Abstract
Phosphodiesterase type 5 inhibitors (PDE5i) is the only approved oral treatment for erectile dysfunction (ED) in the US, and alternative management remains necessary when this treatment fails or is contraindicated. Targeting other pathways than the NO-cGMP pathway and/or combining this approach with PDE5i may introduce new treatments for men who are unresponsive to PDE5i. This study aims to evaluate whether Mirabegron improves erectile function in men with concurrent overactive bladder and mild to moderate ED. Twenty subjects, 40–70 years old, registering International Index of Erectile Function (IIEF) score 11–25 and International Prostate Symptom Score 8–20, were treated with Mirabegron therapy for 12 weeks. Study participants were re-administered IIEF and OAB-q questionnaires on weeks 2, 4, 8, and 12 and assessed for adverse events. The primary and secondary endpoints were an increase in the IIEF-5 score of 4 units and a decrease in the Overactive Bladder questionnaire (OAB-q) symptom severity score of 10 units between study time points. Thirteen men completed the 12-week study. Mirabegron treatment improved the IIEF-5 scores in five patients (38.4%) by 4 points or more, whereas IIEF-5 scores were not affected by Mirabegron treatment in eight patients (61.5%). There were no clinically relevant decreases in the IIEF-5 score. Significant improvements were observed in intercourse satisfaction at week eight compared to baseline (p = 0.01). Orgasmic function and sexual desire were not affected by Mirabegron treatment. As expected, Mirabegron treatment reduced OAB symptoms based on OAB-q short form (p = 0.006) and OAB-q total health-related quality of life (HRQL) scores compared to baseline (p = 0.03). Residual bladder volumes were not affected by treatment. No serious side effects were reported during the study period. This study suggests that Mirabegron may improve both EF and OAB-related symptoms in some individuals without causing serious adverse events.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED), defined as “the inability of men to achieve and/or maintain an erection sufficient for satisfactory sexual performance", affects millions of men with increasing global prevalence [1,2,3,4]. Phosphodiesterase type 5 inhibitors (PDE5i) are usually recommended by clinicians as first-line therapy due to their favorable clinical efficacies and safety profiles. The effectiveness of PDE5i in men with ED for successful sexual intercourse is ~65%, and it is less effective in men with some conditions such as diabetes mellitus and following pelvic surgeries [5,6,7]. PDE5i is the only approved oral treatment for ED in the US, and alternative management remains necessary when this treatment fails or is contraindicated. Targeting other pathways to induce corpus cavernosum (CC) relaxation or inhibit its contraction than the NO-cGMP pathway and/or combining this approach with PDE5i may introduce new treatments for men who are unresponsive to PDE5i.
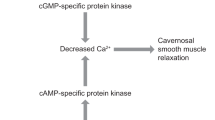
The beta-3 adrenergic receptor (β3-AR) has been demonstrated in human CC, suggesting that it may have a physiologic role in mediating penile erection [8]. The activation of β3-AR mediates CC relaxation via mainly the cAMP-PKA pathway [9]. Other mechanisms also have been implicated in CC relaxation, such as direct activation of K+ channels and closure of voltage-gated Ca2+ channels, cGMP accumulation by NO release, and stimulation of the H2S pathway [10, 11]. However, there are controversial findings in the literature, and the role of β3-AR for penile erection in men is still unclear and needs further studies.
Mirabegron is the first β3-AR agonist that has been approved for the treatment of overactive bladder (OAB) [12]. It exploits the essential role of the β3-adrenergic pathway in detrusor smooth muscle relaxation. OAB symptoms are associated with an increased risk of ED in men and may share a common pathophysiologic mechanism [13]. Mirabegron may act simultaneously in the bladder, urethra, prostate, and CC via β3-AR activation and offers a pharmacologic target for ED. Preclinical studies showed that Mirabegron evokes CC relaxation and increases in vivo erection responses by activating β3-AR independent from the NO-cGMP pathway [14]. However, other studies have described its effect involving alpha-1 adrenergic receptor (α1-AR) blockage independent of β3-AR activation and cAMP accumulation [15].
In the present study, we hypothesized that Mirabegron may act simultaneously in the bladder and CC via β3-AR activation serving as a potential erectogenic treatment for men with ED and OAB. Therefore, this study aimed to evaluate whether Mirabegron improves erectile function (EF) in men with concurrent OAB and mild-to-moderate ED.
Material and methods
Patients
Twenty patients with mild-to-moderate ED and symptoms of OAB, aged 18–70 years, were recruited from the urology clinic at the Johns Hopkins Medical Institutions. Inclusion criteria were the presence of OAB symptoms for at least 3 months with at least eight micturitions per day and at least three episodes of urgency in a 3-day period and mild, mild-to-moderate, or moderate ED based on the International Index of Erectile Function-Erectile Function domain (score 11–25 out of 30) [16]. Exclusion criteria were concurrent ED therapy, prior pelvic surgery (including prostatectomy, cystectomy, transurethral procedures), prior penile surgery, history of priapism, history of neurologic disease (such as spinal cord injury, Parkinson’s disease, multiple sclerosis), uncontrolled hypertension (systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg), chronic kidney disease stage 4 or 5 (estimate creatinine clearance rate <30 ml/min), moderate or severe hepatic impairment (Child-Pugh Class B or C), concomitant use of CYP2D6-metabolized drugs (metoprolol, desipramine, thioridazine, flecainide, propafenone) or digoxin, post-void residual (PVR) amount >150 ml, or evidence of urinary tract infection on urinalysis and/or urine culture. The study was approved by the Institutional Review Board (IRB00097439) and registered at www.ClinicalTrials.gov as NCT02916693.
Study design
This was a single-center, phase 1, non-randomized, single-arm, interventional study conducted prospectively from November 2017 to December 2019. Informed consents were obtained without monetary compensation. After baseline evaluation, consisting of clinical history, physical examination including blood pressure, PVR measurement, urinalysis and/or urine culture, and administration of the IIEF-15 Questionnaire and 5-item short form version (IIEF-5) [17] and Overactive Bladder Questionnaire (OAB-q) [18], patients were consecutively enrolled in a 12-week course consisting of daily Mirabegron 25 mg for 14 days and according to toleration could continue on Mirabegron 25 mg daily or proceed to receive Mirabegron 50 mg daily taken for the next 10 weeks (Astellas Pharma US Inc, IL, USA). Patients were monitored at weeks 2, 4, 8, and 12 by a physician and clinical nurse with clinical visits that included repeat IIEF and OAB-q questionnaires, vital signs, PVR measurement, urinalysis, and clinical interview for recording progress and medication changes and documenting adverse event (AE) occurrences, and adherence to study drug. The principal investigator performed monthly reviews with assessments of trial progress, clinical outcomes, and the occurrence of AEs (Fig. 1).
Outcomes and statistical analysis
The primary efficacy outcome was a total mean increase of 4 or more points from the baseline in the IIEF-5 score. The secondary endpoint was OAB improvement, as reflected by a 10-point decrease from the baseline in the OAB-q symptom severity score. These differences correspond with minimum changes having clinical relevance.
There are no data to suggest the magnitude of the effect of Mirabegron on ED. We performed power calculations that applied a statistical power of 80% and an alpha error of 0.05 for various scenarios characterizing a pattern of means with an absolute increase from pretreatment to 12 weeks of 4 units for IIEF and an absolute decrease of 10 units for OAB, and plausible conservative (higher than expected) values for the within-subject standard deviation.
Pretreatment IIEF and OAB scores were compared to post-treatment scores at 4, 8, and 12 weeks. The primary analysis for both IIEF-15 and OAB scores was repeated measures analysis of variance; if there were missing time points, a mixed model was used. The primary endpoint measurement was determined by TIME effect, i.e., change from the pretreatment value of IIEF or OAB. In addition to absolute change in IIEF-5 or OAB scores, we evaluated models of percentage change from the pretreatment value. If the overall effect of TIME was statistically significant, we used paired t-tests comparing pretreatment to each of the post-treatment values, with a Bonferroni p value adjustment, to determine the time at which an increase in IIEF-5 of at least 4 points or a decrease in OAB of at least 10 points is first detected. Covariates to be evaluated as potential confounding factors include age, comorbidity, or cause of ED, body mass index, and other medications.
Data were analyzed using GraphPad Prism ver. 8 (GraphPad Software, San Diago, CA, USA). A p value < 0.05 was considered statistically significant.
Results
A total of 20 patients were enrolled. Seven patients were lost to follow-up and 13 (65%) remaining patients participated in the study. Baseline demographic and clinical characteristics are shown in Table 1.
At week 12, Mirabegron treatment improved the IIEF-5 scores in five patients (38.4%) by 4 points or more, whereas IIEF-5 scores were not affected by Mirabegron treatment in eight patients (61.5%) (Fig. 2A). There were no clinically relevant decreases in IIEF-5 score.
Mirabegron treatment was associated with significant improvements in the IIEF-5 score at weeks 4 and 8 compared to baseline (p = 0.0003), but not at week 12 (Fig. 2B).
Significant improvements were observed in intercourse satisfaction at week 8 compared to baseline by Mirabegron treatment (p = 0.01). Orgasmic function and sexual desire were not affected by Mirabegron treatment.
As expected, Mirabegron treatment reduced OAB symptoms based on OAB-q short form (p = 0.006) and OAB-q total health-related quality of life (HRQL) scores compared to baseline (p = 0.03) (Fig. 3A, B). Residual bladder volumes were not affected by treatment.
Changes in mean numbers on IIEF, OAB-q scores, and PVR are shown in Table 2.
AEs following Mirabegron treatment were infrequent and transient, consistent with those associated previously with β3-AR agonist use; no serious side effects were reported during the study period. Nonserious expected and unexpected AEs are shown in Table 3.
Discussion
Our study evaluated the effect of Mirabegron, a selective β3-AR agonist, on EF in men with mild, mild-to-moderate, and moderate ED and symptoms of OAB. A clinically meaningful improvement, defined by a change of 4 points or more on IIEF-5 score, was observed in 5 out of 13 (38.4%) men at the end of the 12-week study. However, definitive conclusions about the long-term efficacy of Mirabegron treatment could not be determined, and the increased mean IIEF-5 scores declined following week 8. As expected, Mirabegron improved OAB symptoms and HRQL without increasing PVR. The findings from this study demonstrate potential clinical benefits and safety of this therapy for those men with ED and OAB.
The primary objective of this study was to evaluate the changes in IIEF-5 score by Mirabegron treatment in men with ED and OAB. The use of validated questionnaires such as IIEF in the assessment of ED is well established, and they have been widely used by the clinician to determine the severity of the problem as well as evaluating treatment effectiveness at follow-up visits. Based on previous studies, a clinically meaningful difference in the IIEF-5 score was determined by a 4-point change in the total score with estimated sensitivity and specificity of 0.74 and 0.73, respectively [19]. In addition, the clinically meaningful difference may vary according to ED severity, and it could be as low as two points for mild ED [19]. At the end of our study, the difference in IIEF-5 scores between baseline and week 12 was not significant. However, Mirabegron improved the IIEF-5 scores in seven patients (53.8%) by 2 points or more. The nonsignificant improvements on IIEF-5 scores between study timelines could yet be meaningful clinically. The decline in EF response after week 8 cannot be readily explained.
We also evaluated the treatment effect on other domains of the IIEF questionnaire, such as orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. Significant differences in the intercourse satisfaction domain were found at week 8, which includes questions 6, 7, and 8. This effect could be related to the overall increase in EF that may have improved satisfaction during sexual intercourse. In addition, question 6 assesses the number of attempts for sexual intercourse that could have been promoted by being a participant in this study or relate to an increase in self-confidence due to treatment.
Mirabegron is an alternative treatment option to antimuscarinics in patients with symptoms of OAB. Our results are in line with previous studies that showed Mirabegron improved the symptoms of OAB based on both OAB-q short form and OAB-q total HRQL [20,21,22]. Mirabegron was well-tolerated in our study, and there was no discontinuation due to AEs. The most commonly reported AEs were constipation, back or leg pain, and flu-like symptoms, and no serious AE was reported. Previously, the overall rate of AEs reported for Mirabegron treatment was 47%, while it was 46% in our study [23]. The overall rate of study-related AEs was 30% in our study, which is slightly higher than that described in the literature (19%) [23, 24]. The higher rate of study-related AEs could be related to having a small sample size [25]. However, all AEs were transient, self-limiting, and nonserious.
The β3-AR agonist, Mirabegron, is an effective oral therapy for LUTS associated with OAB. However, its effects on sexual function are unclear. In vitro studies have shown that β3-AR exist in human CC tissue, and activation of these receptors results in CC relaxation, suggesting a potential pro-erectogenic effect in vivo. It is noteworthy that the effect of Mirabegron on human CC relaxation occurs at high concentrations, which may be 2–3 times higher than the therapeutically effective dose in patients with OAB [14, 26]. Previous studies indicate that Mirabegron is well-tolerated at higher doses, and further clinical studies may serve to clarify its dose-dependent effect on EF in men with ED and OAB [27,28,29].
The possible underlying mechanisms for β3-AR-induced CC relaxation may involve a cGMP-dependent and NO-independent pathway, the RhoA/ROCK pathway, or α-1-AR blockade [13, 23]. Previous studies showed that Mirabegron inhibits phenylephrine (α1-AR)-induced smooth muscle contraction by a mechanism dependent on α1-AR blockade [14, 30]. In addition, there is some evidence from in vitro and in vivo studies showing that ROCK inhibitors and PDE5i enhance Mirabegron-induced relaxation in the CC and potentiate its effect [8, 14, 30]. These findings support possible combination therapies between Mirabegron and ROCK inhibitors or PDE5i in men with ED who do not respond to PDE5i.
We acknowledge several limitations due to the design of the study, including a small sample size (a higher rate of lost to follow-up due to consecutively enrolled eligible patients), a single-arm design with no control group, and a single-site study. However, our intent was to perform a small initial investigation to identify a possible signal of EF improvement efficacy. In addition to including a control group, further study should add study arms to evaluate the effect of different dosages of Mirabegron on EF in men with ED. We were unable to ascertain the psychological or general health improvement benefits of our intervention or the possible impact of our attention among all study participants due to the lack of a control group [31].
This study suggests that Mirabegron may improve both EF and OAB-related symptoms in some individuals without causing serious AEs. However, randomized controlled trials with long-term follow-up periods and larger sample sizes are needed to establish its role for ED treatment.
References
NIH Consensus Conference. Impotence: NIH consensus development panel on impotence. JAMA. 1993;270:83–90.
Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3.
Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M. The multinational Men’s Attitudes to Life Events and Sexuality (MALES) study: I. prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607–17.
Braun MH, Sommer F, Haupt G, Mathers MJ, Reifenrath B, Engelmann UH. Lower urinary tract symptoms and erectile dysfunction: comorbidity or typical “aging male” symptoms? Results of the “Cologne Male Survey”. Eur Urol. 2003;44:588–94.
Hatzimouratidis K, Hatzichristou DG. A comparative review of the options for treatment of erectile dysfunction: which treatment for which patient? Drugs. 2005;65:1621–50.
Limoncin E, Gravina GL, Corona G, Maggi M, Ciocca G, Lenzi A, et al. Erectile function recovery in men treated with phosphodiesterase type 5 inhibitor administration after bilateral nerve-sparing radical prostatectomy: a systematic review of placebo-controlled randomized trials with trial sequential analysis. Andrology. 2017;5:863–72.
Liao X, Qiu S, Bao Y, Wang W, Yang L, Wei Q. Comparative efficacy and safety of phosphodiesterase type 5 inhibitors for erectile dysfunction in diabetic men: a Bayesian network meta-analysis of randomized controlled trials. World J Urol. 2019;37:1061–74.
Cirino G, Sorrentino R, di Villa Bianca RD, Popolo A, Palmieri A, Imbimbo C, et al. Involvement of beta 3-adrenergic receptor activation via cyclic GMP- but not NO-dependent mechanisms in human corpus cavernosum function. Proc Natl Acad Sci U S A. 2003;100:5531–6.
Uchida H, Shishido K, Nomiya M, Yahamaguchi O. Involvement of cyclic AMP-dependent and –independent mechanisms in the relaxation of rat detrusor muscle via β–adrenoceptors. Eur J Pharmacol. 2005;518:195–202.
Hristov KL, Cui X, Brown SM, Liu L, Kellet WF, Petkov GV. Stimulation of beta-3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2008;295:1344–53.
Mitidieri E, Tramontano T, Gurgone D, Imbimbo C, Mirone V, Fusco F, et al. β(3) adrenergic receptor activation relaxes human corpus cavernosum and penile artery through a hydrogen sulfide/cGMP-dependent mechanism. Pharmacol Res. 2017;124:100–4.
Maggiore ULR, Cardozo L, Ferrero S, Sileo F, Cola A, Del Deo F, et al. Mirabegron in the treatment of overactive bladder. Expert Opin Pharmacother. 2014;15:873–87.
Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol. 2003;44:637–49.
Gur S, Peak T, Yafi FA, Kadowitz PJ, Sikka SC, Hellstrom WJ. Mirabegron causes relaxation of human and rat corpus cavernosum: could it be a potential therapy for erectile dysfunction? BJU Int. 2016;118:464–74.
Alexandre EC, Kiguti LR, Calmasini FB, Silva FH, da Silva KP, Ferreira R, et al. Mirabegron relaxes urethral smooth muscle by a dual mechanism involving beta3-adrenoceptor activation and alpha1-adrenoceptor blockade. Br J Pharm. 2016;173:415–28.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26.
Coyne KS, Thompson CL, Lai J-S, Sexton CC. An overactive bladder symptom and health-related quality of life short-form: validation of the OAB-q SF. Neurourol Urodyn. 2015;34:255–63.
Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010.
Carlson KV, Rovner ES, Nair KV, Deal AS, Kristy RM, Schermer CR. Factors associated with improvements in patient-reported outcomes during mirabegron or antimuscarinic treatment of overactive bladder syndrome: A Registry Study (PERSPECTIVE). Adv Ther. 2019;36:1906–21.
Herschorn S, Staskin D, Tu LM, Fialkov J, Walsh T, Gooch K, et al. Patient-reported outcomes in patients with overactive bladder treated with mirabegron and tolterodine in a prospective, double-blind, randomized, two-period crossover, multicenter study (PREFER). Health Qual Life Outcomes. 2018;16:69.
Ko KJ, Choo MS, Chang YS, Kim JC, Lee KS. A multicenter prospective study for overactive bladder patient treatment satisfaction with mirabegron after being unsatisfied with antimuscarinic therapy (FAVOR study). Neurourol Urodyn. 2020;39:2417–24.
Wagg A, Staskin D, Engel E, Herschorn S, Kristy RM, Schermer CR. Efficacy, safety, and tolerability of mirabegron in patients aged ≥65yr with overactive bladder wet: a phase IV, double-blind, randomised, placebo-controlled study (PILLAR). Eur Urol. 2020;77:211–20.
Chapple CR, Cruz F, Cardozo L, Staskin D, Herschhorn S, Choudhury N, et al. Safety and efficacy of mirabegron: analysis of a large integrated clinical trial database of patients with overactive bladder receiving mirabegron, antimuscarinics, or placebo. Eur Urol. 2020;77:119–28.
Lessing C, Schmitz A, Albers B, Schrappe M. Impact of sample size on variation of adverse events and preventable adverse events: systematic review on epidemiology and contributing factors. Qual Saf Health Care. 2010;19:1–5.
de Oliveira MG, Rojas-Moscoso JA, Bertollotto GM, Candido TZ, de A Kiguti LR, et al. Mirabegron elicits rat corpus cavernosum relaxation and increases in vivo erectile response. Eur J Pharmacol. 2019;858:172447.
Iitsuka H, Tokuno T, Amada Y, Matsushima H, Katashima M, Sawamoto T, et al. Pharmacokinetics of mirabegron, a β3-adrenoceptor agonist for treatment of overactive bladder, in healthy Japanese male subjects: results from single- and multiple-dose studies. Clin Drug Investig. 2014;34:27–35.
Krauwinkel W, van Dijk J, Schaddelee M, Eltink C, Meijer J, Strabach G, et al. Pharmacokinetic properties of mirabegron, a β3-adrenoceptor agonist: results from two phase I, randomized, multiple-dose studies in healthy young and elderly men and women. Clin Ther. 2012;34:2144–60.
Chapple CR, Dvorak V, Radziszewski P, Van Kerrebroeck P, Wyndaele JJ, Bosman B, et al. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J. 2013;24:1447–58.
Frazier EP, Peters SL, Braverman AS, Ruggieri MR Sr., Michel MC. Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:449–62.
McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–77.
Funding
This investigator-sponsored research study was provided funding and drug support by Astellas Pharma Global Development Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
ALB served as principal investigator on this clinical trial and he is a consultant/advisor for Astellas Pharmaceuticals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karakus, S., Musicki, B. & Burnett, A.L. Mirabegron improves erectile function in men with overactive bladder and erectile dysfunction: a 12-week pilot study. Int J Impot Res 34, 588–592 (2022). https://doi.org/10.1038/s41443-021-00455-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-021-00455-2
- Springer Nature Limited
This article is cited by
-
Overactive bladder negatively affects erectile function and promotes premature ejaculation: findings from large representative population-level study
World Journal of Urology (2024)
-
Conservative Non-surgical Options for Erectile Dysfunction
Current Urology Reports (2023)