Abstract
Erectile dysfunction (ED) is a global health problem that commonly occurs due to multiple factors, particularly by a vascular abnormality with the activation of platelet (PLT). Mean platelet volume (MPV), a PLT activity marker, has been hypothesized to be associated with ED. The present meta-analysis aims to evaluate the MPV and its contribution to ED diagnosis. A systematic searching to summarize the association of MPV as a predictive marker for ED was conducted on two databases, including MEDLINE (PubMed) and CINAHL (EBSCOhost). We included all English studies that measured MPV levels in ED and non-ED subjects. A total of 168 publications were initially retrieved and screened systematically. 12 studies with 1643 subjects were included for both qualitative and quantitative analysis. The MPV mean difference between ED patients and healthy subjects; vasculogenic and non-vasculogenic ED showed significant differences. Our findings show PLT is associated with the development of ED. Higher MPV level was found in the ED subjects compared to the healthy controls. Nevertheless, the evidence is still limited due to the small number of studies and further investigations are required to support the utilization of MPV for ED diagnosis.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED) is defined as the lack of ability to initiate or sustain a penile erection adequate for sexual intercourse [1]. It is a mishap for both patients and their partners with significant influence on the quality of life globally [2]. The mechanism underlying ED is thought to be a vascular abnormality since the risk factors of ED such as aging, metabolic syndrome, vascular disease, smoking, and obesity [3,4,5]. Vasculogenic ED can result from several pathological factors occurring in blood vessels, including atherosclerosis [6], endothelial dysfunction, and inflammation [7]. These pathological factors disrupt the endothelial cells and lead to disturbance of vascular homeostasis [4, 5]. Platelet activation contributes to the development of atherothrombosis and the inflammatory vascular response [4, 5, 8].
Recently, several studies reported that men with vasculogenic ED have an increased value of MPV [9,10,11,12,13,14]. MPV is considered an indicator of PLT dimension that is automatically measured by blood counter devices [15]. MPV measurement has a relatively low cost and reflects PLT activity indirectly [16]. The high number of large platelet could associate with the development of atherothrombosis since the metabolic rate and enzymatic activity of large PLTs are higher than small PLTs. The production of thromboxane as the most potent vasoconstrictor substance by large PLT tends to be higher [17]. Moreover, the MPV was elevated in subjects with higher blood pressure, diabetes mellitus, hypercholesterolemia, obesity, and smoking [12,13,14]. These findings support the statement that high MPV may associate with the risk for cardiovascular problems, specifically vasculogenic ED. The increase in PLT activity contributes to the development of atherosclerosis [4, 5]. The process of atherogenesis involves the aggregation of thrombocytes, production of thromboxane, and synthesis of adhesion molecules [4, 17,18,19]. Increased PLT activity could contribute to the development of vasculogenic ED through a similar process of atherogenesis [9, 10].
Several studies have studied the association between vasculogenic ED and PLT activity; [9,10,11,12,13,14] however, the results are conflicting. For instance, while Ciftci et al. [13] stated that both PLT count and MPV increased in vasculogenic ED, Aldemir et al. [14] reported that PLT count was normal. Previously, a meta-analysis investigated the relationship between MPV and ED; however, the studies that were included were only up to 2018 [20]. The present study aims to broaden the previous meta-analysis to confirm the association of MPV and ED.
Methods
The target population included in this study is patients with ED and the intervention is the MPV blood test with healthy subjects as the control for the main comparison. Another comparison for analysis in this review are between vasculogenic and non-vasculogenic ED; severe ED and mild ED patients. The expected outcome was higher MPV levels in ED patients as it might be considered as a predictive marker of ED diagnosis. The methods for conducting and writing this review based on the guidance from the Preferred Reporting Items for Systematic Reviews and Meta-analyses [21] informed the methods for conducting and reporting this review.
Literature searching
The databases included in the search were: MEDLINE (PubMed) and CINAHL (EBSCOhost). The combination of subject heading index terms and the free text was used to expand the literature searching. The keywords used are presented in Table 1. Non-English language articles were excluded from this review. Additional studies were further explored from the reference lists of all qualified studies, systematic reviews, and meta-analyses. Searches were finished in September 2021.
Measurement of ED
The instruments involving ED measurement in the qualified studies including (1) the five-item International Index of Erectile Dysfunction (IIEF-5) questionnaire [22]; (2) the standard 15-item International Index of Erectile Function (IIEF-15) questionnaire [23]; (3) history taking and physical examination; and (4) penile Doppler ultrasound.
Study selection
Two reviewers (NR and MH) were independently involved in selecting titles and abstracts to select appropriate studies to be included or excluded. The studies were considered suitable using the eligibility criteria in Table 2.
Data extraction
Extraction of the relevant data was conducted independently by two authors (NR and MH) in compliance with the eligibility criteria. Extracted studies were noted in the collection form. Discussion and consensus finding was performed if a disagreement occurred. In the present meta-analysis, we gathered these variables for each literature: (1) the first author’s name, year of publication, region; (2) sample size of the study case and control groups; (3) data including the MPV.
Statistical analysis
Meta-analysis was conducted based on guidance from the Cochrane Collaboration [24]. The strength of the association between MPV and the ED risks was evaluated using weighted mean differences (MDs) and the 95% confidence interval (CI). Pooling eligible studies’ data was conducted using both fixed and random effect models. All numerical variables were reported as mean ± SD. The criteria for significant heterogeneity was p < 0.10. I2 values of 25, 50, and 75% were considered low, medium, and high levels of heterogeneity. The Z-test was conducted to evaluate the significance of the pooled results. A two-tailed xi value was statistically significant if it was <0.05. The statistical analysis was conducted with RevMan 5.2. Estimation of possible publication bias was performed by using Egger’s test and funnel plots. Sensitivity analysis was conducted to assess the stability of the results. Estimation of the pooled MDs was performed by excluding one study each time to assess the impact of individual studies.
Random effect model (method of Dersimonian and Laird) analysis was used when items had high levels of heterogeneity (with I2 > 75%); otherwise, the fixed-effects model was applied. In a random-effects model, there is an assumption of variation between studies, and thus the measured OR represents a more conservative value.
Results
Literature search
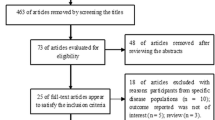
A total of 168 publications were initially retrieved (Fig. 1). Twenty studies were excluded for being duplicates. Of these, 136 were excluded during abstract screening, and 12 articles were considered for both qualitative and quantitative analysis. The risk of bias and applicability concern in each study was identified using the QUADAS-2 tool. The risk of bias and applicability concern graph and summary were showed in Figs. 2 and 3.
Study characteristics
A total of 12 studies were included in this study, with 1643 subjects. Nine studies were case-control studies, two were retrospective studies, and one was cross-sectional study. All included publications were published from 2004 to 2020. We divided the included studies into three sections: (1) ED patients vs. healthy subjects (2), vasculogenic ED vs. non-vasculogenic ED patients, and (3) mild vs. severe ED patients. Two studies belonged to two groups. The characteristics of the studies are presented in Tables 3–5.
Synthesis of results
We found that the MD of the MPV between ED patients and healthy subjects was 0.74 (95% CI 0.33–1.16). There was a statistically significant heterogeneity and overall effect among included studies ((I2 = 87%, p = 0.0004), and a random-effects model was used for the analysis (Fig. 4). The MD of MPV between vasculogenic ED and non-vasculogenic ED patients was 1.09 (95% CI: 0.69–1.50) (Fig. 5). There was also statistically significant heterogeneity and overall effect among included studies (I2 = 84%, p = < 0.00001), and a random-effects model was used for the analysis. The MD of the MPV between mild ED and severe ED patients was 0.57 (95% CI: 0.11–1.03) (Fig. 6). There was statistically significant heterogeneity, whereas the overall effect among included studies was statistically significant (I2 = 91%, p = 0.01).
Discussion
ED is a common problem amongst sexually active male individuals [25]. Despite neuro or psycho-genic disorders, vascular disorders are the most common underlying cause [26, 27]. The risk factors for ED, such as diabetes mellitus, vascular disease, smoking habits, high-level lipid profile, desk-bound lifestyle, overweight, and metabolic disorders, parallel to risk factors for coronary artery disease (CAD) [28,29,30]. This indicates that ED is associated with CAD development. Several shreds of evidence stated that ED could independently predict cardiovascular disease in the future [8, 13]. Previous studies suggested that ED can be the initial feature of CAD symptoms [31]. A study stated that 19% of subjects with erectile problems had silent CADs revealed by angiography. This finding suggests comprehensive cardiovascular examinations for subjects with vasculogenic ED [32]. Other study reported that ED is a fundamental finding for future CADs [33]. These indicate that ED is closely related to CVD progression. PLTs have been considered to have a significant role in vascular disorders such as atherosclerosis and ED [4, 5, 8].
As MPV is an essential representation of PLT function, activity, production, and stimulation rate, the association between ED, PLT, and MPV becomes essential [11]. Large platelets have a higher metabolic rate and enzymatic activity than small platelets. The production rate of Thromboxane A2 (TXA2) as the most potent vasoconstrictor substance by large PLTs is higher. Increased platelet activity contributes to atherosclerosis development through mechanisms including aggregation of thrombocytes, production of thromboxane, and synthesis of adhesion molecules [7, 8, 11, 16, 17]. Some studies report that raised MPV induces and accelerates atherogenesis, resulting in thrombosis, CAD, and myocardium infarction [34, 35]. These are recognized as the basis of atherogenesis in penile arterial deficiency [9, 11, 13]. In vasculogenic ED, the adherence of PLTs to the cavernosal walls was proposed. PLTs produce TXA2, which increases oxidative stress during erection [14]. TXA2 secreted more abundantly in a larger PLT. Thus, its function mirrored the MPV raise [13]. Several studies stated that a rise of MPV parallel with CAD risk factors, such as smoking habit, metabolic disorder, overweight, hypertension, and abnormal lipid profile [19].
The present meta-analysis found that ED subjects’ mean MPV was significantly higher than healthy subjects (p < 0.0004). We also found that the mean MPV of vasculogenic ED subjects was also higher than those with non-vasculogenic ED. Furthermore, there was a significant difference in MPV level between mild ED and severe ED (p < 0.00001), suggesting that MPV levels also could be used to differentiate ED severity. This result showed vital evidence of the association between MPV and ED development. Commonly used blood counters automatically measure this marker of platelet size. It can be found easily and has a low cost. In addition, it indirectly represents the platelet activity.
Limitations
Several limitations in this review were low to moderate quality and a low number of included studies in each analysis group. From the study design, we also included retrospective studies that were less appropriate for this review and analysis. The results from the analysis also showed high heterogeneity among studies included and making it difficult to generalize the conclusions. In addition, most of the articles did not exclude subjects with psychological factors, associated comorbidities, and past illness history that could cause a potential bias.
Conclusion
There is a possible association between a high level of MPV and the development of ED. We found that the difference in MPV levels between ED subjects and healthy controls showed significant results. The level of MPV between mild and severe ED was also found to be significantly different. However, due to the limitations, these findings cannot justify the use of MPV as a predictive marker for ED and differentiate between ED severity.
References
NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90.
Wagner G, Fugl-Meyer KS, Fugl-Meyer AR. Impact of erectile dysfunction on quality of life: patient and partner perspectives. Int J Impot Res. 2000;12:S144–6. https://doi.org/10.1038/sj.ijir.3900594.
Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–78. https://doi.org/10.1016/j.eururo.2013.08.023.
Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–19. https://doi.org/10.1161/CIRCRESAHA.113.300512.
Sopova K, Tatsidou P, Stellos K. Platelets and platelet interaction with progenitor cells in vascular homeostasis and inflammation. Curr Vasc Pharm. 2012;10:555–62. https://doi.org/10.2174/157016112801784486.
Ibrahim A, Ali M, Kiernan TJ, Stack AG. Erectile dysfunction and ischaemic heart disease. Eur Cardiol. 2018;13:98–103. https://doi.org/10.15420/ecr.2017.21.3.
Vlachopoulos C, Aznaouridis K, Ioakeimidis N, Rokkas K, Vasiliadou C, Alexopoulos N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J. 2006;27:2640–8. https://doi.org/10.1093/eurheartj/ehl341.
Yang SW, Cho SH, Kwon HS, Sohn IS, Hwang HS. Significance of the platelet distribution width as a severity marker for the development of preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2014;175:107–11. https://doi.org/10.1016/j.ejogrb.2013.12.036.
Otunctemur A, Bozkurt M, Beşiroğlu H, Polat EC, Ozcan L, Ozbek E. Erectile dysfunction is positively correlated with mean platelet volume and platelet count, but not with eosinophil count in peripheral blood. Urol J. 2015;12:2347–52.
Bayraktar Z, Albayrak S. Blood platelet activity in men with vasculogenic erectile dysfunction. Arch Ital di Urol e Androl. 2017;89:51–4. https://doi.org/10.4081/aiua.2017.1.51.
Sonmez MG, Goger YE, Sonmez LO, Aydin A, Balasar M, Kara C. Can eosinophil count, platelet count, and mean platelet volume be a positive predictive factor in penile arteriogenic erectile dysfunction etiopathogenesis? Am J Men’s Health. 2017;11:678–83.
La Vignera S, Condorelli RA, Burgio G, Vicari E, Favilla V, Russo GI, et al. Functional characterization of platelets in patients with arterial erectile dysfunction. Andrology. 2014;2:709–15.
Ciftci H, Gumuş K, Yagmur I, Sahabettin S, Çelik H, Yeni E, et al. Assessment of Mean Platelet Volume in men with vasculogenic and nonvasculogenic erectile dysfunction. Int J Impot Res. 2015;27:38–40. https://doi.org/10.1038/ijir.2014.17.
Aldemir M, Akdemir F, Okulu E, Ener K, Ozayar A, Gudeloglu A. Evaluation of blood platelet count and function in patients with erectile dysfunction. Andrologia. 2016;48:189–92. https://doi.org/10.1111/and.12430.
Korniluk A, Koper-Lenkiewicz OM, Kamińska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. https://doi.org/10.1155/2019/9213074.
Pogorzelska K, Krętowska A, Krawczuk-Rybak M, Sawicka-Żukowska M. Characteristics of platelet indices and their prognostic significance in selected medical condition—a systematic review. Adv Med Sci. 2020;65:310–5.
Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32:443–60. https://doi.org/10.1016/0049-3848(83)90255-4.
Abdel-rahman TM. Mean platelet volume and prognosis of unstable angina. World J Cardiovasc Dis. 2015;5:32–41.
Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56. https://doi.org/10.1111/j.1538-7836.2009.03584.x.
Yang G, Muzepper M. Platelet indices and erectile dysfunction: a systematic review and meta-analysis. Andrologia. 2019;51:e13248. https://doi.org/10.1111/and.13248.
Moher D, Liberati A, Tetzlaff J, Altman DG,PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Rhoden EL, Teloken C, Sogari PR, Souto CAV. The use of simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int J Impot Res. 2002;14:245–50.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. https://doi.org/10.1016/s0090-4295(97)00238-0.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. 2021. www.training.cochrane.org/handbook.
Rastrelli G, Maggi M. Erectile dysfunction in fit and healthy young men: psychological or pathological? Transl Androl Urol. 2017;6:79–90. https://doi.org/10.21037/tau.2016.09.06.
Ludwig W, Phillips M. Organic causes of erectile dysfunction in men under 40. Urol Int. 2014;92:1–6.
Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. https://doi.org/10.1016/j.amjmed.2006.06.010.
Meena BL, Kochar DK, Agarwal TD, Choudhary R, Kochar A. Association between erectile dysfunction and cardiovascular risk in individuals with type-2 diabetes without overt cardiovascular disease. Int J Diabetes Dev Ctries. 2009;29:150–4. https://doi.org/10.4103/0973-3930.57345.
Ponholzer A, Temml C, Rauchenwald M, Madersbacher S. Vascular risk factors and erectile dysfunction in a cohort of healthy men. Int J Impot Res. 2006;18:489–93. https://doi.org/10.1038/sj.ijir.3901468.
Vrentzos GE, Paraskevas KI, Mikhailidis DP. Dyslipidemia as a risk factor for erectile dysfunction. Curr Med Chem. 2007;14:1765–70. https://doi.org/10.2174/092986707781058931.
Roumeguère T, Wespes E, Carpentier Y, Hoffmann P, Schulman CC. Erectile dysfunction is associated with a high prevalence of hyperlipidemia and coronary heart disease risk. Eur Urol. 2003;44:355–9. https://doi.org/10.1016/s0302-2838(03)00306-3.
Vlachopoulos C, Rokkas K, Ioakeimidis N, Aggeli C, Michaelides A, Roussakis G, et al. Prevalence of asymptomatic coronary artery disease in men with vasculogenic erectile dysfunction: a prospective angiographic study. Eur Urol. 2005;48:996–1002. https://doi.org/10.1016/j.eururo.2005.08.002.
Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, Lieber MM, et al. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108–13. https://doi.org/10.4065/84.2.108.
Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58:1378–85. https://doi.org/10.1016/j.jacc.2011.06.024.
Pizzulli L, Yang A, Martin JF, Lüderitz B. Changes in platelet size and count in unstable angina compared to stable angina or non-cardiac chest pain. Eur Heart J. 1998;19:80–4. https://doi.org/10.1053/euhj.1997.0747.
Wang XS, Guo LQ, Xiao ZY, Guan Y, Zhang JY, Li M, et al. Mean platelet volume might be an effective indicator of arterial erectile dysfunction. Asian J Androl. 2018;21:62–6. https://doi.org/10.4103/aja.aja_74_18.
El Taieb MA, Hegazy EM, Maklad SM, Khairy R. Platelet Indices as a marker for early prediction of erectile dysfunction in diabetic patients. Andrologia. 2019;51:e13163 https://doi.org/10.1111/and.13163.
Senturk AB, Yaytokgil M, Yilmaz AH, Ekici M, Aydin C, Demir E, et al. Can platelets be the early biomarkers of erectile dysfunction? J Pak Med Assoc. 2018;68:515–518.
Resorlu M, Adam G, Doluoglu OG, Bozkurt S, Sancak BE, Alpaslan A, et al. Mean platelet volume and platelet distribution as a diagnostic marker for penile vascular disease in patients with erectile dysfunction. Rev Int Androl. 2016;14:41–5. https://doi.org/10.1016/j.androl.2015.11.001.
Guo LQ, Liu YQ, Sun WD, Yuan MZ, Xiao ZY, Song HB, et al. significance of platelet distribution width as a severity marker of erectile dysfunction. Andrologia. 2017;49:1–7. https://doi.org/10.1111/and.12628.
Tangal S, Ozayar A, Ener K, Gokçe MI, Haliloglu AH. Does mean platelet volume (MPV) have a role in evaluation of erectile dysfunction and its severity? Rev Int Androl. 2020;18:1–6. https://doi.org/10.1016/j.androl.2018.07.007.
Culha MG, Atalay HA, Canat HL, Alkan I, Ozbir S, Can O, et al. The relationship between erectile dysfunction severity, mean platelet volume and vitamin D levels. Aging Male. 2020;23:173–8. https://doi.org/10.1080/13685538.2018.1459544.
Acknowledgements
The authors would like to thank and appreciate all of the supports from the Department of Urology, Dr. Cipto Mangunkusumo Hospital for allowing this manuscript to be appropriately written and published.
Funding
This research received funding from Universitas Indonesia under PUTI 2020 grant program, project number NKB-1867/UN2.RST/HKP.05.00/2020.
Author information
Authors and Affiliations
Contributions
NR, PB, and MH provided the presented idea. NR and MH wrote the manuscript. MH performed the data analysis. PB and WA did critical reading and editing the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasyid, N., Harjanggi, M., Atmoko, W. et al. Mean platelet volume as a predictive marker of erectile dysfunction: a meta-analysis. Int J Impot Res 34, 746–752 (2022). https://doi.org/10.1038/s41443-021-00523-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-021-00523-7
- Springer Nature Limited
This article is cited by
-
Preanalytical and analytical factors in the mean platelet volume: a potential cause of heterogeneity in studies of erectile dysfunction
International Journal of Impotence Research (2022)