Abstract
Traditionally, semen analysis has been viewed solely as a tool for assessing male fertility. However, emerging research suggests that abnormal semen parameters may serve as indicators of broader health issues beyond reproductive function. Studies have revealed significant associations between abnormal semen parameters and an increased risk of chronic diseases such as prostate cancer, diabetes, ischemic heart disease, and metabolic disorders. These findings challenge the conventional understanding and position semen analysis as a potential screening tool for overall male health. The correlation between abnormal semen parameters and conditions like erectile dysfunction further underscores the multifaceted implications of semen quality. This suggests that abnormal semen parameters may be a risk factor for poorer overall health and a higher likelihood of developing comorbidities over time. Given these compelling associations, there is a growing call to integrate semen analysis into routine health assessments for young men, particularly in conjunction with established general health screenings. This proactive approach aligns with a preventative healthcare paradigm, facilitating early detection of underlying health concerns and timely interventions. However, overcoming cultural, logistical, and cost-related barriers is crucial for the successful implementation of this shift in reproductive health.
Similar content being viewed by others
Introduction
For decades, the clinical utility of semen analysis has been limited to investigating suspected issues related to male fertility. Traditionally, its scope was limited to evaluating the quantity and quality of seminal fluid, offering insights into a man’s reproductive capabilities. However, contemporary research is beginning to broaden the implications of semen parameters to measures of male health beyond fertility assessment.
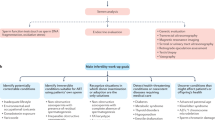
Recent studies have revealed significant correlations between abnormal semen parameters and various chronic diseases [1]. These findings suggest that semen analysis may provide valuable insights into overall well-being, transcending beyond its traditional role. Semen quality acts as a metaphorical “window” into the intricacies of male physiology, indicating a connection to the balance of physiological processes that influence not only reproductive function but also the development and progression of systemic diseases (Fig. 1).
Semen analysis, traditionally viewed as a tool for evaluating male fertility, is now being recognized for its potential to provide insights into overall male health. By interpreting subtle abnormalities in semen parameters, we may gain an early glimpse into underlying imbalances or pathologies that could manifest as chronic diseases or sexual and erectile dysfunction (ED) later in life. This evolving understanding opens a novel perspective: leveraging this routine test as a screening tool for the general well-being of young men, particularly in relation to sexual and ED. Semen analysis thus holds promise as a non-invasive yet informative assessment, poised to enhance the proactive management of male health concerns beyond just reproductive issues.
The link between abnormal semen parameters and sexual dysfunction
Supporting this viewpoint, a study by Lotti et al. found a significant correlation between abnormal semen parameters and ED among infertile men [2]. In their investigation, involving 448 infertile men with a mean age of 36.8 ± 7.9 years, the prevalence of ED demonstrated a clear increase alongside the severity of semen abnormalities, compared to healthy, fertile controls of similar age. ED prevalence increased as a function of semen quality impairment severity (p < 0.0001), even after adjusting for confounders such as age, chronic disease burden, and prostatitis-like symptoms. Interestingly, the adverse impact of abnormal semen parameters on erectile function appeared to stand independently of hormonal, metabolic, and vascular factors typically associated with ED pathogenesis. Azoospermic men exhibited the highest rates of ED, with impaired erectile function scores, as well as premature ejaculation compared to those with fewer semen irregularities and fertile controls. In these azoospermic men, erectile function was associated with both psychological disturbances and a less overall healthy phenotype (higher Chronic Disease Score (CDS); p = 0.015).
Despite comparable testosterone levels, glycemic profiles, and penile vascular status across the groups, the prevalence and severity of ED demonstrated an inverse correlation with semen quality. This observation suggests that the mechanisms underlying the relationship between semen abnormalities and ED may extend beyond conventional etiological pathways. However, it is important to note that this is a cross-sectional study and therefore causality cannot be inferred. There likely exists a multi-factorial process affecting underlying health including sexual health, and a semen analysis may serve as a useful objective screening tool. These findings align with the recent meta-analysis examining the relationship between sexual dysfunction and male infertility, which found a significantly higher prevalence of sexual dysfunction (OR = 2.66, 95% CI [1.69, 4.19], p < 0.0001) and lower International Index of Erectile Function scores in infertile men compared to fertile controls (SMD = −0.47, 95% CI [−0.63, −0.31], p < 0.0001) [3]. Given that ED is widely recognized as a significant risk factor for cardiovascular diseases in the general population, its occurrence in infertile men, particularly those with azoospermia, could prompt physicians to assess for potential underlying subclinical morbidities as well as sexual dysfunction. Therefore, the assessment of semen quality alongside ED may serve as an important indicator for identifying individuals who warrant further cardiovascular evaluation and intervention.
The link between abnormal semen parameters and other diseases
Recent studies have shown an association between abnormal semen parameters and an increased risk of cancer diagnosis. A large-scale cohort study involving over 22,000 men undergoing evaluation for infertility revealed a clear link – those with male factor infertility were twice as likely to be diagnosed with high-grade prostate cancer compared to the general population (SIR, 2.0; 95% CI, 1.2–3.0) [4]. This disparity persisted after accounting for potential confounding factors through multivariate analyses, with men afflicted by male factor infertility being more than 2.5 times more likely to develop high-grade prostate cancer (HR, 2.6; 95% CI, 1.4–4.8). Additionally, Walsh et al. found that men seeking infertility treatment had an increased risk of subsequently developing testicular cancer (SIR, 1.3; 95% CI, 0.9–1.9), with a markedly higher risk among those with known male factor infertility (SIR, 2.8; 95% CI, 1.5–4.8), suggesting the existence of common etiologic factors for infertility and testicular cancer [5].
Furthermore, abnormal semen analysis results may also correlate with metabolic disorders. A register-based study that followed multiple in-vitro fertilization (IVF) registration cycles identified 651 cases of diabetes. Adjusted hazard ratios for this condition among men with male factor infertility ranged from 1.08 to 1.45 when compared to the reference group [6]. Additionally, when assessing the effects of specific causes of male factor infertility, the risks were amplified – men with oligospermia, azoospermia, and aspermia exhibited adjusted hazard ratios of 1.44, 2.10, and 3.20, respectively, highlighting the intricate interplay between semen parameters and metabolic dysregulation.
Moreover, a study involving over 36,000 men revealed that those diagnosed with male factor infertility had a higher risk of developing not only diabetes (HR 1.30, 95% CI 1.10–1.53) but also ischemic heart disease (HR 1.48, 95% CI 1.19–1.84), alcohol abuse (HR 1.48, 95% CI 1.07–2.05), and drug abuse (HR 1.67, 95% CI 1.06–2.63) compared to their counterparts who received fertility testing alone -presumably not found to be infertile [7]. The association between male factor infertility and later health outcomes was further reinforced when comparing infertile men to fertile men, with the association being strongest for men with longer follow-up times. Specifically, the incidence of hyperlipidemia, renal disease, and chronic pulmonary disease was significantly higher among infertile men. However, the incidence of these conditions was lower among infertile men with shorter follow-up times (less than 3 years) compared to those with longer follow-up times (more than 3 years). This suggests that the increased incidence of these health issues after the diagnosis of infertility is not entirely due to pre-existing illnesses present before the diagnosis but conditions that may develop or worsen over time.
Setting aside specific diagnoses, there is also evidence that abnormal semen analysis results correlate with worse overall health status and mortality rates. In a study by Salonia et al., infertile men displayed significantly higher rates of comorbidities than fertile controls, as indicated by their elevated Charlson Comorbidity Index (CCI) scores (0.33 vs. 0.14, p < 0.001) where a higher score indicates the increased burden of comorbidity [8]. Even after adjusting for factors like age, BMI, and educational status, the CCI was significantly lower for fertile men compared to male factor infertility patients. This suggests that the association between male infertility and later health outcomes is not entirely due to pre-existing conditions, but rather, abnormal semen parameters may be an indicator or risk factor for overall poorer health and a higher likelihood of developing comorbidities over time.
Rationale for early screening and intervention
The recognition that abnormalities in semen analysis can indicate underlying health conditions is driving calls for early screening and intervention strategies utilizing this valuable diagnostic tool. Semen analysis is emerging as a crucial resource, providing unique insight into overall physiological well-being beyond just reproductive health. The concept of semen analysis serving as a “window” into broader physiological well-being underscores its potential as a proactive health measure. Notably, men undergoing fertility evaluations are often in an age group that may not regularly visit a primary care physician. Therefore, abnormal findings during semen analysis should prompt recommendations for comprehensive general health screening. In conjunction with established assessments such as blood pressure, lipid profiles, and HbA1c levels, integrating semen analysis into routine health evaluations holds promise for early detection of underlying concerns. This proactive approach not only addresses reproductive health but also serves as a sentinel for potential systemic conditions, facilitating timely interventions and fostering holistic health management.
Discussion
The findings presented underscore the emerging perspective of semen analysis as a potential screening tool for overall health in young men. Traditionally viewed solely through the lens of reproductive health assessment, semen analysis has taken on a broader significance, with abnormal semen parameters demonstrating compelling associations with various chronic diseases. There is a clear correlation between abnormal semen parameters and ED among infertile men, with the prevalence of ED increasing alongside the severity of semen abnormalities. Notably, this association appeared independent of hormonal, metabolic, and vascular factors typically implicated in ED pathogenesis. Furthermore, extensive evidence points to the link between abnormal semen parameters and an increased risk of prostate cancer, testicular cancer, diabetes, ischemic heart disease, and metabolic disorders. Infertile men, particularly those with severe forms of male factor infertility such as azoospermia, exhibited higher rates of comorbidities and overall poorer health status when compared to fertile controls.
These findings collectively suggest that semen analysis may serve as a valuable screening tool, providing insights into the overall well-being of young men beyond the realm of reproductive function. By recognizing and interpreting the subtle signs embedded within semen parameters, clinicians may gain an early glimpse into the underlying imbalances or pathologies that could manifest as chronic diseases later in life.
Despite the promising potential of semen analysis as a screening tool for overall health and its integration into routine health assessments, several potential barriers must be addressed. Cultural factors may influence individuals’ willingness to undergo fertility-related evaluations, and misconceptions or stigma surrounding infertility may hinder uptake [9]. Moreover, logistical challenges, such as access to specialized reproductive health services or the availability of trained personnel to perform semen analyses, may pose barriers, particularly in underserved or rural areas [10]. However, with the recent development of accurate at-home testing kits that can be mailed to expert analysis labs, certain barriers to access have improved significantly and men can now deposit samples in the comfort of their homes [11]. Additionally, the cost of semen analysis and associated diagnostic tests could present financial obstacles for individuals without adequate insurance coverage or financial resources [12].
While current evidence links abnormal semen parameters to chronic conditions, it does not establish causation. Further research is needed to understand the mechanisms and causal pathways between semen abnormalities and systemic health issues. Prospective longitudinal studies with multiple semen analysis points are essential, as existing evidence is mostly cross-sectional and retrospective. These studies could reveal how semen parameter changes predict chronic disease development or progression.
Identifying optimal age groups for routine semen analysis screening is crucial to maximize the impact on long-term health outcomes and allocate resources effectively. Interventional studies should explore lifestyle modifications, pharmacological interventions, and other therapies to improve semen parameters. If improvements correlate with better chronic disease outcomes, such as reduced diabetes, cardiovascular disease, or sexual dysfunction, semen analysis could become a valuable health marker.
Understanding the biological mechanisms linking semen abnormalities to chronic diseases is vital. Mechanistic studies could lead to targeted interventions and personalized treatments. Addressing these limitations and pursuing comprehensive research could integrate semen analysis into routine health assessments for young men, promoting preventative healthcare, early detection, and timely interventions for various health concerns.
Conclusion
Integrating semen analysis into routine health evaluations for young men marks a significant shift in reproductive health, highlighting connections between abnormal semen parameters and chronic diseases. This approach extends semen analysis beyond fertility assessment, offering insights into underlying physiological processes. Detecting abnormalities early could lead to prompt investigations and early detection of chronic conditions. Additionally, semen analysis can aid in assessing sexual dysfunction severity. However, to utilize semen analysis as a proactive health measure, challenges like cultural, logistical, and cost barriers must be addressed. More research is needed to define its screening role and identify the most beneficial age groups. Incorporating semen analysis into health evaluations could enhance young men’s overall well-being and longevity.
References
Kasman AM, Del Giudice F, Eisenberg ML. New insights to guide patient care: the bidirectional relationship between male infertility and male health. Fertil Steril. 2020;113:469–77. https://doi.org/10.1016/j.fertnstert.2020.01.002
Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol. 2018;15:287–307. https://doi.org/10.1038/nrurol.2018.20
Liu Y, Wang Y, Pu Z, Wang Y, Zhang Y, Dong C, et al. Sexual dysfunction in infertile men: A systematic review and meta-analysis. Sex Med. 2022;10:100528. https://doi.org/10.1016/j.esxm.2022.100528
Walsh TJ, Schembri M, Turek PJ, Chan JM, Carrol PR, Smith JF, et al. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010;116:2140–7. https://doi.org/10.1002/cncr.25075
Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6. https://doi.org/10.1001/archinternmed.2008.562
Glazer CH, Bonde JP, Giwercman A, Vassard D, Pinborg A, Schmidt L, et al. Risk of diabetes according to male factor infertility: a register-based cohort study. Hum Reprod. 2017;32:1474–81. https://doi.org/10.1093/humrep/dex097
Eisenberg ML, Li S, Cullen MR, Baker LC. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril. 2016;105:629–36. https://doi.org/10.1016/j.fertnstert.2015.11.011
Salonia A, Matloob R, Gallina A, Abdollah F, Saccà A, Briganti A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol. 2009;56:1025–32. https://doi.org/10.1016/j.eururo.2009.03.001
Kuug AK, James S, Sihaam JB. Exploring the cultural perspectives and implications of infertility among couples in the Talensi and Nabdam Districts of the upper east region of Ghana. Contracept Reprod Med. 2023;8:28. https://doi.org/10.1186/s40834-023-00225-z
Onofre J, Geenen L, Cox A, Van Der Auwera I, Willendrup F, Andersen E, et al. Simplified sperm testing devices: a possible tool to overcome lack of accessibility and inconsistency in male factor infertility diagnosis. An opportunity for low- and middle-income countries. Facts Views Vis ObGyn. 2021;13:79–93. https://doi.org/10.52054/FVVO.13.1.011
Samplaski MK, Falk O, Honig S, Shin D, Matthews W, Smith JF. Development and validation of a novel mail-in semen analysis system and the correlation between one hour and delayed semen analysis testing. Fertil Steril. 2021;115:922–9. https://doi.org/10.1016/j.fertnstert.2020.10.047
Larsen RG, Bowdino CS, Mathes MA, Gustin SL, Deibert CM. Minimal access to male fertility prices online: an analysis of the Society for Assisted Reproductive Technology (SART) clinics. Transl Androl Urol. 2020;9:2107–12. https://doi.org/10.21037/tau-20-944
Acknowledgements
We would like to express our deepest gratitude to the following individuals and organizations for their invaluable contributions to this manuscript. Dr. Mohit Khera at the Department of Urology, Baylor College of Medicine, and Dr. Nathan Starke at the Department of Urology, Houston Methodist Hospital for their insightful guidance and constructive feedback throughout the research process. We would also like to thank IJIR: Your Sexual Medicine Journal and its editorial team for considering our manuscript for publication and for their dedication to advancing research in the field of urology and sexual medicine.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
GS: Writing- Original draft preparation, LNT: Writing- Original draft preparation, NS: Writing- Reviewing and Editing, MK: Writing- Reviewing and Editing, AK: Conceptualization, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Ethical approval
Not necessary as this is an opinion-driven perspective paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saffati, G., Thompson, L.N., Starke, N. et al. Can semen analysis be utilized as a screening tool for overall health in young men?. Int J Impot Res (2024). https://doi.org/10.1038/s41443-024-00949-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-024-00949-9
- Springer Nature Limited