Abstract
There is insufficient evidence that angiotensin-converting enzyme inhibitors (ACEIs) can reduce pneumonia by inducing a dry cough that confers a protective effect on the airway. To increase the evidence base on the clinical use of ACEIs for pneumonia prevention, this retrospective cohort study aimed to comparatively examine the risk of pneumonia-related hospitalization between ACEI initiators and angiotensin II receptor blocker (ARB) initiators using claims data from two Japanese municipalities. We identified persons who were newly prescribed any ACEI or ARB as their first antihypertensive agent between April 2016 and March 2020. The Fine-Gray method was applied to a Cox proportional hazards model to estimate the subdistribution hazard ratio (HR) of ACEI use (reference: ARB use) for pneumonia-related hospitalization, with death treated as a competing risk. Sex, age, comorbidities, medications, and pneumococcal immunization were included as covariates. The analysis was conducted on 1421 ACEI initiators and 9040 ARB initiators, and the adjusted subdistribution HR of ACEI use was estimated to be 1.21 (95% confidence interval: 0.89–1.65; P = 0.22). ACEI initiation did not demonstrate any significant preventive effect against pneumonia-related hospitalization relative to ARB initiation. There remains a lack of strong evidence on the protective effects of ACEIs, and further research is needed to ascertain the benefits of their use in preventing pneumonia.

We conducted a large-scale retrospective cohort study using real-world healthcare data from a Japanese population. In this study, ACEI initiation did not indicate a significant preventive effect against pneumonia-related hospitalization.
Similar content being viewed by others
Introduction
Pneumonia is one of the most common diseases throughout the world, and has a wide spectrum of severity that ranges from very mild to life-threatening cases. Approximately 2.38 million people died of lower respiratory tract infections worldwide in 2016, making it the sixth leading cause of death from illness [1]. Before the COVID-19 pandemic, the pneumonia-related hospitalization rate in Japan was 310 per million population in 2011 [2], with nearly 100,000 pneumonia deaths in 2019 [3].
Several studies have reported effective measures for preventing pneumonia, including smoking cessation and immunization against influenza and pneumococcus [4,5,6]. Oral care is also believed to be effective because micro-aspiration and silent aspiration are important pathogenic mechanisms of pneumonia, even in healthy persons [7]. Additionally, angiotensin-converting enzyme inhibitors (ACEIs), which are frequently used to treat hypertension and chronic heart failure, could potentially reduce the risk of pneumonia through the induction of a dry cough (a common adverse effect) that confers a protective effect on the airway. A possible mechanism for this effect is that ACEIs elevate bradykinin and substance P levels, which stimulate the sensory nerves of the airway and enhance the cough reflex [8,9,10]. A systematic review and meta-analysis published in 2012 reported that ACEI use was significantly associated with a 34% reduction in pneumonia risk when compared with control treatments, whereas angiotensin II receptor blockers (ARBs) did not impact pneumonia risk [11]. However, the current clinical evidence lacks strength, and studies have yielded conflicting results [12,13,14].
Accordingly, there is a need to increase the evidence base on the clinical use of ACEIs for pneumonia prevention. This observational study was conducted to comparatively examine the risk of pneumonia-related hospitalization between ACEI initiators and ARB initiators using claims data from two Japanese municipalities. We set pneumonia-related hospitalization as the study outcome in order to focus on severe conditions that require in-hospital care.
Methods
Data source
This retrospective cohort study utilized data provided by the Longevity Improvement & Fair Evidence (LIFE) Study, which is an ongoing database project managed by Kyushu University (Fukuoka, Japan) [15]. The LIFE Study collects claims data for medical care and long-term care (LTC) services from participating municipalities in Japan, and creates databases for research. Medical care claims data are acquired from residents enrolled in either of Japan’s public medical care insurance systems (National Health Insurance and Latter-Stage Elderly Healthcare System), and include information on patient characteristics and reimbursement claims for all insurance-covered medical services delivered in inpatient and outpatient settings. National Health Insurance enrollees include the self-employed, agricultural and fishery workers, part-time workers, retirees, and their dependents aged 0–74 years; this system covers more than 80% of the population aged 65–74 years. Latter-Stage Elderly Healthcare System enrollees include residents aged ≥ 75 years. Next, Japan’s LTC system provides coverage for persons with certified care needs. Its enrollees mainly include individuals aged ≥ 65 years, and the data contain LTC claims (for use of insurance-covered LTC services) and certified LTC needs levels (indicating the degree of LTC required by a person). The number of municipalities participating in the LIFE Study varies over time depending on differences in individual contracts, with the earliest participant providing data from April 2014.
For this study, data from April 2016 to March 2020 were acquired from two municipalities. The datasets comprised recorded diagnoses, prescribed medications, treatment and hospitalization dates, comorbidities, government-subsidized vaccinations, LTC claims, and certified LTC needs levels. Diagnoses and prescribed medications were identified using the International Classification of Diseases, 10th revision (ICD-10) codes and Anatomical Therapeutic Chemical (ATC) codes, respectively.
Study design and population
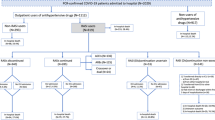
From the 4 years of data, we set the observation period as the 3 years spanning April 2017 to March 2020. First, we identified participants with claims data in the study database in April 2017. Next, we identified those who were newly prescribed any ACEI (ATC code: C09A; including captopril, enalapril, lisinopril, perindopril, ramipril, quinapril, benazepril, cilazapril, fosinopril, trandolapril, spirapril, delapril, moexipril, temocapril, zofenopril, and imidapril) or ARB (C09C-D; including losartan, eprosartan, valsartan, irbesartan, tasosartan, candesartan, telmisartan, olmesartan medoxomil, azilsartan medoxomil, and fimasartan) as their first antihypertensive agent during the observation period. Participants were categorized into an ACEI group or ARB group based on their initially prescribed antihypertensive agent, and the date of this initial prescription in each participant was designated his/her cohort entry date (CED). We excluded (i) individuals who lacked an adequate lookback period (i.e., 1 year before their respective CEDs), (ii) individuals who were previously prescribed any type of antihypertensive agent (ATC codes: C01D, C02K, C07A, C08C-E, C09A, C09C-D, C09X, C02L) during the lookback period, and (iii) individuals who were previously hospitalized for pneumonia or other lower respiratory tract infection (ICD-10 codes: J13–18, J20–22, J69) during the lookback period. Figure 1 presents an overview of the study design and its follow-up process.
Study design. The inclusion criterion (INCL) is records of claims data on April 1st, 2017. Exclusion criterion 1 (EXCL-1) is the lack of an adequate lookback period (1 year before the CED). Exclusion criterion 2 (EXCL-2) is a previous prescription for any type of antihypertensive agent during the lookback period. Exclusion criterion 3 (EXCL-3) is a previous hospitalization for pneumonia or other lower respiratory tract infection during the lookback period. The first set of covariates (Covariates 1) comprises each participant’s sex and age at the time of his/her CED, and the second set of covariates (Covariates 2) comprises each participant’s comorbidities, medications, and pneumococcal immunization during the year before his/her CED. CED cohort entry date
Outcome measure
The outcome measure was pneumonia-related hospitalization, which was identified using the combination of records simultaneously indicating a hospital admission and the occurrence of pneumonia or other lower respiratory tract infection (ICD-10 codes: J13–18, J20–22, J69). This set of ICD-10 codes included pneumonia, lower respiratory tract infection, and pneumonitis due to solids and liquids (including aspiration pneumonia); and excluded chronic pulmonary diseases (e.g., asthma, chronic obstructive pulmonary disease), suppurative and necrotic conditions (e.g., empyema, lung abscess), pleural disease (e.g., pleuritis), and infections caused by other specific organisms (e.g., tuberculosis). Participants in either group were followed-up from their CEDs until the earliest occurrence of any of the following events: outcome, death, disappearance from the database, cessation of ACEI or ARB prescription (defined as no records of these prescriptions within 89 days after the last prescription date), overlapping prescriptions of ACEI and ARB, or the end of the observation period (March 2020).
Covariates
For this study, we identified two sets of covariates. First, we determined each participant’s sex and age at the time of his/her CED. Second, we analyzed each participant’s history of comorbidities, medications, and pneumococcal immunization (lookback period) (Supplementary Table S1). Comorbidities included cancer, diabetes mellitus, chronic heart failure (CHF), coronary artery disease, cerebrovascular disease (CVD), dementia, chronic lower respiratory disease (including asthma and chronic obstructive pulmonary disease), chronic kidney disease, chronic liver failure, gastroesophageal reflux disease, and dysphagia. Medications included immunosuppressants, antacids, and sedatives (including hypnotics). Pneumococcal immunization referred to immunization with the 23-valent pneumococcal polysaccharide vaccine (PPSV23).
Statistical analysis
We performed a survival analysis to assess the relationship between the outcome of interest (pneumonia-related hospitalization) and multiple predictor variables measured either at the CED or during the lookback period. A cumulative incidence function, estimated by modeling the cause-specific hazard function, was utilized due to the need to account for death as a competing risk in this study design. Cumulative incidence curves were generated for each group from CED to the end of follow-up, and differences were assessed using Gray’s test. Additionally, we applied the Fine-Gray competing risk approach to a Cox proportional hazards model to minimize the effects of confounding factors while estimating the adjusted subdistribution hazard ratio (HR) and 95% confidence interval (CI) of ACEI use (reference: ARB use) for pneumonia-related hospitalization. The model adjusted for sex, age, comorbidities, medications, and PPSV23 immunization.
To increase the robustness of our findings, we repeated the analysis following propensity score matching between the ACEI group and ARB group. Propensity scores were estimated using a logistic regression model with ACEI or ARB use as the dependent variable (Supplementary Table S2). The covariates included sex, age, comorbidities, medications, and PPSV23 immunization. We conducted 1:1 nearest-neighbor matching with a maximum caliper of 0.20. The subdistribution HR was then calculated using the same method as the main analysis.
Two-tailed P values below 0.05 were considered to be statistically significant. During the follow-up period, individuals with missing data (“N/A” values) for any covariate were also excluded from the analysis. The statistical analysis was performed using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio version 6.1.524 (Posit PBC, Boston, MA, USA) software.
Subgroup analyses
We performed a set of subgroup analyses to examine the protective effects of ACEIs in specific subpopulations. The following six subgroups were created using the covariate data: male, female, aged ≥ 65 years, aged ≥ 75 years, history of CHF, and history of CVD. Furthermore, individuals receiving LTC services were identified based on their certified LTC needs levels and activities of daily living classification at the time of their CEDs. LTC needs levels range from levels 1 to 5, with higher levels indicating a greater requirement for LTC services. Activities of daily living were assessed using the Japanese government’s “Criteria for Determination of the Daily Life Independence Level (Bedridden level) of the Elderly with Disability” scale, which ranges from grade J (mostly independent) to grade C (completely bedridden) [16, 17]. Using these data, the following three subgroups were created: LTC needs levels 1–5 (persons requiring ≥ 32 min of LTC services/day), LTC needs levels 3–5 (persons requiring ≥ 70 min of LTC services/day), and activities of daily living grades B–C (i.e., bedridden persons) [18].
For each of the nine subgroups, we estimated the subdistribution HRs and 95% CIs using the same method as the main analysis. The definitions of LTC needs levels and bedridden levels under Japan’s LTC system are shown in Supplementary Table S3 [16,17,18].
Sensitivity analysis
Recognizing that an observed association may be affected by the presence of an unmeasured confounder with potential imbalance between the ACEI and ARB groups, we performed a quantitative sensitivity analysis to evaluate the magnitude of this effect [19]. Assuming that an HR can be reasonably considered to be equivalent to a relative risk (RR), we estimated the adjusted RRs for cases in which the strength of association between an unmeasured confounder and disease outcome (RRCD) ranged from 1.5 to 3.0 according to various prevalences of the unmeasured confounder in both cohorts.
Results
From the two participating municipalities, we initially identified 469,951 individuals with claims data in April 2017. After applying the exclusion criteria, the analysis was conducted on 1421 ACEI initiators and 9040 ARB initiators (Fig. 2). Table 1 summarizes the baseline characteristics of the ACEI and ARB groups. ACEI initiators were observed to be older than ARB initiators, and had a higher prevalence of the following comorbidities: cancer, CHF, coronary artery disease, CVD, dementia, chronic kidney disease, gastroesophageal reflux disease, and dysphagia. In addition, ACEI initiators had a higher proportion of antacid prescriptions but a lower proportion of PPSV23 vaccinations during the year before their CEDs.
Flow diagram of study participant selection. Exclusion criterion 1 (EXCL-1) is the lack of an adequate lookback period (1 year before the CED). Exclusion criterion 2 (EXCL-2) is a previous prescription for any type of antihypertensive agent during the lookback period. Exclusion criterion 3 (EXCL-3) is a previous hospitalization for pneumonia or other lower respiratory tract infection during the lookback period. The proportions of individuals among those newly prescribed ACEIs or ARBs are indicated in parentheses. There is overlap among the excluded participants as some individuals meet two or more exclusion criteria. ACEI angiotensin-converting enzyme inhibitor; ARB angiotensin II receptor blocker; CED cohort entry date
Table 2 outlines the results from the follow-up observations and statistical analysis. The mean treatment durations for ACEI and ARB were 156.3 and 258.6 days, respectively. The unadjusted subdistribution HR of ACEI use (reference: ARB use) for pneumonia-related hospitalization was 1.72 (95% CI: 1.35–2.19; P < 0.05). In the multivariable regression analysis that incorporated the covariates, the adjusted subdistribution HR of ACEI use (reference: ARB use) for pneumonia-related hospitalization was 1.21 (95% CI: 0.89–1.65; P = 0.22); the association between each covariate and pneumonia-related hospitalization is shown in Supplementary Table S4. Additionally, 1412 ACEI initiators were matched with the same number of ARB initiators based on their propensity scores. In this group, the subdistribution HR of ACEI use (reference: ARB use) for pneumonia-related hospitalization was 1.37 (95% CI: 0.87–2.14; P = 0.17) (Supplementary Table S2).
Results of the subgroup analyses
Supplementary Table S5 presents the baseline characteristics of each subgroup. The cumulative incidence curves of pneumonia-related hospitalization and the statistical analysis results for the original groups and subgroups are shown in Figs. 3 and 4, respectively. In all analyzed subgroups, the cumulative probability of ACEI initiators for pneumonia-related hospitalization was likely to be equivalent to or higher than that of ARB initiators at any point during the observation period (Fig. 3). The unadjusted subdistribution HRs in the original groups and subgroups tended to be higher than the adjusted subdistribution HRs (Fig. 4). When examining the adjusted subdistribution HRs, ACEI was not significantly associated with pneumonia-related hospitalization in all subgroups, including those of older age and with CVD. Only the subgroups of LTC needs levels 1–5 and 3–5 had unadjusted and adjusted subdistribution HRs that were both below 1.00.
Cumulative incidence curves for pneumonia-related hospitalization. The figure shows cumulative incidence curves of pneumonia-related hospitalization in A the original groups and the following subgroups: B male, C female, D aged ≥ 65 years, E aged ≥ 75 years, F history of CHF, G history of CVD, H LTC needs levels 1–5, I LTC needs levels 3–5, and J bedridden. Death is treated as a competing risk. The Y-axes show changes in the cumulative probability of pneumonia-related hospitalization. The X-axes show time (days) of follow-up. ACEI angiotensin-converting enzyme inhibitor; ARB angiotensin II receptor blocker; CHF chronic health failure; CVD cerebrovascular disease; LTC long-term care
Subdistribution HRs of pneumonia-related hospitalization for ACEI use compared with ARB use. The forest plot shows the subdistribution HRs in the original groups and the following subgroups: male, female, aged ≥ 65 years, aged ≥ 75 years, history of CHF, history of CVD, LTC needs levels 1–5, LTC needs levels 3–5, and bedridden. The further a subdistribution HR lies to the left of 1.00, the more effective ACEI is for preventing pneumonia-related hospitalization. ACEI angiotensin-converting enzyme inhibitor; ARB angiotensin II receptor blocker; CHF chronic health failure; CI confidence interval; CVD cerebrovascular disease; HR hazard ratio; LTC long-term care
Results of the sensitivity analysis
The quantitative sensitivity analysis was conducted with the assumptions that the observed RR (which did not account for the unmeasured confounding effect) was 1.20 (based on the observed results in Table 2) and that the prevalence of this confounder in the ARB group was 0.16. The assumed prevalence of 0.16 was based on a prior report about the rate of tobacco use in Japan [20]. By examining the combinations of RRCD values and unmeasured confounder prevalences in the ACEI cohort, we found that a high RRCD (e.g., 2.5–3.0) and considerable imbalance in unmeasured confounder distribution between comparison groups (e.g., prevalence of 0.16 vs 0.4–0.5) would be required to demonstrate the protective effects of ACEIs. Supplementary Table S6 shows the adjusted RRs that accounted for the potential confounding effect in each hypothetical situation.
Discussion
In this observational study, ACEI initiation did not demonstrate a significantly lower risk of pneumonia-related hospitalization when compared with ARB initiation in a Japanese population. Although a significantly higher unadjusted HR was initially estimated from the original groups, this relationship lost significance after adjusting for the covariates. The results of the subgroup analyses based on various pneumonia risk factors were similar to those of the main analysis. By omitting milder cases treated in outpatient settings, our study provides greater insight into the relationship between ACEI use and more severe conditions that require in-hospital care.
The main finding of this study is that the use of ACEI was not associated with a lower risk of pneumonia-related hospitalization after adjusting for major risk factors that were available in our database. The reason for selecting ARB as the comparator against ACEI was that we expected the characteristics to be similar between initiators of either drug. In contrast to our expectations, the ACEI group had higher proportions of several known risk factors of pneumonia, such as older age, CHF, CVD, and chronic kidney disease. These trends, possibly influenced by guideline recommendations and physicians’ preferences, have also been previously observed [14]. The presence of these differences suggests that there were still potentially unmeasured confounding factors that could influence the results, leading to an underestimation of ACEI’s protective effects.
The subgroup analyses confirmed the findings of the main analysis. These analyses were conducted to highlight important risk factors of pneumonia and to control for bias due to unmeasured confounders. Additionally, by utilizing the LTC needs certification data in the LIFE Study’s database, we were able to homogenize characteristics within restricted populations of both groups, which helped to verify the true effects. In all subgroups—including those restricted to persons with CVD, high LTC needs, and bedridden status—ACEI initiation did not confer any significant protective effect for pneumonia-related hospitalization. We believed these findings are noteworthy and meaningful because ACEI’s airway protective effects were expected to have a strong impact in these high-risk subgroups. However, the unadjusted and adjusted subdistribution HRs were both below 1.00 in the high LTC needs groups, suggesting that these populations may be highly susceptible to ACEIs for pneumonia prevention.
To improve the robustness of our study, we also performed a quantitative sensitivity analysis to address potential unmeasured confounding effects. The results indicated that a highly imbalanced distribution of unmeasured confounders between comparison groups would be required to explain ACEI’s protective effect. We assumed that smoking is the most important confounding factor that we could not measure in this study. Prior studies have reported that the rate of tobacco use is 16.7% in Japan [20], and that the adjusted odds ratio of tobacco use for pneumonia is 1.61 (95% CI: 1.53–1.69) [21]. However, the extremely high RRCD (e.g., 2.5–3.0) and considerable imbalance in unmeasured confounder distribution between comparison groups (e.g., prevalence of 0.16 vs 0.4–0.5) needed to indicate the protective effect of ACEIs suggest that smoking as a single uncontrolled factor would not be sufficient to reverse our results.
There are potential drawbacks when using ACEI with the aim of inducing coughing to protect the airway. Coughing is one of the most common reasons for people to consult a physician, and can detrimentally affect a person’s quality of life. Moreover, coughing may also have a considerable economic impact, especially in the COVID-19 era. The findings from previous reports and the present study remain insufficient to establish conclusive evidence on the protective effects of ACEIs. Given the potential trade-off between inducing a persistent dry cough and preventing pneumonia, physicians must carefully consider whether to recommend ACEIs to patients as a preventive measure.
This study was conducted using real-world medical claims data acquired from a large cohort comprising more than 10,000 ACEI and ARB initiators. While the nature of observational studies makes it challenging to effectively control the risk of biases and confounders, we believe that the exclusion criteria (which removed patients who were previously hospitalized for lower respiratory infectious disease or prescribed any antihypertensive agent during the year before their CEDs) contributed to the equalization of characteristics between the comparison groups. Furthermore, the LIFE Study database enabled us to link claims data, LTC needs data, and vaccination records. This was especially useful for the subgroup analyses on persons with high LTC needs and bedridden status, as these allowed us to examine the target association in subpopulations with an elevated risk of aspiration and pneumonia.
This study has three limitations. First, the outcomes and covariates were identified from claims data, which may include coding errors and diagnostic inaccuracies. Second, there were several potentially important confounders that we could not measure in this study, such as smoking status, influenza vaccination, and socioeconomic status. The lack of these factors may have skewed the results. Nevertheless, our sensitivity analysis indicated that a highly unequal distribution of confounders between the comparison groups would be needed to demonstrate the protective effects of ACEIs. Third, this study was limited to National Health Insurance enrollees and Latter-Stage Elderly Healthcare System enrollees residing in two municipalities. Accordingly, they may not be representative of the entire Japanese population. On the other hand, the study population is likely to have a high coverage of persons with older age, high LTC needs, and bedridden status.
Perspective of Asia
A systematic review and meta-analysis published in 2012 suggested that Asian populations could benefit more from the putative preventive effects of ACEI use against pneumonia, but the underlying rationale is unclear [11]. Potential ethnicity-specific differences in the pneumonia prevention effects of ACEIs should be considered when interpreting the results of our study of Japanese healthcare data. Further research is needed to identify specific populations that could benefit from ACEI use.
Conclusion
In this large-scale retrospective cohort study using real-world healthcare data from a Japanese population, ACEI initiation did not show any significant preventive effect against pneumonia-related hospitalization, even in high-risk subgroups with CVD, older age, high LTC needs, and bedridden status. On the other hand, the results suggested that the high LTC needs groups are potentially susceptible to ACEIs for pneumonia prevention. Due to the current lack of strong evidence, clinicians must carefully consider the potential benefits and risks of prescribing ACEIs to prevent pneumonia.
References
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210.
Ministry of Health, Labour and Welfare. Patient Survey 2011. [In Japanese] https://www.mhlw.go.jp/toukei/saikin/hw/kanja/11/dl/toukei.pdf. Published 2012. Accessed 9 May 2024.
Ministry of Health, Labour and Welfare. Vital Statistics 2019. [In Japanese] https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai19/dl/h6.pdf. Published 2020. Accessed 9 May 2024.
Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 Influenza Season. MMWR Recomm Rep. 2022;71:1–28.
Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–5.
Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816–9.
Mandell LA, Niederman MS. Aspiration Pneumonia. N Engl J Med. 2019;380:651–63.
Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med. 1996;2:814–7.
Morice AH, Lowry R, Brown MJ, Higenbottam T. Angiotensin-converting enzyme and the cough reflex. Lancet. 1987;2:1116–8.
Tomaki M, Ichinose M, Miura M, Hirayama Y, Kageyama N, Yamauchi H, et al. Angiotensin converting enzyme (ACE) inhibitor-induced cough and substance P. Thorax. 1996;51:199–201.
Caldeira D, Alarcao J, Vaz-Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ. 2012;345:e4260.
Kumazawa R, Jo T, Matsui H, Fushimi K, Yasunaga H. Association between angiotensin-converting enzyme inhibitors and post-stroke aspiration pneumonia. J Stroke Cerebrovasc Dis. 2019;28:104444.
Lai CC, Wang YH, Wang CY, Wang HC, Yu CJ, Chen L. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on the risk of pneumonia and severe exacerbations in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:867–74.
Chang CH, Lin JW, Ruan SY, Lee YC, Wu LC, Lin MS, et al. Comparing individual angiotensin-converting enzyme inhibitors with losartan in the risk of hospitalization for pneumonia and related mortality: a nationwide cohort study. J Hypertens. 2015;33:634–42.
Fukuda H, Ishiguro C, Ono R, Kiyohara K. The Longevity Improvement & Fair Evidence (LIFE) Study: overview of the study design and baseline participant profile. J Epidemiol. 2023;33:428–37.
Ministry of Health, Labour and Welfare. Criteria for Determination of the Daily Life Independence Level (Bedridden level) of the Elderly with Disability. https://www.mhlw.go.jp/english/database/db-hss/dl/siel-2010-04.pdf. Published 2012. Accessed 9 May 2024.
Ministry of Health, Labour and Welfare. Text of Certification Investigators for Long-Term Care Needs 2009 (revised April 2024). [In Japanese] https://www.mhlw.go.jp/content/001249525.pdf. Published 2009. Accessed 9 May 2024.
Ministry of Health, Labour and Welfare. Text of Certification Committee Members for Long-Term Care Needs 2009 (revised April 2021). [In Japanese] https://www.mhlw.go.jp/content/000819417.pdf. Published 2009. Accessed 9 May 2024.
Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
Ministry of Health, Labour and Welfare. National Health and Nutrition Survey 2019. [In Japanese] https://www.mhlw.go.jp/content/10904750/000722240.pdf. Published 2020. Accessed 9 May 2024.
Vinogradova Y, Hippisley-Cox J, Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009;59:e329–338.
Funding
This study was funded by a grant from the JST FOREST Program (Grant Number: JPMJFR205J).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Kyushu University Institutional Review Board for Clinical Research (Approval No. 22114-05).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uemura, R., Hieda, M., Maeda, M. et al. Risk of pneumonia-related hospitalization after initiating angiotensin-converting enzyme inhibitors compared with angiotensin II receptor blockers: a retrospective cohort study using LIFE Study data. Hypertens Res 47, 2275–2283 (2024). https://doi.org/10.1038/s41440-024-01768-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01768-7
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Do angiotensin-converting enzyme inhibitors not reduce the risk of pneumonia?
Hypertension Research (2024)
-
Preface-risk of hypertension to cardiovascular disease and beneficial effects of drugs
Hypertension Research (2024)