fetal prefrontal and midbrain, as well as in hybrid neurons in the human cortex. Furthermore, the CPS-related genes exhibited significant enrichment in inhibitory lysosome-associated membrane protein 5 (LAMP5) in the lateral geniculate nucleus (LGN) and GABA in the hippocampus. In contrast, the CPA-related genes showed significant enrichment in GABAergic cells in the prefrontal cortex at 16 gestational weeks (GW16) and in excitatory neurons in the psychENCODE developmental dataset (Fig. 5d and Supplementary Data 20). These findings suggested that the two components had both common and distinct elements at the cellular level.
The polygenic score analysis revealed the genetic variation of cognitive processing speed and accuracy was significantly associated with cognitive development in young adolescents
Here, we investigated whether the two cognitive factors can elucidate individual variations in cognitive abilities in children and early adolescents, using polygenic score (PGS) analyses on 8–10 year-old adolescents from the ABCD study. PGS scores for both factors demonstrated a significant correlation with all 7 cognitive abilities and 2 composite scores (Bonferroni-corrected p < 0.05, Supplementary Data 20). Considering the high correlation between CPA and CPS, we further included the PGS scores of both factors in the same regression model to explore their specific effects on children’s cognitive scores. The results showed that the PGS score of CPS was significantly associated with flanker, list sorting working memory, dimensional change card sort, pattern comparison processing speed, and the picture sequence memory task. In contrast, the PGS score of CPA was significantly associated with picture vocabulary, list sorting working memory, picture sequence memory, and oral reading recognition (Fig. 6 and Supplementary Data 20). For the composite scores, we revealed that PGS for CPS was significantly associated with the fluid intelligence composite (b = -0.114, se = 0.017, p < 10-10) but not the crystallized intelligence composite (b = -0.016, se = 0.017, p > 0.3). Conversely, the PGS for CPA was strongly associated with the crystallized intelligence composite (b = 0.229, se = 0.017, p < 10-39) and showed a weaker association with the fluid intelligence composite (b = 0.081, se = 0.017, p < 10-5, Fig. 6 and Supplementary Data 20). These findings demonstrated the separation of the two gene scores in relation to fluid and crystallized intelligence tasks and highlighted their substantial contributions to the development of diverse cognitive abilities in children.
The forest plots showed the estimation of the effects of PGS scores on all 7 cognitive abilities and 2 composite cognitive scores in the ABCD datasets (n = 4968). The x-axis indicates the estimation of the regression coefficient in the linear mixed-effects model and the error band indicates the estimation ± 95%CI.
Discussion
Using genomic SEM, we segregated two latent factors, namely, cognitive processing speed and accuracy that deciphered independent dimensions of general cognitive function. Notably, the CPS factor exhibited distinctions from previously identified cognitive phenotypes, while the CPA factor demonstrated similarity to general cognitive ability. Genetic correlations based on neuroimage phenotypes revealed that microstructural variations in white matter tracts underpinned CPS but not CPA. Health-related traits, including screen exposure, drinking, smoking, and sleep status, exhibited differential genetic correlations with CPS and CPA. We further demonstrated a significant causal relationship between these cognitive factors and psychiatric conditions (e.g., schizophrenia), implying a crucial covariation between cognitive ability and mental health. In addition, enrichment analysis highlighted diverse neurobiological annotations for these two factors, suggesting unique neural mechanisms underlying them. Lastly, the PGS score of the two cognitive factors could significantly explain the individual variations in multiple cognitive abilities in young adolescents, unveiling the important contributions of the two factors to normal cognitive development.
Before delving into our results, it’s crucial to examine the relationship between the two latent factors and prior studies to avoid confusion in cognitive terminology. For CPS, our review identified two types of similar measurements in previous studies: one focused on general processing speeds from multiple tasks7,9 and the other on reaction time tasks alone5,6,11,12. The former, with a limited sample size (n = 1311–32,070), identified a single effective SNP (rs17518584), which did not survive the threshold in our study (p = 4.73 e-5). The latter, despite large sample sizes (n = 282,217–432,297), showed inconsistent results across studies. Utilizing a GWAS summary with the most significant SNPs from these studies, we found a moderate genetic correlation between reaction time with the latent factor of CPS (rg = 0.55), signifying a different genetic basis between the CPS and simple reaction time. Concerning CPA, our analysis may not yield new elements compared to general cognitive ability but provided an independent measure in contrast to CPS. Notably, our current study employed different cognitive tasks compared to previous studies10,22,24,25, yet the results exhibited a high level of correlation, supporting the reliability of the extracted components using genomic structural equation modeling.
We performed a comprehensive analysis that revealed distinct neuroimaging and genetic features underlying the two factors. A notable difference is that the CPS but not the CPA exhibits a significant correlation with white matter fibers. This finding aligns with prior imaging-behavior studies that demonstrated a significant link between fluid intelligence and white matter microstructure41,42,43. Given the pivotal role of these white matter fibers as information highways in the brain, higher integrity or myelination (higher FA) of fiber tracts might enhance information processing speed44. Annotation analyses further revealed significant enrichment during late infancy, a critical period for white matter myelination development45. The significantly associated fibers mainly included the superior longitudinal fasciculus, superior frontal-occipital fasciculus, corona radiata, and corpus callosum. The dorsal association fibers such as the superior frontal-occipital fasciculus connect the occipital and frontal lobes, serving as an important bridge between visual processing and executive functions, thus could be an important basis for the cognitive processing speed in vision-based tasks46,47. The corpus callosum is the largest interhemispheric commissure and an important pathway for information processing between the two hemispheres. Previous studies have also reported associations between the myelination of the corpus callosum and processing speed in healthy adults48. As for the cortical cortex, CPS-related regions were located in the anterior and dorsal occipital lobes, which may reflect that CPS is more dependent on basic sensory abilities. While the CPA-related regions were located in the insula and the anterior cingulate gyrus, the insula has been linked to various cognitive functions, such as executive functions, and the anterior cingulate gyrus is involved in error monitoring, both of which are crucial for accurately completing tasks49,50.
Previous studies have reported a notable genetic correlation between the g factor and various traits, including vascular-metabolic and neuropsychiatric aspects5. Extending these findings, our study identified a significant correlation between CPA/CPS and several health-related behaviors, such as screen exposure and sleep status. For most health-related traits, the correlations were negative, meaning higher cognitive abilities were associated with healthier lifestyles or states (lower alcohol and smoking frequency or lower risk of psychiatric disorders). There were some exceptions such as computer use, playing computer gaming, and ASD. The positive link between computer use and intelligence may be because most jobs today, especially mental jobs, rely on computers. The high correlation between computer games and CPS may be because video games benefit cognitive processing speed51,52, but more causal evidence is needed to verify this point. The positive correlation between ASD and cognitive ability was also reported in the previous study20,53, which we thought was an interesting result, but the mechanism is not well studied yet. Future studies may be able to delve deeper into the genetic pleiotropy between neurological disease and cognitive ability.
Furthermore, we established a significant causal relationship between these cognitive factors and psychiatric disorders. In particular, we found a bidirectional causal interaction between schizophrenia and both CPA/CPS. Deficits in multiple cognitive domains, especially cognitive processing speed, are a core clinical feature of schizophrenia54,55. Our finding also supported the hypothesis of schizophrenia for general cognitive impairment56. In addition, other Mendelian randomization analyses have reported causal associations between white matter microstructure and schizophrenia57. Therefore, one may speculate a complex interaction between schizophrenia, brain white matter, and cognitive processing speed.
Several limitations should be noted in interpreting our results. First, the cognition-related traits used in the present study were limited and lacked the phenotypes for sensorimotor or language abilities. The inclusion of more comprehensive cognitive tasks may lead to more representative estimates of general cognitive abilities at the time of factor extraction. Second, in the current study, our primary focus was on structural aspects (gray matter and white matter) rather than functional components. Nevertheless, we appreciate the importance of extending this analysis to include brain functionality measures in future studies. Third, we only estimated the genetic correlation but not the causal relationship between general cognitive ability and other health-related traits, which was due to the sample overlap between the datasets. Therefore, whether cognitive ability leads to a healthy lifestyle (or vice versa) still needs further evidence.
In summary, through the genomic SEM, we found that two latent cognitive factors underlined multiple cognitive phenotypes, namely cognitive processing speed and accuracy. Those two factors showed distinct neuroimaging signatures, genetic architecture, and associations with health-related traits. The findings not only delineate the diverse neurobiology associated with general cognitive abilities but also shed light on their connections with brain health and a broad spectrum of cognitive development.
Methods
GWAS datasets
We utilized GWAS summary statistics on cognitive abilities obtained from a previous investigation19, which employed fastGWA to analyze 2173 traits across 456,422 individuals in the UK Biobank. This dataset provided 14 cognition-related traits with sample sizes ranging from 24,713 – 455,496, detailed in Supplementary Data 2. These traits predominantly originated from seven cognitive tasks, including fluid intelligence/reasoning, numeric memory, pairs matching, prospective memory, reaction time, symbol digit substitution, and trial-making tests. The fluid intelligence score we used was derived from the UK Biobank, where it was calculated based on the unweighted sum from 13 other tasks (https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=100027).
GenomicSEM analyses
We employed the GenomicSEM13 package in R (version 4.2.2) to perform factor analyses on the 14 cognitive traits. First, we preprocessed the 14 GWAS summary statistic files using the munge function. Specifically, we retained all HapMap3 SNPs with allele frequency > 0.01, information scores > 0.9, and those outside the major histocompatibility complex (MHC) regions. This step ensured that our analyses were not confounded by the complex genetic structures in the MHC regions. Subsequently, we applied the Linkage Disequilibrium Score Regression (LDSC20) method to calculate a genetic covariance matrix (S) and a sampling covariance matrix (V). An exploratory factor analysis with two factors and Promax rotation was performed on the S matrix to examine the separation between the two factors. These two factors, namely, cognitive processing speed (CPS) and cognitive processing accuracy (CPA), effectively distinguished components related to speed and accuracy, accounting for 58% of the genetic variation (Supplementary Data 3). In a follow-up step, a confirmatory factor analysis (CFA) with the two factors demonstrated a good model fit with χ2(69) = 1125, Akaike Information Criteria (AIC) = 1197, Comparative Fit Index (CFI) = 0.9992, and Standard Root Mean Square Residual (SRMR) = 0.0736. Finally, we standardized the 14 cognition-related GWAS summary data using the sumstats function and used the userGWAS function to estimate the significant SNPs for CPA/CPS factors. We estimated the QSNP heterogeneity statistic for the two factors and removed the SNPs showing significant effect (p < 5e−8), resulting in 3750 SNPs removed in the CPA factor and 404 SNPs removed in the CPS factor.
We found that in the data-driven model mentioned above, the CPS factor loaded on some measurements related to accuracy. Therefore, we further compared the data-driven EFA results with another CFA model purely based on hypothesis, where the F1 factor only included items measuring reaction time, and the F2 factor included items measuring accuracy. This model showed a lower goodness-of-fit compared to the data-driven results (Supplementary Fig. 4), which might be because although some cognitive measures were labeled as “accuracy” or “speed” by name, they may reflect cognitive ability from another domain. For instance, “RT_acc” which was defined as “mean time to correctly identify matches”, inherently reflects cognitive processing speed. Therefore, we chose to use the data-driven framework for the following analysis in our study. We also conducted genetic correlation analyses between the factors obtained from the two models and the cognitive components reported in previous literature (Supplementary Data 7) and found that the genetic correlations were similar between the two models.
Genetic correlation
We estimated the genetic correlation in the context of the factor model between the two factors (i.e. CPS and CPA) and various traits, including previously reported cognitive phenotypes (n = 9; reaction time5; common executive function11; two general intelligence10,22; educational attainment10; cognitive and noncognitive skills24; cognitive and noncognitive performance25), brain structure (n = 40826), socioeconomic status (n = 2, household income and social deprivation19), risk behaviors (n = 1519), psychiatric disorders (n = 1028,29,30,31,32,33,34,35,36,58), personality (n = 527), and sleep (n = 919). False Discovery Rate (FDR) correction was applied in brain structure considering the small effect size and large number of the phenotypes while Bonferroni correction was applied on other phenotypes. For a comprehensive understanding of these traits, a detailed description was provided in Supplementary Data 7 and 10. For the brain structure phenotypes, we used the cortical brain volume, cortical thickness of 148 cortical regions, and the mean fractional anisotropy (FA), and diffusivity (MD) value along the 48 white matter tracts. Only phenotypes with significant heritability were included in our analysis. A looser criterion was used to include all potential brain phenotypes, and all 408 brain phenotypes survived this threshold (FDR-corrected p < 0.05).
The comparison of genetic correlation between CPS and CPA with other traits was computed using the standard z-test formula:
Where b is the estimate of the genetic correlation, and se is the standard error of the estimate. The comparison of effect sizes between the two factors in the subsequent analyses followed a similar formula.
Annotation analyses
All analyses in this section were conducted using the FUMA platform38, and Bonferroni corrections were applied to account for multiple tests in all subsequent analyses.
Leading SNPs
The leading SNPs for CPS/CPA were chosen based on a significance threshold of p-value < 2.5e−8, a window size <250 kb, and two criteria for defining leading SNPs: a linkage disequilibrium (r2) threshold of 0.6 and a lead SNP definition threshold of 0.1. The reference panel used was 1000 G Phase 3 European.
SNP-to-gene mapping
This analysis was conducted using the default parameters in the FUMA processes, without the application of any optional SNP filtering.
Gene-set analysis
Gene-set analysis was conducted using MAGMA, covering a total of 10,526 gene sets categorized into three classes of Gene Ontology (GO39) terms—biological processes, molecular function, and cellular components. The gene sets were obtained from MsigDB (v7.059,60).
MAGMA expression analysis
We utilized MAGMA37 to investigate whether genes associated with the two factors (i.e. CPS and CPA) exhibited selective expression in the BrainSpan gene expression data61 and GTEx tissue data61. The BrainSpan data covered 11 developmental periods spanning from early prenatal to middle adulthood and the GTEx tissue data encompassed 54 different tissue types.
Cell type analysis
We utilized the cell type function40 within the FUMA platform to assess whether genes associated with CPS /CPA demonstrated specificity in certain cell types based on human brain expression datasets.
Two-sample Mendelian randomization analysis
In the two-sample MR analyses14,15, instrumental SNPs for exposures were selected using the clump function in PLINK software (v1.9). Genomic data from the European superpopulation in the 1000 Genomes Project62 served as the linkage disequilibrium (LD) reference, with a restriction to bi-allelic SNPs and a minor allele frequency > 0.1. LD pruning parameters were set at r2 = 0.001, window size = 10,000 kilobase pairs, and a p-value threshold of 5e-8 for exposures. Subsequently, SNPs significantly associated with outcomes or three potential cofounders (alcohol intake frequency, smoking frequency, and household income, p < 5e-8) were removed. Data harmonization was achieved using the harmonise_data function in the TwoSampleMR v0.5.6 R package, ensuring consistent allele usage for genetic variant association estimation. The primary analysis method employed was the Inverse Variance-Weighted (IVW63) regression with multiplicative random effects. For the significant results with the IVW method, we conducted four other methods to assess the robustness of the results including weighted median method, weighted mode, MR-Egger, and MR-PRESSO64. The Wald ratio method65 was used when only one instrument was available. MR-Egger regression66 and MR-PRESSO test were applied to evaluate potential directional pleiotropy bias, and a leave-one-out analysis was performed to check for the influence of individual SNPs on the causal association.
To prevent sample overlap between the two factors and the GWAS summary for psychiatric disorders, only the dataset without the UK Biobank sample was considered. The analysis included nine psychiatric disorders: attention deficit hyperactivity disorder (ADHD29), anxiety disorder67, autism spectrum disorder (ASD30), bipolar disorder (BD34), major depressive disorder (MDD32), obsessive-compulsive disorder (OCD31), panic disorder58, schizophrenia36, and Tourette syndrome (TS35). For subsequent analyses, the two factors were initially treated as exposures (forward MR) and then as outcomes (inverse MR). Bonferroni corrections were applied for the multiple tests.
ABCD datasets
Genotype data
The polygenic scores (PGS) analysis16 of CPS and CPA defined based on the primary results were applied to the genetic data from the Adolescent Brain Cognitive Development (ABCD) study (v418,68). The ABCD data used in this study included genetic data69 and cognitive scores17 collected at the baseline, from 11,875 children aged 9 and 10 from 21 research sites across the United States between October 2016 and October 2018. All study procedures received approval from institutional review boards at individual sites, and written consent was obtained from parents, with verbal assent from the children70.
We referred to the previous methods in the related paper71,72 to estimate population stratification and combined the pre-imputed genotype data with the 1000 Genome Phase 3 data (https://www.internationalgenome.org/). We used the first five principal components to identify population clusters using UMAP, resulting in 7 broad populations including Africans, Americans, Bengali, Finnish Europeans, Non-Finnish Europeans, East Asians, and South Asians. For the PGS analyses, only non-Finnish Europeans (n = 6161) were included. Post-imputation quality control measures were implemented, eliminating SNPs with low minor allele frequencies (MAF < 0.01), poor imputation (r2 < 0.3), high missingness (>0.05), and those failing the Hardy-Weinberg disequilibrium test (p < 10-6). Finally, 11,540,083 autosomal SNPs were retained for the subsequent analysis.
Cognitive variables
Cognitive ability was evaluated using nine age-corrected standard scores obtained from the NIH Toolbox17,73. These scores encompassed picture vocabulary, flanker, pattern comparison processing speed, picture sequence memory, oral reading recognition, list sorting working memory, dimensional change card sort, fluid intelligence composite, and crystallized intelligence composite.
We excluded preterm-born infants with gestational age <37 weeks and randomly excluded one participant from each pair with a closed gene relationship (pi-hat > 0.1875) estimated with individual genotype data using PLINK (v1.9). We also excluded individuals with incomplete data (e.g. cognitive scores and demographic data). Finally, 3843 subjects (mean age: 9.92 ± 0.61 years, 2039 males) remained in the following PGS analysis.
PGS analysis
PRScs (https://github.com/getian107/PRScs)16 was used for the PGS analysis. The European data from the 1000 Genomes Project Phase 3 were used as the LD reference, with all other parameters set to default. After obtaining the PGS for CPS/CPA, we conducted a regression analysis of the PGS scores and the cognitive scores of adolescents. We used a linear mixed-effects model, with age, sex, and PGS scores as fixed effects, and the data collection sites as random effects to predict each cognitive score. Given the high correlation between CPS and CPA, we included both PGS scores simultaneously in the regression analysis to obtain the specific effects of CPS/CPA. To address multiple tests, Bonferroni corrections were applied.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The GWAS summary statistics are available following the corresponding reference, the main data comes from the Psychiatric Genomics Consortium (https://pgc.unc.edu/for-researchers/download-results/), Oxford Brain Imaging Genetics Server (https://open.win.ox.ac.uk/ukbiobank/big40/), GWAS Catalog (https://www.ebi.ac.uk/gwas/home), and fastGWA summary statistics for UKB imputed data (https://yanglab.westlake.edu.cn/data/ukb_fastgwa/imp/). The ABCD data (https://nda.nih.gov/abcd) are not openly available due to data privacy laws, but access can be obtained upon application at NDA website (https://nda.nih.gov/abcd/request-access). The genetic data of the 1000 Genomes project is available online (https://www.internationalgenome.org/). The GWAS results of the CPS and CPA generated in this study have been deposited in the GWAS Catalog (CPS: GCST90446168: https://www.ebi.ac.uk/gwas/studies/GCST90446168 and CPA: GCST90446169: https://www.ebi.ac.uk/gwas/studies/GCST90446169), noted that the effective sample size for the factors reported in the data was calculated following the method on the GenomicSEM website (https://github.com/GenomicSEM/GenomicSEM/wiki/5.-Multivariate-GWAS).
Code availability
Code used to create the two factors is available at https://github.com/zjuwulab/GenomicSEM-CognitiveProcessing. Other softwares or packages used in this study include MATLAB (v2018a), R (v4.2.2), GenomicSEM (https://github.com/GenomicSEM/GenomicSEM), TwoSampleMR (v0.1.2), Plink (v1.90b7), and FUMA.
References
Baker, L. A., Vernon, P. A. & Ho, H. Z. The genetic correlation between intelligence and speed of information processing. Behav. Genet. 21, 351–367 (1991).
Vernon, P. A. Speed of information processing and general intelligence. Intelligence 7, 53–70 (1983).
Heitz, R. P. & Schall, J. D. Neural mechanisms of speed-accuracy tradeoff. Neuron 76, 616–628 (2012).
Bogacz, R., Wagenmakers, E. J., Forstmann, B. U. & Nieuwenhuis, S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 33, 10–16 (2010).
Davies, G. et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 9, 1–16 (2018).
Davies, G. et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112 151). Mol. Psychiatry 21, 758–767 (2016).
Ibrahim-Verbaas, C. A. et al. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol. Psychiatry 21, 189–197 (2016).
Uffelmann, E. et al. Genome-wide association studies. Nat. Rev. Methods Prim. 1, 59 (2021).
Luciano, M. et al. Whole genome association scan for genetic polymorphisms influencing information processing speed. Biol. Psychol. 86, 193–202 (2011).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Hatoum, A. S. et al. Genome-wide association study shows that executive functioning is influenced by GABAergic processes and is a neurocognitive genetic correlate of psychiatric disorders. Biol. Psychiatry 93, 59–70 (2023).
Schoeler, T. et al. Participation bias in the UK biobank distorts genetic associations and downstream analyses. Nat. Hum. Behav. 7, 1216–1227 (2023).
Grotzinger, A. D. et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat. Hum. Behav. 3, 513–525 (2019).
Burgess, S. et al. Guidelines for performing mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 4, 186 (2023).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Prim. 2, 6 (2022).
Ge, T., Chen, C. Y., Ni, Y., Feng, Y. C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1–10 (2019).
Luciana, M. et al. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci. 32, 67–79 (2018).
Karcher, N. R. & Barch, D. M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology 46, 131–142 (2020).
Jiang, L. et al. A resource-efficient tool for mixed model association analysis of large-scale data. Nat. Genet. 51, 1749–1755 (2019).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
de la Fuente, J., Davies, G., Grotzinger, A. D., Tucker-Drob, E. M. & Deary, I. J. A general dimension of genetic sharing across diverse cognitive traits inferred from molecular data. Nat. Hum. Behav. 5, 49–58 (2021).
Savage, J. E. et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 50, 912–919 (2018).
Lee, P. H. et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482.e11 (2019).
Demange, P. A. et al. Investigating the genetic architecture of noncognitive skills using GWAS-by-subtraction. Nat. Genet. 53, 35–44 (2021).
Lam, M. et al. Collective genomic segments with differential pleiotropic patterns between cognitive dimensions and psychopathology. Nat. Commun. 13, 6868 (2022).
Smith, S. M. et al. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat. Neurosci. 24, 737–745 (2021).
De Moor, M. H. M. et al. Meta-analysis of genome-wide association studies for personality. Mol. Psychiatry 17, 337–349 (2012).
Watson, H. J. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214 (2019).
Demontis, D. et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 55, 198–208 (2023).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Arnold, P. D. et al. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181–1188 (2018).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018).
Nievergelt, C. M. et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 10, 1–16 (2019).
Mullins, N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021).
Yu, D. et al. Interrogating the genetic determinants of Tourette’s syndrome and other tiC disorders through genome-wide association studies. Am. J. Psychiatry 176, 217–227 (2019).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: Generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, 1–19 (2015).
Watanabe, K., Taskesen, E., Van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1–10 (2017).
Consortium, T. G. O. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000)
Watanabe, K., Umićević Mirkov, M., de Leeuw, C. A., van den Heuvel, M. P. & Posthuma, D. Genetic mapping of cell type specificity for complex traits. Nat. Commun. 10, 3222 (2019).
Rabin, J. S. et al. Global white matter diffusion characteristics predict longitudinal cognitive change independently of amyloid status in clinically normal older adults. Cereb. Cortex 29, 1251–1262 (2019).
Borghesani, P. R. et al. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 51, 1435–1444 (2013).
Jacobs, H. I. L. et al. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum. Brain Mapp. 34, 77–95 (2013).
Fields, R. D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370 (2008).
Monje, M. Myelin plasticity and nervous system function. Annu. Rev. Neurosci. 41, 61–76 (2018).
Wang, X. et al. Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct. Funct. 221, 2075–2092 (2016).
Meola, A., Comert, A., Yeh, F. C., Stefaneanu, L. & Fernandez-Miranda, J. C. The controversial existence of the human superior fronto-occipital fasciculus: connectome-based tractographic study with microdissection validation. Hum. Brain Mapp. 36, 4964–4971 (2015).
Chopra, S. et al. More highly myelinated white matter tracts are associated with faster processing speed in healthy adults. Neuroimage 171, 332–340 (2018).
Gasquoine, P. G. Contributions of the insula to cognition and emotion. Neuropsychol. Rev. 24, 77–87 (2014).
Apps, M. A. J., Rushworth, M. F. S. & Chang, S. W. C. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707 (2016).
Dye, M. W. G., Green, C. S. & Bavelier, D. Meta-analysis of action video game impact on perceptual, attentional, and cognitive skills. Curr. Dir. Psychol. Sci. 18, 321–326 (2009).
Bediou, B. et al. Meta-analysis of action video game impact on perceptual, attentional, and cognitive skills. Psychol. Bull. 144, 77–110 (2018).
Clarke, T. K. et al. Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol. Psychiatry 21, 419–425 (2016).
Kochunov, P. et al. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry 74, 958–966 (2017).
McCutcheon, R. A., Reis Marques, T. & Howes, O. D. Schizophrenia - an overview. JAMA Psychiatry 77, 201–210 (2020).
Schaefer, J., Giangrande, E., Weinberger, D. R. & Dickinson, D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr. Res. 150, 42–50 (2013).
Guo, J. et al. Mendelian randomization analyses support causal relationships between brain imaging-derived phenotypes and risk of psychiatric disorders. Nat. Neurosci. 25, 1519–1527 (2022).
Forstner, A. J. et al. Genome-wide association study of panic disorder reveals genetic overlap with neuroticism and depression. Mol. Psychiatry 26, 4179–4190 (2021).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Liberzon, A. et al. The molecular signatures database hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Lonsdale, J. et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Wald, A. The fitting of straight lines if both variables are subject to error. Ann. Math. Stat. 11, 284–300 (1940).
Bowden, J., Smith, G. D. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Duncan, L. et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am. J. Psychiatry 174, 850–858 (2017).
Jernigan, T. L. & Brown, S. A. Introduction. Dev. Cogn. Neurosci. 32, 1–3 (2018).
Uban, K. A. et al. Biospecimens and the ABCD study: rationale, methods of collection, measurement and early data. Dev. Cogn. Neurosci. 32, 97–106 (2018).
Clark, D. B. et al. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: the ABCD experience. Dev. Cogn. Neurosci. 32, 143–154 (2018).
Warrier, V. et al. Gene-environment correlations and causal effects of childhood maltreatment on physical and mental health: a genetically informed approach. Lancet Psychiatry 8, 373–386 (2020).
Li, M. et al. Causal relationships between screen use, reading, and brain development in early adolescents. Adv. Sci. 2307540, 1–11 (2023).
Barch, D. M. et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev. Cogn. Neurosci. 32, 55–66 (2018).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. NeuroImage automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15 (2010).
Mori, S. et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582 (2008).
Acknowledgements
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2021ZD0200202), the National Natural Science Foundation of China (81971606, 82122032), and the Science and Technology Department of Zhejiang Province (202006140, 2022C03057) to D.W., the Zhejiang Provincial Natural Science Foundation of China (LQ23C090008) and Project of Zhejiang Province Education Science Planning (2023SCG341) to X.D., the National Key Research and Development Program of China (2023YFE0210300) to M.L. Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Author information
Authors and Affiliations
Contributions
M.L., X.D., and D.W. conceptualized the study; M.L. and Y.C. designed the analytic approach; M.L. and X.D. analyzed the data and visualized the results; M.L. and D.W. wrote the manuscript; Z.C. and X.X. helped in the analyses; X.X., and Z.Z. helped in the writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Andrew Grotzinger and Zhiqiang Sha for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, M., Dang, X., Chen, Y. et al. Cognitive processing speed and accuracy are intrinsically different in genetic architecture and brain phenotypes. Nat Commun 15, 7786 (2024). https://doi.org/10.1038/s41467-024-52222-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52222-8
- Springer Nature Limited