Abstract
Literature concerning corporotomy location in multicomponent inflatable penile prosthetic surgery via a penoscrotal approach is scarce if not nonexistent. Aim of our study was to report practices in low-, moderate-, and high-volume penile implant centers regarding corporotomy location and evaluate its potential impact on intraoperative and short-term postoperative complications. Data from 18 (13 European and 5 American) implant centers were collected retrospectively between September 1st, 2018 and August 31st, 2019. Variables included: intraoperative proximal and distal corpus cavernosum length measurement, total corporal length measurement, total penile implant cylinder length, and length of rear tip extenders. Eight hundred and nine virgin penile implant cases were included in the analysis. Mean age of participants was 61.5 ± 9.6 years old. In total, 299 AMS 700™ (Boston Scientific, USA) and 510 Coloplast Titan® (Minneapolis, MN USA) devices were implanted. The mean proximal/distal corporal measurement ratio during corporotomy was 0.93 ± 0.29 while no statistical difference was found among low-, moderate-, and high-volume penile implant centers. A statistically significant correlation between lower proximal/distal measurement ratio and higher age (p = 0.0013), lower BMI (p < 0.0001), lower use of rear tip extenders (RTE) (p = 0.04), lower RTE length (p < 0.0001), and absence of diabetes (p = 0.0004) was reported. In a 3-month follow up period, 49 complications and 37 revision procedures were reported. This is the first study reporting the current practices regarding corporotomy location during IPP placement in a multicenter cohort, particularly when including such a high number of patients. Nevertheless, the retrospective design and the short follow up period limits the study outcomes. Corporotomy location during penoscrotal IPP implantation does not correlate with intraoperative or short-term postoperative complication rates. Future studies with longer follow up are needed in order to evaluate the association of corporotomy location with long-term complications.
Similar content being viewed by others
Introduction
Inflatable penile prosthesis (IPP) implantation emerged almost 5 decades ago [1] and continues to represent a definitive and valuable treatment modality for men with erectile dysfunction (ED) refractory to conservative approaches. Moreover, it is the only therapeutic solution offering high efficacy rates for ED patients in whom nonsurgical approaches are contraindicated due to intolerable side effects or are not acceptable to the patient due to low satisfaction rates [2]. Three piece IPPs are the most popular devices used for motivated men suffering from severe ED, and they have demonstrated high patient and partner satisfaction over time [3, 4].
Numerous studies have described the various IPP implantation techniques (penoscrotal, infrapubic, or subcoronal) including comprehensive reporting of comparative data between each approach [5, 6]. Moreover, there is an emerging interest in defining the criteria for the proper selection and consultation of IPP candidates [7]. Additional research has been performed regarding IPP optimization to achieve more patient friendly, efficacious, and durable devices [8, 9]. While the majority of studies describing IPP implantation techniques focus mainly on IPP reservoir placement or on surgical methods of managing difficult virgin or revision cases, in our knowledge, the current literature lacks assessment of the crucial step of corporotomy location during IPP implantation. Corporotomy location has direct implications with regards to the basic and defining steps of IPP implantation such as surgical exposure of the corpora, corporal dilatation process, IPP cylinder placement, rear tip extender (RTE) use, and tubing location. Therefore, is often an issue of controversy between IPP implanters during relevant congresses and “hands on” masterclasses. Our multicenter study aims to report data upon contemporary practices regarding corporotomy location during penoscrotal IPP implantation and evaluate its potential impact on intraoperative and short-term postoperative complications.
Materials and methods
After obtaining Ethical Committee approval (Number 29.39-uro20.02; Jessa Hospital Hasselt; Belgium), data were collected retrospectively from 13 European and 5 US penile implant centers between September 1st, 2018 and August 31st, 2019. Data collection was conducted independently by each participant center on standardized forms in Microsoft Excel. The predefined variables (common for all penile implant centers) included: proximal and distal corporal length measurement, total corporal length measurement, total cylinder length, and length of RTEs. Furthermore, patient characteristics including indication for penile implant, operative time, type of implant, reservoir characteristics, and short-term (3-month follow-up period) complications were reported. IPP centers were categorized to high (>50 IPP/year), moderate (10–50 IPP/year), and low (<10 IPP/year) volume.
Statistical analysis
Continuous variables were estimated as mean ± standard deviation while categorical variables were reported as relative frequencies. We used multiple imputation (five imputations) to impute patients’ missing covariates. The association between proximal/distal corporal length and the recorded covariates was examined using a linear mixed model, a hierarchical model that accounts for the correlation within the patient (left-right measurements) and also within the hospital. For all tests, two-tailed p values lower than 0.05 were considered statistically significant.
Results
A total of 809 patients who underwent virgin placement of a multicomponent (3-piece) IPP were included in the analysis. Mean age of participants was 61.5 ± 9.6 years old. In total, 299 AMS 700™ (Boston Scientific, USA) and 510 Coloplast Titan® (Minneapolis, MN, USA) devices were implanted. Patient characteristics are shown in Table 1.
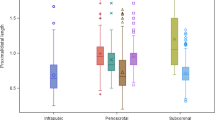
The mean proximal and distal corporal length measurement based on each total corporal measurement length are shown in Table 2. The mean proximal/distal measurement ratio during corporotomy was 0.93 ± 0.29 (Fig. 1). No statistically significant difference regarding mean proximal/distal measurement ratio during corporotomy was revealed among high (>50 IPP/year), moderate (10–50 IPP/year), and low volume IPP (<10 IPP/year) surgeons (p = 0.44). In the majority of cases (n = 670, 82.8%), the proximal/distal measurement ratio was between the range 0.45–1.2. A statistically significant correlation between lower proximal/distal measurement ratio and higher age (p = 0.0013), lower BMI (p < 0.0001), lower use of RTE (p = 0.04), shorter RTE length (p < 0.0001), and absence of diabetes (p = 0.0004) was reported (Table 3). The RTE use rate was 78.9% (638/809). Our study results showed no difference in the mean proximal/distal measurement ratio between Coloplast Titan® and AMS (Boston Scientific, USA) implant devices, which were 0.95 ± 0.20 and 0.84 ± 0.24 (p = 0.42).
Over a 3-month follow up period, 49 cases of complications and 37 revision procedures were reported and none of them was correlated to corporotomy location. No corporotomy-related complications were reported. Specifically, 30 cases of IPP infection (3.7%) and 19 cases (2.3%) of scrotal hematoma were reported.
Discussion
Since its introduction to the market in 1973, IPP implantation has been established as a definitive surgical treatment option for patients with severe ED [1]. Improvement of surgical outcomes for IPP implantation can be achieved by innovations in device design or with refinement of surgical technique. However, to achieve maximal results with regard to patient safety and satisfaction, a combination of both strategies should be employed.
In the early years of IPP implantation, mechanical problems occurred in as many as 50% of cases within the first 5 postoperative years [10]. As is the case in medical prosthetic technology as whole, many improvements have been made regarding IPP implantation as limitations became apparent over time [11]. Improvements regarding IPP cylinder design, materials, reservoirs, pumps, connections, and tubings have been implemented leading to a decreased rate of IPP mechanical failure as well as infection rates [12]. Also, numerous variations in surgical technique have been introduced over the past 5 decades. Many studies have presented different approaches (penoscrotal, infrapubic, or subcoronal) regarding accessing the corporal bodies during IPP surgery [13,14,15]. Moreover, the serious reservoir placement-related complications and increased application of surgical approaches, which compromise the space of Retzius, led prosthetic urologists to introduce innovative Retzius sparing reservoir placement techniques [16,17,18].
In contrast to the choice of reservoir placement and skin incision, little attention has been given to refinement of the location of the corporotomies during IPP surgery and the possible impact on short- and long-term outcomes.
A more distal corporotomy site leads to longer tubing length intracorporeally and subsequently to more friction of tubing against the cylinder wall. In the past, as reported by Scarzella et al. [19] friction due to physical contact of the components over time was accused for causing erosion and leakage of the cylinder, generally 12–18 months post implantation, which was termed “input tubing wear.” Technical improvements and adaptations have been made to overcome this issue. AMS tried to solve this problem by manufacturing a polytetrafluoroethylene sleeve around the tubing as it exits each intracorporeal cylinder to prevent friction. A sleeve, which is now unnecessary with the triple layer cylinder, thus many urologists strip it off completely before cylinder placement. Moreover, during removal of cylinders with the sleeve in place it can add 10–15 min to the procedure to remove the ingrowth of tissue into the sleeve since it is not silicone-coated. The parts of the sleeve sometimes left during an implant repair can be a nidus for infection and they should be removed completely. Also, regarding Coloplast Titan, the bioflex covering has not had issued with input tubing wear.
Moreover, RTEs were developed in 1981 and quickly became popular among implanters as a measure to minimize friction of the tubing against the cylinder wall. The reported high RTE use rate, from 58% up to 73%, during IPP surgeries suggests that this innovation gave surgeons flexibility in placement of corporotomies and minimization of intracorporeal tubing [16].
However, it has been separately reported that RTE utilization should be kept to a minimum. DeLay et al. in their study included 65,448 IPP implantation cases between 2000 and 2015, and revealed that the use of RTE significantly increased revision rate (1.34% vs. 2.65%) [20]. Moreover, Thirumavalavan et al. showed in a laboratory study [21] and in a review article [22] that limiting the use of RTE results in more natural erectile quality because the RTEs are not involved in penile rigidity during inflation of the device. By increasing the length of RTE, the authors demonstrated in a lab model that IPP bending deflection increased, which was correlated with a decrease in axial rigidity [21]. However, in this point we must highlight that clinically in the vast majority of cases where multiple RTEs are used the rigidity of the 3-piece inflatable cylinders is still more than adequate to avoid any buckling during penetration. Thirumavalavan et al. conceded that use of RTE was underexplored in penile implant surgery literature, and they consequently focused on mechanical component ameliorations that could be possible. Based on the findings, it would be more logical to limit the use of RTE or at least their length by focusing on the surgical technique. In our study, it seems that the lower proximal/distal measurement ratio led to lower use of RTE (p = 0.04) and shorter RTE length (p < 0.0001). Thus, theoretically, by focusing on achieving a proximal corporotomy, the prosthetic surgeon can overcome or at least minimize the aforementioned biomechanical deficiencies related to RTE utilization. Another advantage of this technical adaptation is that tubing exiting from a low proximal corporotomy can be more easily buried in the scrotum avoiding the problem of visible tubing, which is a source of patient dissatisfaction as it leads to what some refer to as “Maserati” penis with so called “tail pipes” within the scrotum. However, even when the corporotomy is more distally there is always the solution of tubing excision followed by a new connection or even the straightening of tubing by setting the pump lower in the scrotum.
Illustrations in Fig. 2a, b from both available implant models demonstrate that there is a difference between the devices regarding the distance from the proximal tip of the cylinder to the connection of the tubing with the cylinder, which is measured 3.3 cm in AMS 700™ implant device (Fig. 2a) and 4.4 cm in the Coloplast Titan® (Fig. 2b). The proximal tip to tubing distance is independent from total cylinder length in both devices. Some implanters believe that the proximal extent of the corporotomy incision should be at the site of this tubing-cylinder junction to be able to prevent friction inside the corporal body from the tubing against the cylinder wall. Nevertheless, in cases in which the required overall length of the implanted IPP is increased, achieving such proximal placement of the corporotomy is more difficult. Conclusively, based on the tubing-cylinder junction theory, the tubing should ideally exit the corporotomy at the lower angle of the corporotomy, which correlates to an ~1 cm more proximal corporotomy in AMS 700™ compared to Coloplast Titan® devices according to the aforementioned difference in the tubing-cylinder connection location. Our study results showed no difference in the mean proximal/distal measurement ratio between Coloplast Titan® and AMS 700™ (Boston Scientific, USA) implant devices.
In our study, the mean proximal length measurement according to the total corporal measurement length ranged from 7.6 to 12.4 cm (Table 2), which represents a major difference compared to the theoretically ideal proximal length measurement of 3.3 and 4.4 cm depending on which device is utilized. Also, the RTE use rate was extremely high at 78.9% (638/809) and a statistically significant correlation between lower proximal/distal measurement ratio and lower use of RTE (p = 0.04) and shorter RTE length (p < 0.0001) was observed. The finding supports that a relationship exists between RTE use and a more distal corporotomy site. Nevertheless, we must underline that despite the aforementioned theoretical advantages of the lower proximal/distal measurement ratio no difference was found regarding intraoperative and short-term postoperative complications but also revision rates compared to the cases where the corporotomy was performed more distally.
Our data regarding the significant correlation of lower proximal/distal measurement ratio with lower BMI (p < 0.0001) and absence of diabetes (p = 0.0004) could be attributed to the fact that performing a more proximal corporotomy is more challenging in obese patients, potentially due to a more demanding dissection process or in diabetic patients perhaps due to fibrosis resulting in a lack of tissue compliance.
Regardless, it must be emphasized that performing a proximal corporotomy is technically more challenging. The technical difficulty in a proximal corporotomy, besides reaching and dissecting this area properly, which incidentally can lead to increased bleeding rates, lies in dilating the corporal bodies distally and also inserting the IPP cylinders with the Furlow. Thus, in some cases of low proximal corporotomies, distal corporal dilation, and also positioning of the cylinders, by the use of the straight and unbendable Furlow, will be hindered due to the emergence of an acute angle that is difficult to overcome. For that reason, in many cases there is a necessity of an extended corporotomy to pass the dilating instruments parallel to the penile shaft. Consequently, the extension of the corporotomy leads to the need for additional closure sutures, which could potentially damage the cylinders. Moreover, the aforementioned technical challenges theoretically should increase the operation time, a factor which has been proven to impact negatively the infection rates in IPP implantation cases [23]. Nevertheless, our study results showed no correlation of lower corporotomy with higher surgery duration or higher bleeding events.
Our study sought to also investigate whether volume of IPP implantation cases affected corporotomy location. Interestingly, no significant difference was revealed among high (>50 IPP/year), moderate (10–50 IPP/year), and low volume (<10 IPP/year) IPP surgeons, who in their vast majority avoid to perform their corporotomy too proximally. One explanation may be that most implanters, regardless of surgical volume, are either not aware or convinced by the theoretical aforementioned positive benefits of a more proximal corporotomy. However, in our opinion the main reason is the most favorable surgical plan offered by the more distal corporotomy due to the avoidance of the aforementioned technical difficulties emerging from the more proximal incision of the corporal bodies. A choice, which according to our study results, seems to be totally justified as no higher complication rate was reported compared to the more difficult to perform proximal corporotomy.
Certain crucial limitations exist within our study including its retrospective character and the absence of a validated questionnaire assessing patient satisfaction. Moreover, due to the relatively short follow-up period (~3 months), we were unable to record later complications, which could be linked potentially to the site of corporotomy, such as the IPP cylinder herniation and leakage, and which are usually reported in longer follow-up periods. The multicenter design of our study and the fact that, to our knowledge, it is a unique report of current practices regarding corporotomy location during IPP implantation are important strengths of the present project.
Conclusively, in this study we demonstrated that the mean proximal/distal measurement ratio during corporotomy was 0.93 ± 0.29 without deferring significantly among high (>50 IPP/year), moderate (10–50 IPP/year), and low volume IPP (<10 IPP/year) surgeons. Moreover, no impact of the corporotomy location choice to intraoperative and short-term postoperative complication rate was reported. Future prospective studies with longer follow-up periods investigating the relationship between the corporotomy site and the use of RTE, the long-term complication rate and patient satisfaction may further elucidate the importance of corporotomy location choice during IPP insertion.
References
Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology. 1973;2:80–2.
Levine LA, Becher EF, Bella AJ, Brant WO, Kohler TS, Martinez-Salamanca JI, et al. Penile prosthesis surgery: current recommendations from the international consultation on sexual medicine. J Sex Med. 2016;13:489–518.
Barton GJ, Carlos EC, Lentz AC. Sexual quality of life and satisfaction with penile prostheses. Sex Med Rev. 2019;7:178–88.
Jorissen C, De Bruyna H, Baten E, Van Renterghem K. Clinical outcome: patient and partner satisfaction after penile implant surgery. Curr Urol. 2019;13:94–100.
Palmisano F, Boeri L, Cristini C, Antonini G, Spinelli MG, Franco G, et al. Comparison of infrapubic vs penoscrotal approaches for 3-piece inflatable penile prosthesis placement: do we have a winner?. Sex Med Rev. 2018;6:631–9.
Otero JR, Manfredi C, Wilson SK. The good, the bad, and the ugly about surgical approaches for inflatable penile prosthesis implantation. Int J Impot Res. 2020. https://doi.org/10.1038/s41443-020-0319-4. [Epub ahead of print].
Fraile Poblador A, Díaz Pérez D, Hevia Palacios M, Burgos Revilla FJ. Analysis of preoperative and postoperative expectations of penile implant candidates. Actas Urol Esp. 2020;44:345–50.
Madiraju SK, Wallen JJ, Rydelek SP, Carrion RE, Perito PE, Hakky TS. Biomechanical studies of the inflatable penile prosthesis: a review. Sex Med Rev. 2019;7:369–75.
Wallen JJ, Barrera EV, Ge L, Pastuszak AW, Carrion RE, Perito PE, et al. Biomechanical comparison of inflatable penile implants: a cadaveric pilot study. J Sex Med. 2018;15:1034–40.
Mulcahy JJ. The development of modern penile implants. Sex Med Rev. 2016;4:177–89.
Pastuszak AW, Lentz AC, Farooq A, Jones L, Bella AJ. Technological improvements in three-piece inflatable penile prosthesis design over the past 40 years. J Sex Med. 2015;12:415–21.
Chung E. Penile prosthesis implant: scientific advances and technological innovations over the last four decades. Transl Androl Urol. 2017;6:37–45.
Houlihan MD, Köhler TS, Wilson SK, Hatzichristodoulou G. Penoscrotal approach for IPP: still up-to-date after more than 40 years? Int J Impot Res. 2020;32:2–9.
Picola Brau N, Torremadé J. Infrapubic surgical approach for penile prosthesis surgery: indications and technique. Actas Urol Esp. 2020;44:301–8.
Weinberg AC, Pagano MJ, Deibert CM, Valenzuela RJ. Sub-coronal inflatable penile prosthesis placement with modified no-touch technique: a step-by-step approach with outcomes. J Sex Med. 2016;13:270–6.
Mykoniatis I, Osmonov D, van Renterghem K. A modified surgical technique for reservoir placement during inflatable penile prosthesis implantation. Sex Med. 2020;8:378–82.
Hakky T, Lentz A, Sadeghi-Nejad H, Khera M. The evolution of the inflatable penile prosthesis reservoir and surgical placement. J Sex Med. 2015;12:464–7.
Clavell-Hernández J, Martin C, Wang R. Orgasmic dysfunction following radical prostatectomy: review of current literature. Sex Med Rev. 2018;6:124–34.
Scarzella GI. Cylinder reliability of inflatable penile prosthesis. Experience with distensible and nondistensible cylinders in 325 patients. Urology 1988;31:486–9.
DeLay K, Gabrielson A, Yafi F, Hellstrom W. Pd22-06 rear tip extenders during inflatable penile prosthesis placement: impact on need for revision. J Urol. 2017;197:e443–4.
Thirumavalavan N, Cordon BH, Gross MS, Taylor J, Eid J-F. Rear tip extenders and penile prosthesis rigidity: a laboratory study of coloplast prostheses. J Sex Med. 2018;15:1030–3.
Thirumavalavan N, Cordon BH, Gross MS, Taylor J, Eid J-F. The rear tip extender for inflatable penile prostheses: introduction of “rigidity factor” and review of the literature. Sex Med Rev 2019;7:516–20.
Köhler TS, Wen L, Wilson SK. Penile implant infection prevention part 1: what is fact and what is fiction? Wilson’s Workshop #9. Int J Impot Res. 2020. https://doi.org/10.1038/s41443-020-0326-5. [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Renterghem, K., Jacobs, B., Yafi, F. et al. Current practices regarding corporotomy localization during penoscrotal inflatable penile implant surgery: a multicenter cohort study. Int J Impot Res 34, 302–307 (2022). https://doi.org/10.1038/s41443-021-00431-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-021-00431-w
- Springer Nature Limited
This article is cited by
-
50th year anniversary of penile implants: an ongoing worldwide triumph
International Journal of Impotence Research (2023)
-
Analysis of the effects of different surgical approaches on corporotomy localization in inflatable penile implant surgery performed by expert implant surgeons
International Journal of Impotence Research (2023)