Abstract
Atrial fibrillation (AF), the most common cardiac arrhythmia, is an important contributor to mortality and morbidity. Ubquitin-specific protease 7 (USP7), one of the most abundant ubiquitin-specific proteases (USP), participated in many cellular events, such as cell proliferation, apoptosis, and tumourigenesis. However, its role in AF remains unknown. Here, the mice were treated with Ang II infusion to induce the AF model. Echocardiography was used to measure the atrial diameter. Electrical stimulation was programmed to measure the induction and duration of AF. The changes in atrial remodeling were measured using routine histologic analysis. Here, a significant increase in USP7 expression was observed in Ang II-stimulated atrial cardiomyocytes and atrial tissues, as well as in atrial tissues from patients with AF. The administration of p22077, the inhibitor of USP7, attenuated Ang II-induced inducibility and duration of AF, atrial dilatation, connexin dysfunction, atrial fibrosis, atrial inflammation, and atrial oxidase stress, and then inhibited the progression of AF. Mechanistically, the administration of p22077 alleviated Ang II-induced activation of TGF-β/Smad2, NF-κB/NLRP3, NADPH oxidases (NOX2 and NOX4) signals, the up-regulation of CX43, ox-CaMKII, CaMKII, Kir2.1, and down-regulation of SERCA2a. Together, this study, for the first time, suggests that USP7 is a critical driver of AF and revealing USP7 may present a new target for atrial fibrillation therapeutic strategies.

Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is a common sustained cardiac arrhythmia which is associated with an increased risk of mortality, stroke, heart failure (HF), and other heart-related complications [1]. According to the Global Burden of Disease, there may be up to 33.5 million patients with AF [2]. So far, despite the implementation of successful treatment techniques and the administration of antiarrhythmic drugs, there is still a high recurrence rate and a high burden of medical costs [3]. Therefore, identifying modifiable risk factors for AF and appropriate preventative actions may significantly improve community health and reduce costs to the health-care system. The development of AF has been linked to a number of processes, including electrical and structural remodeling of atrial tissue [4].
Ubiquitin-proteasome system (UPS) plays an indispensable role in cell biology functions. Deubiquitinating enzymes (DUBs) are known as the element of UPS by specific remove ubiquitin from modified proteins or disassemble the chain of ubiquitin. Growing studies demonstrated that DUBs play a significant role in a variety of diseases especially in cardiovascular events such as atherosclerosis, myocardial fibrosis, myocardial hypertrophy, and myocardial ischemia/reperfusion injury [5,6,7,8]. Ubiquitin-specific protease 7 (USP7), also called HAUSP, is a USP-family DUB which has been first discovered as a viral binding protein that preferentially cleaves K11-, K63- and K48-linked ubiquitin chains [9]. USP7 regulates cell function such as gene expression, transcription, DNA damage, viral infection, and immune reaction, mainly expressed in the cell nucleus [10]. Moreover, several studies have demonstrated that USP7 regulates multiple biological functions including apoptosis, proliferation, tumorigenesis, acute or chronic inflammation and oxidative stress [11,12,13,14]. Interestingly, it has been revealed that USP7 plays an important role in several cardiovascular diseases based on these cell and biologic functions [10]. USP7 obviously increases expression in heart-failure mice with cardiac hypertrophy while its inhibitor prevents myocardium from cardiac hypertrophy [15]. USP7 responses to inflammasome-activating signals and is necessary for inflammasome activation. NLRP3 is a crucial component of NLRP3 inflammasome, whose ubiquitination status is affected by the administration of USP7 inhibition [16]. However, there is limited research to expose the relationship between USP7 and AF occurrence and progression.
Here, we demonstrated that USP7 was upregulated in serum and atrial tissues from AF patients and in Ang II-induced atrial tissues of AF. The administration of the ubiquitin-specific protease USP7 inhibitor p22077 ameliorates Ang II-induced atrial remodeling, atrial fibrosis, inflammation, and oxidase stress, which lead to AF. Mechanistically, p22077 reduced Ang II-induced activation of TGF-β/Smad2, NF-κB/NLRP3, NADPH oxidases (NOX2 and NOX4) signals, the elevated expression of CX43, ox-CaMKII, CaMKII, Kir2.1, and the degraded expression of SERCA2a in the atrial samples, which are participated in fibrosis, oxidative stress, inflammatory response and electrical remodeling. Together, we identified that USP7 participate in the AF pathogenesis and provide that USP7 could act as a novel treatment target of AF.
Method
Study subjects
There are altogether 41 fresh serum samples that recruited from persons with AF in our study. The followings are exclusion criteria: patients with chronic HF, myocardial structural lesions, severe valvular heart disease, endocrine disease (diabetes, thyroid disease), stroke, hematologic system diseases (chronic myeloid leukemia, anemia), surgery or trauma in the past 3 years, infectious disease (acute or chronic), cancerous condition, and acute or chronic renal dysfunction with an estimated glomerular filtration rate of 30 ml/min per 1.73 m2 and others infectious and immune diseases (sepsis, parasitic diseases, arthritis, inflammatory bowel disease). And the normal sinus rhythm controls (n = 44) with normal liver function were also recruited if they showed no family history of AF, as well as any obvious abnormalities in physical examination including blood routine examination, biochemical test, clinical diagnosis, and B-ultrasonography reports [5, 17]. Blood samples were collected for the Elisa. The study, which included six patients with atrial fibrillation and six age- and sex-matched controls, was carried out in conformity with the Helsinki Declaration, and the patients agreed on a written informed consent form before undergoing the procedure, which had been authorized by the Institutional Ethics Committee of Dalian Medical University’s First Affiliated Hospital. Atrial tissues were preserved in neutral buffered formalin solution for 48 hours before being paraffin-embedded and processed for histologic pathology and Studies involving human subjects were approved by Dalian Medical University’s First Affiliated Hospital (PJ-KS-KY-2021-229, to X.-H. Y.).
Animal experiments
8-week-old male mice that weight about 22 g were administered with p22077 (15 mg/kg/day; S7133, Selleck Chem, USA), a USP7 inhibitor, or DMSO (control) in abdominal cavity for 1 week, then injected 2.5% tribromoethanol (0.02 ml/g; Sigma-Aldrich, St. Louis, MO, USA) into abdominal cavity to anesthetize mice and infused them utilizing osmotic mini-pumps (Alzet model 1004, Durect, Cupertino, CA, USA) with Ang II (2,000 ng/kg/min; MB1677, Meilunbio, Dalian, CHN) or saline for 3 weeks. Finally anesthetized the mice using 2.5% tribromoethanol with an intraperitoneal injection, induced atrial fibrillation and recorded it, then removed the hearts. In this study, the Animal Care and Use Committee of Dalian Medical University (No. LCKY2016-31) approved all the experiments and conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Neonatal rat atrial cardiomyocyte isolation and culture
We isolated the atrial tissues from the hearts of rats belonging to Sprague-Dawley aged 1-to 3-day-old. We then digested the atria at 37 °C trypsin (C0201, Beyotime, Shanghai, CHN) for 8-10 cycles each lasted 2–5 min. Next, we collected and incubated these cells for 90 min in a 37 °C environment. And then, we collected suspended cells and centrifuged at a speed of 1000 rpm for 15 min, then incubated cells in culture medium contained DMEM/F12 (SH30023.01B, Hyclone, South Logan, UT, USA) with 20% FBS (16140071, Gibco, Grand Island, NY, USA), 1% penicillin/streptomycin, and 100 mM BrdU for 36 h. Finally, we starved the cells by the method of serum-starved for 12 h and then induced with Ang II (100 nM) or saline for 24 h.
Blood pressure measurement
All the mice’s blood pressures were measured once every 3 days in the same amount of time in the mornings after intraperitoneal injection of p22077. This work has lasted until the pump was buried for 3 weeks. During this process, the mice were in stable state and the ambient temperature was controlled at 37 °C.
Echocardiographic measurement
We anesthetized mice with 2.5% tribromoethanol (0.02 ml/g; Sigma-Aldrich, St. Louis, MO, USA) and measured echocardiographic evaluation of left atrial diameter in mice with Vevo 2100 High-Resolution Imaging System (Visual Sonics, Inc, Toronto, Ontario, Canada).
Induction and duration of atrial fibrillation
We anesthetized mice with 2.5% tribromoethanol by an intraperitoneal injection, and detected the AF progression by using a Millar 1.9 F octapolar electrophysiology catheter (Scisense, London, Ontario, Canada), mouse right jugular vein and right atrium were inserted with recording/stimulating electrophysiology catheters to achieve intracardiac pacemaking. We administered 33 burst pacing impulses at the voltage magnitude of 3, 5, and 8 and different frequencies for each individual mouse was used for stimulating AF. With varying impulses, we recorded the AF durations after burst pacing. According to previous study [18], AF was considered rapid with the atrial rates greater than 1500 beat/min and completely irregular atrial waving lasting greater than or equal 0.5 s.
Histological analysis
After fixing atrial samples in 4% paraformaldehyde, dehydrating and embedding them in paraffin, we performed electrocardiography. Atrial samples were cut into a series of 4 µm thickness thin slices and attached to slides in the 42 °C water. After dewaxing treatment, a Masson staining kit (DC0032, Leagene, Beijing, CHN) and a H&E staining kit (D006, Nanjing Jiancheng Bio Inc, Nanjing, Jiangsu, CHN) were used to stain the fibrosis areas. ImageJ was used to measure the quantitative analysis of the fibrosis areas. Incubated paraffin sections in immunohistochemistry (IHC) with the following first antibodies: anti-USP7 (1:100; GTX125894) (Genetex, San Antonio, Texas, USA); anti-CD68 (1:100; 28058-1-AP), anti-α-SMA (1:200; 67735-1-Ig), anti-NLRP3 (1:150; 19771-1- AP) (Proteintech, Wuhan, Hubei, CHN), incubated with first antibodies at 4 °C all night and with appropriate rabbit or mouse derived secondary antibodies for 2 h at room temperature and detected by DAB Kit (GK347010, Genetech, Shanghai, CHN) the next day, stained nucleus with hematoxylin (D006, Nanjing Jiancheng Bio Inc). The histologic staining’s images were taken by ECLIPSE Ni-U (Nikon Instruments Inc, Tokyo, JPN) and measured by ImageJ.
Immunofluorescence
Sections had been blocked at room temperature for 30 min in blocking solution containing Triton X-100, 0.3 M glycine and 1% BSA, and then incubated with anti-CX43 (26980-1-AP) overnight at 4°C and cleaned with PBS. The secondary antibody (A0453, Beyotime) has incubated these sections in darkness at room temperature for a period of 2 h before labeling the nucleus with DAPI. Using a Leica STELLARIS 5 confocal microscope captured those images. ImageJ was applied to calculate the intensity of cell fluorescence.
Quantitative real-time PCR analysis
TRIzol (SM139-02, Seven Biotech, Beijing, CHN) had been utilized to extract the whole RNA from atrial tissues. Following the manufacturer’s instructions, we utilized a reverse transcription enzyme mix (11141ES60, Yeasen, Shanghai, CHN) for the production of first-strand cDNA from the whole RNA [19]. We determined the amount of mRNA including USP7, the markers of fibrosis collagen I (COL1A1) and collagen III (COL3A1), the markers of inflammation IL-1β and IL-6, the NADPH oxidase enzymes NOX2 (CYBB) and NOX4, the potassium ion channel family members Kcna5, Girk1 and Girk4 by real-time PCR (qPCR) analysis as described [20]. We standardized each gene’s expression to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). As shown in supplemental Table 1, primer sequences were obtained from Sango Biotech (Shanghai, CHN).
Immunoblotting analysis
Heart tissue’s entirety of proteins have been extracted with the RIPA buffer (Solarbio Science Technology Co, Beijing, CHN) and ground, centrifuged. The concentrations were then measured by BCA (P0010; Beyotime, Shanghai, CHN) protein assay in 562 nm, quantitated and heated at 100 °C. An equivalent quantity of protein (40–60 μg) was separating by 8–12% SDS-PAGE and transported to a PVDF membrane (ISEQ00010, Millipore, Shanghai, CHN), mixed with indicated first antibodies overnight at 4 °C and with the secondary antibodies for 2 h at room temperature. The whole blots were developed using a chemiluminescent system, and analyzed signal intensities using a Gel-Pro 4.5 Analyzer (Media Cybernetics, Rockville, MD, USA), and normalized to Tubulin levels.
Antibodies
All primary and secondary antibodies for use in immunoblot are as listed here: USP7 (1:1,500; GTX125894) and ox-CaMKII (1:1,000; GTX36254) were purchased from GeneTex (San Antonio, Texas, USA). TGF-β (1:1,000; 21898-1-AP), CaMKII (1:1,500; 20667-1-AP), SERCA2a (1:800; 67248-1-lg), Kir2.1 (1:1,500; 19965-1-AP), CX43 (1:1,500; 26980-1-AP), NLRP3 (1:800; 19771-1-AP), NOX4 (1:1,500; 14347-1-AP), p65 (1:1,000; 10745-1-AP) and Tubulin (1:3,500; 66031-1-Ig) were bought from Proteintech Group Inc (Wuhan, Hubei, CHN). p-Smad2 (1:1,500; ab280888) was obtained from Abcam (Cambridge, MA, UK). Smad2 (1:1,500; WL02286) was acquired from Wanleibio (Shenyang, Liaoning, CHN). NOX2 (1:1,500; abs124860) was got from Absin (Shanghai, CHN). p-p65 (1:1,000; 310013) was gained from Zen Bioscience (Chengdu, Sichuan, CHN). Beyotime (Shanghai, CHN) provided IgG secondary antibodies against mice (1:6,000; A0216) or rabbits (1:6,000; A0208).
Elisa
In a word, these samples were comprised of 41 serum specimens from subjects with AF and 44 serum specimens from persons with SR. Total USP7 proteins in serum were then measured with a human USP7 ELISA kit (FS11681, Westang Biologicals, Shanghai, CHN) according to the manufacturer’s directions [15].
Statistical analysis
GraphPad Prism 8.0 was used to evaluate the statistical calculations. First, a normalcy test was performed. If all of the groups met the standard deviation criteria and the variations were equivalent, our method was the Student’s t-test or one-way ANOVA (with the Bonferroni post hoc test) as appropriate. We employed the nonparametric Mann-Whitney U test if the aforementioned conditions hadn’t been satisfied. P < 0.05 was considered statistically significance. Values are to be reported as mean ± SD.
Univariable and multivariable logistic regression were performed to evaluate potential risk factors for AF. Model 1 was a crude model with no correction for covariates; model 2 was a multifactorial model that adjusting for sex and age; and model 3 was an entire risk adjustment model that modified potentially confounding factors that were essential on single-variate analysis and those identified to be linked to AF, such as sex, age, LA diameter, LVEF, and SCr.
Results
The expression of USP7 is up-regulated in atrial tissues from patients with AF and in Ang II-induced atrial tissues
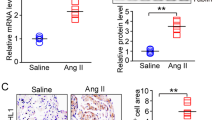
To confirm the function of USP7 in heart tissues from AF patients, immunohistochemistry (IHC) had been employed to assess the expression of USP7 in AF patients and normal control group. In AF patients’ heart tissues, compared with normal group, USP7 expression was clearly enhanced (Fig. 1A, B), suggesting that USP7 may involve in the progression of AF. Moreover, α-SMA was increased in AF tissue vascular walls compared with that in the SR group. Masson staining and HE staining were used to measure the fibrotic area and inflammatory infiltration. The data exhibited significantly greater atrial fibrosis and inflammation in AF human heart tissues than that in SR group (Fig. 1A). qPCR analysis and immunoblotting assay further confirmed that the mRNA and protein levels of USP7 was significantly more extensive in heart tissues from AF patients than control group (Fig. 1C, D). Moreover, to ensure whether USP7 plays a crucial role in patients with AF, we tested the serum level of USP7 in subjects with AF and in SR counterparts. The serum level of USP7 was vastly greater in patients with AF than SR counterparts which was measured by human USP7 Elisa kit (Fig. 1E). The clinical data of AF patients and SR control were illustrated in Table 1. We found that AF patients had greater LA diameter and SCr than SR control. The relationship between AF and serum USP7 was then evaluated using univariable and multivariable logistic regression models (supplemental Table 2). The data shown that the odds ratio (OR) of AF for every rise of one standard deviation (SD) in USP7 was 2.296 [95% CI, 1.049–5.024; p = 0.038].
The expression of USP7 is up-regulated in atrial tissues from patients with AF and in Ang II-induced atrial tissue. A Immunohistochemical (IHC) staining in control group and patients’ atrial tissues with anti-USP7 and α-SMA. Atrial fibrosis stained with Masson and inflammatory cell infiltration stained with HE (blue). Scale bar = 50 µm. B Bars illustrate the quantification of USP7 positive area (n = 6). C qPCR assay of USP7 mRNA level in human atrial tissues (n = 6). D Representative immunoblotting analysis and quantification of USP7 in atrial tissues from AF patients and SR group (n = 4). E The serum USP7 concentration of SR group (n = 44) and patients with AF (n = 41) was quantified by ELISA. **P < 0.01 vs normal SR control. F qPCR evaluation of the mRNA level of USP7 in mice atrial tissues with saline or Ang II (n = 6). G Representative immunoblotting assay and quantification of USP7 protein in mice atrial material from each group that had been stimulated by Ang II (2,000 ng/kg/min) (n = 6). H IHC staining of USP7 (left) and the quantification of the USP7-positive region (right) in atrial material with Ang II or saline treated mice (n = 6). Scale bar = 50 μm. I Primary atrial cardiomyocytes (ACMs) received stimulation with Ang II (100 nM) or saline for 24 h. Immunoblot analysis (upon) and quantification (below) were utilized to determine the amount of USP7 protein in ACMs (n = 4). The statistics are shown as the mean ± SD, with n denoting the number of animals in each group. *p < 0.05, and **p < 0.01 vs saline-treated WT mice
Then, to evaluate the correlations between USP7 and Ang II-induced atrial remodeling, we initially identified the alterations of USP7 expression in mice atrial tissues treated with Ang II. After 3 weeks of Ang II or saline infusion, qPCR and immunoblotting analysis revealed that the expression of USP7 in atrial tissues infused with Ang II was considerably higher than that in saline controls (Fig. 1F, G). Additionally, immunohistochemical analysis has further shown that the expression of USP7 was elevated in the atrial tissues treated with Ang II (Fig. 1H). Atrial cardiomyocytes (ACMs) treated with Ang II similarly showed a considerable rise in USP7 protein levels (Fig. 1I). These findings indicated that USP7 may associate with AF.
Administration of USP7 inhibitor p22077 attenuates Ang II-induced atrial remodeling and the induction of AF in mice
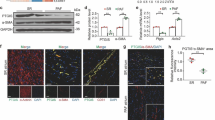
To determine whether USP7 was closely associated with the progression of AF, we used p22077, a strong and sensitive USP7 inhibitor, to detect the effect of USP7 in Ang II-induced AF. WT mice were treated with p22077 (15 mg/kg, per day) at 8-week in parallel with an Ang II infusion (2000 ng/kg/min) at 9-week to induce AF, and then echocardiography, electrical stimulation and sacrifice at 12-week (Fig. 2A). Immunoblotting assay shown that the expression of USP7 was enhanced in Ang II- injected atrial tissues. However, the inhibitor p22077 did not affect USP7 protein levels (Fig. 2B). Additionally, with Ang II infusion, an elevation in systolic blood pressure (SBP) was observed in DMSO-injected mice compared to baseline levels, and this effect was inhibited by the administration of p22077 (Fig. 2C). Considering atrial structure modification is a fundamental pathophysiological aspect of AF, we applied echocardiography to investigate the impact of USP7 on atrial dilation. The elevated left atrial (LA) dilatation caused by Ang II was diminished in the p22077 group (Fig. 2D).
Administration of USP7 inhibitor p22077 attenuates Ang II-induced atrial remodeling and the induction of AF in mice. A Measures of injection p22077 in mice. Mice were dosed with p22077 (15 mg/kg, each day) for 1 week followed by Ang II (2,000 ng/kg/min) infusion and then together for another 3 weeks. B Immunoblotting analyzed USP7 protein in mice injected with p22077 and following Ang II or saline injection (left). The USP7 value was quantified (right, n = 4). C Systolic blood pressure (SBP) was monitored with a tail-cuff every three days following Ang II treatment (n = 6). D Echocardiographic measurement of LA dilation (left) and quantification (right) in mice treated with Ang II or saline (n = 6). E Representative atrial electrogram recordings for AF or SR control. The solid lines represent burst pacing, whereas the dotted lines represent AF. F Bar graph showing percent AF inducibility (n = 9–10). G Total AF duration in DMSO and p22077-injected mice receiving Ang II or saline perfusion. The number n denotes the number of animals utilized in each group. *P < 0.05, **P < 0.01
To further confirm whether USP7 plays an important role in the progression of AF, we next investigated the susceptibility to AF via programmed electrical stimulation to AF (Fig. 2E), which was calculated by the inducibility and duration of Ang II-injected AF in mice administered with DMSO or p22077. The induction of AF was substantially increased in Ang II injected mouse groups with DMSO or p22077 in comparison to saline-injected mice (Fig. 2F, 77.8 vs. 20%, 55.6 vs. 10%). Nevertheless, inhibition of USP7 reduced the inducibility of Ang II-triggered AF (55.6 versus 77.8%). The total duration of AF was also reduced in the p22077 group infused with Ang II. (Fig. 2G). In addition, heart rate in each group of mice did not change significantly between the DMSO- or p22077-injected groups and the saline or Ang II-infused groups. The DMSO or p22077 injection groups did not differ statistically (Fig. 2C–G).
p22077 decreases Ang II-induced atrial fibrosis
In the next step, USP7 is evaluated in mice with atrial fibrosis, which is known to stimulate the onset and progression of AF [21]. The fibrosis areas of Masson staining exhibited an expanded in Ang II-infused tissues of atrial, suggested atrial fibrosis was increased under treatment of Ang II-induced. This growth was reversed by the administration of p22077 (Fig. 3A). Moreover, immunohistochemical staining (IHC) shown that the Ang II-infused increase of the number of α-SMA positive cells was decreased in p22077-treated group (Fig. 3B). qPCR assay exhibited an increase of mRNA level of collagen I (COL1A1), collagen III (COL3A1) in Ang II-induced group, which were decreased after the administration of p22077 (Fig. 3C, D). Western blot analysis shown an elevation in TGF-β, p-Smad2 protein expression in Ang II-induced group, which were decreased in p22077-treatment group (Fig. 3E). All above results suggested that administered p22077 could decrease Ang II-induced atrial fibrosis.
p22077 decreases Ang II-induced atrial fibrosis. A A representative Masson staining for fibrosis (left) and quantification of the fibrotic area on the Masson-stained sections of the saline-infused and Ang II-infused mice atrial tissues (right; n = 6). Scale bar = 20 μm. B Representative images of IHC analyses (left) and quantification of α-SMA-positive cells level (right; n = 6). Scale bar = 20 μm. C, D mRNA expression level analyses of collagen I (COL1A1) and collagen III (COL3A1) by qPCR in Ang II-induced mice atrial tissues and saline groups (n = 6). E Immunoblot analyses (left) and each protein level quantification (right, n = 4) of TGF-β, p-Smad2, and Smad2 in mice atrial tissues. The number n indicates how many animals were used in each group. *P < 0.05, **P < 0.01
Administration of p22077 attenuates Ang II-induced re-distribution of CX43 and dysregulation of ion channel and Ca2+ transporter expression in mouse atria
Electrical propagation can be impaired by aberrant connexin number, abnormal function, or anomalous positioning which can also lead to occurrence and/or development of reentry circuits that assist AF [22]. The next step was to determine whether USP7 affects the distribution and expression of CX43, which may be involved in Ang II induces electrical remodeling in atria. CX43 immunofluorescence (red) in atrial tissues shown it mainly focus on cell ends in saline groups, but disorderly distributed among the cell-ends and lateral margins in Ang II-infused groups. Meanwhile, the CX43 fluorescence staining’s intensity was elevated in groups of Ang II-infusion (Fig. 4A, red). However, administration of p22077 attenuated this dysfunction of CX43 (Fig. 4B). In addition, the Kcna5, Girk1 and Girk4 mRNA levels were significantly higher in Ang II-induced atrial groups compare to saline groups, but this effect was reversed by p22077 adminstration (Fig. 4C). Consistent, the administration of p22077 reduced the Ang II-induced upregulation of ox-CaMKII, CaMKII, CX43, and Kir2.1, while increased the Ang II-induced decreased of SERCA2a (Fig. 4D). Together, p22077 reduces Ang II-induced CX43 re-distribution and deregulation of ion channel and Ca2+ transporter expression in atrial tissues.
Administration of p22077 attenuates Ang II-induced re-distribution of CX43 and dysregulation of ion channel and Ca2+ transporter expression in mouse atria. A, B Cx43 representative images of immunofluorescence (red) and quantification of the intensity of CX43 in atrial tissues of Ang II-induced mice and saline-induced mice (n = 6). Scale bar = 20 μm. C mRNA levels analysis of Kcna5, Girk1 and Girk4 in atrial tissues (n = 6). D Immunoblot analyses of calcium ion channel related protein, potassium channel related protein, and gap junction proteins ox-CaMKII, CaMKII, SERCA2a, CX43, and Kir2.1 in atrial tissues (left). Quantification of protein levels (right, n = 4). n represents the amount of used animals (n = 4). *P < 0.05, **P < 0.01
Administration of p22077 decreases Ang II-induced atrial inflammation and atrial oxidative stress
It has been reported that inflammation and oxidative stress were attached great importance in regulating Ang II-induced cardiac remodeling. We further tested whether USP7 had a potential role on inflammation and producing reactive oxygen species (ROS) in Ang II-infused AF mouse model [23, 24]. As it is shown in hematoxylin-eosin staining, a greater increase of inflammation cells occurred in Ang II-induced groups than saline groups, including CD68-positive macrophages and NLRP3-positive cells, and this impact was diluted by administration of p22077 (Fig. 5A, B). In addition, DHE staining confirmed that the Ang II injection caused significance enhancement of peroxide production, but this alteration was inhibited in p22077-treated group (Fig. 5A, B). Moreover, the mRNA quantitative analysis of pro-inflammatory cytokines IL-1β and IL-6 and NADPH oxidases NOX2 (CYBB) and NOX4 was significantly elevated in Ang II-induced mice atrial tissues (Fig. 5C, D), but was decreased in the p22077-treated mice. Meanwhile, western blot assay shown that the Ang II-induced augment of NLRP3, p-p65, NOX2 and NOX4 was significantly reserved in the group treated with p22077 (Fig. 5E, F). In saline groups all above parameters treated with and without p22077 show no obvious alterations (Fig. 5A–F). Together, these data demonstrate that USP7 is attached great importance in Ang II-induced inflammation, oxidative stress as well as the development of AF, and it’s possible that p22077 could blunted this process.
Administration of p22077 decreases Ang II-induced atrial inflammation and atrial oxidative stress. A Hematoxylin-eosin (H&E) staining, CD68, NLRP3 staining by IHC and staining of atrial sections with dihydroethidium (DHE). Scale bar = 20 μm. Representative images are shown. B The quantification of the positive cells of CD68 and NLRP3 in the IHC-stained sections from Ang II-induced mice heart tissues (upon, n = 6). Quantification of DHE content (below, n = 6). C, D mRNA expression level analyses of pro-inflammatory cytokines IL-1β and IL-6 and NADPH oxidoreductase NOX2 (CYBB) and NOX4 in atrial tissues (n = 6). E Representative immunoblot analysis in Ang II-induced atrial tissues (NLRP3, p-p65, p65, NOX2, NOX4, and Tubulin). F Quantification of NLRP3, p-p65/p65, NOX2 and NOX4 in mice atrial tissues (n = 4). The data are presented as the mean ± SD. The number n indicates how many animals were used in each group. *P < 0.05, **P < 0.01
We further tested whether USP7 had a potential role on producing reactive oxygen species (ROS) in Ang II-infused AF mouse model [24]. In our study, DHE staining confirmed that the Ang II injection caused significance enhancement of superoxide production, but this alteration was inhibited in p22077-treated group (Fig. 5A, B). Moreover, qPCR assay and immunoblotting assay demonstrated that the elevation of the mRNA and protein level of NADPH oxidases NOX2 and NOX4 in Ang II-induced mice was attenuated in the p22077-treated mice. No significant differences were observed in either of the saline-treated groups in any of these parameters (Fig. 5D–F). Therefore, USP7 is responsible for the oxidative stress and the development of AF caused by Ang II.
Discussion
This study, for the first time, revealed that the deubiquitinase USP7 may play an important role in Ang II-induced AF. Our investigation generated three significant conclusions, which are listed below. (1) USP7 protein level was found to be higher in atrial tissues and serum from AF individuals, as well as in Ang II-induced atrial tissues and atrial myocytes. (2) The administration of p22077, the inhibitor of USP7, significantly attenuated the Ang II-induced atrial enlargement, atrial remodeling, and the progression of atrial fibrillation (3) On the mechanism, the administration of p22077, the USP7 inhibitor, recovered the multiple Ang II-induced regulators and signal pathways, including ox-CaMKII, CaMKII, CX43, Kir2.1, SERCA2a, TGF-β/SMAD, NF-κB/NLRP3, NOX2 and NOX4, which has a connection to the atrial fibrillation. Together, our results demonstrated that the deubiquitinase USP7 serves an essential part in Ang II-induced atrial fibrillation and may represent an innovative treatment option for AF. As shown in graphical abstract, we proposed a working model.
Electrical remodeling and structural remodeling are the pathophysiological basis form the development and maintenance of AF, including atrial dilation, calcium overload, atrial fibrosis, cell death, inflammation and oxidase stress [25, 26]. Growing evidence demonstrates that some certain interactome-related nodal points are the molecular driving factors of AF progression, such as CaMKII and NLRP3 [22]. Here, our results shown that inhibition of USP7 attenuated atrial electrical remodeling, atrial fibrosis, inflammation, and oxidase stress (Figs. 3–5). Interestingly, the expression of CaMKII and NLRP3, two key regulators in AF, were increased in Ang II-induced atrial tissues but were rescued by the administration of the USP7 inhibitor, p22077. CaMKII is involved in fibrotic signaling in the myocardium. Angiotensin II increases oxidation of CaMKII, leading to atrial cell death and atrial fibrosis, whereas inhibition of CaMKII has a protective effect against atrial cell death and fibrosis [27, 28]. Interestingly, our data found that administration of USP7 inhibitor p22077 downregulated the protein levels of both ox-CaMKII and total-CaMKII.
It is well known that AF and HF are closely related and that similar pathophysiological mechanisms coexist, which explains the reduced cardiac output observed in patients with AF and the increased incidence of AF in patients with HF. However, despite the clear interconnectedness of AF and HF, finding targets that can affect both diseases simultaneously is a major challenge. Therefore, it is valuable to identify the molecular mechanisms that simultaneously facilitate the treatment of AF and HF. And in our previous experiments, we found that USP7 expression was increased in the blood and ventricular tissue of patients with HF [15]. Meanwhile, in this paper, we demonstrated that USP7 was observed in patients with AF with similar results. The baseline of action is the same, both during pathological myocardial hypertrophy and during atrial fibrillation, in which fibrosis, inflammation, and oxidative stress are connected to the pathological processes of both diseases [29,30,31,32], and our experiments revealed that inhibition of USP7 significantly inhibited Ang II-induced atrial fibrosis, inflammation, and oxidative stress, indicating that USP7 may be a potential marker and a novel therapeutic target both for AF and HF.
It has been reported that several substrate proteins, such as FOXO1/3, FOXO3/4, HIF-1 alpha, NOX2, SMAD3, PTEN, NF-κB, Keap1, NLRP3 and others, are stabilized and deubiquitinated by the deubiquitinase USP7 in many kinds of biological processes [10, 12, 33]. Recently, growing studies corroborated that USP7 acts a critical part in the control of redox homeostasis and inflammation. Xue et al. corroborated that the increase expression of USP7 was observed in H9c2 cells cultured under hypoxia. Overexpression of USP7 exacerbated LV remodeling and reduced LV function in rats and promoted hypoxia-induced apoptosis of cardiomyocytes, which was accompanied by increased secretion of cytokines (IL-1β, TNF-α, and IL-6) and myocardial injury markers (LDH, cTnI, and CK-MB) [34]. Moreover, knockdown or inhibition of USP7 decreased chondrocyte proliferation, accelerated apoptosis and inflammatory response, activated endoplasmic reticulum stress (ERS), and NF-κB/p65 signaling by BiP-eIF2α-ATF4-CHOP signaling [35], suggesting that USP7 may play an important role in inflammation. A study has revealed that the administration of USP7 inhibitor p22077 inhibited the activation of NLRP3 inflammasome and inflammation response, indicating that USP7 may be involved in inflammation [16]. Thus, these studies suggested that inhibiting USP7 may be a viable treatment for cardiovascular disease. Our data, which is consistent with these reports, showed that administration of USP7 inhibitor p22077 reduced the Ang II-induced activation of NF-κB, the increase expression of NLRP3, IL-1β, IL-6, NOX2, NOX4, and the content of ROS, which are important regulator in inflammation response, oxidative stress, and closely related with the progression of AF (Fig. 4A, E). A number of substrate proteins that are crucial for atrial fibrosis, inflammation, oxidative stress, and the development of AF appear to be targeted and stabilized by the USP7 inhibitor p22077, these findings suggesting that inhibition of USP7 may exert a protective effect on atrial fibrillation. Interestingly, a study has demonstrated that USP7 plays a beneficial role in angiogenesis induced by angiotensin-converting enzyme inhibitor (ACEI) in a hindlimb ischemia model: p22077 canceled the ACEI-induced angiogenesis [36]. The opposing effects of USP7 in two models, angiotensin-converting enzyme inhibitor-induced angiogenesis and hypertension-induced atrial fibrillation, may be dependent on the modifications of the USP7 or the target substrates.
Hypertension is a risk factor of AF. Increasing animal experiments and clinical data suggest that the renin-angiotensin system (RAS) and inflammation are involved in hypertension and AF [37]. In our study, we found that the administration of p22077 decreased both systolic blood pressure (SBP) and AF in Ang II-infused mice. Growing evidence shows that Ang II exerts the effects on the increased of SBP and AF closely associated with the activation of NF-κB/NLRP3 signaling [38, 39]. Meanwhile, NF-κB and NLRP3 are stabilized and deubiquitinated by the deubiquitinase USP7, suggesting that USP7 may associate with Ang II-induced increased SBP and AF [16, 40, 41]. Thus, in our study, the administration of p22077 not only decreased systolic blood pressure in Ang II-infused mice, but also attenuated Ang II-induced AF, which might through NF-κB/NLRP3 inflammation signaling.
Several limitations of this study should be addressed. First, all the mRNA and protein expression levels in Ang II-induced atrial tissues and atrial tissues from patients with AF of USP7 was up-regulated (Fig. 1). The mechanism of increased USP7 expression during atrial fibrillation is likely to be related to the regulation of transcription factors. Top Transcription factor binding sites in the USP7 gene promoter, predicted by Genecards database and QIAGEN database, are including AREB6, Arnt, c-Myc, E47, Max, Max1, NF-κB, PPAR-gamma1, and PPAR-gamma2. Among them, NF-κB plays a major role in the pathogenesis of atrial fibrillation as a central response element to two triggering factors: inflammation and oxidative stress [42]. As thus, the Ang II-induced increase expression of USP7 might be regulated by the transcription factor NF-κB, which needs to be determined in the future. Second, USP7 acts as a deubiquitinating enzyme and plays a role in the progression of different diseases by deubiquitinating and stabilizing the different substrates. In Ang II-induced atrial fibrillation, which substrate (or substrates) are regulated by USP7 to influence the development of AF is a question that we need to address in the future. Third, the present study used USP7-specific inhibitors, which may affect different organs in mice, so the possibility that USP7 inhibitors affect the atria by affecting other organs cannot be ruled out. Future studies could investigate the effect of atrial myocyte-specific knockdown of USP7 on Ang II-induced atrial fibrillation.
Conclusion
The current study revealed that USP7 was up-regulated in Ang II-injected atrial cardiomyocytes and atrial tissues in mice, and the atrial tissues from AF individuals. The administration of p22077, a USP7 inhibitory agent, reduced Ang II-induced atrial enlargement, atrial fibrosis, inflammation and oxidase stress, thereby attenuated the progression of AF. Our findings demonstrate that the deubiquitinating enzyme USP7 may serve as a biomarker for the clinical diagnosis of AF and a novel therapeutic strategy for AF.
Data availability
Every single record generated in the current investigation remains accessible to the corresponding authors following reasonable request. All the data obtained in the current study were available from the corresponding authors on reasonable request. All data necessary for evaluating the conclusions of the paper are included in the paper. The authors of this paper will provide any additional data which are requested.
References
Dan GA. Rhythm control in AF: Have we reached the last frontier? Eur Cardiol. 2019;14:77–81.
Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NAM. The state of the art: Atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin Proc. 2016;91:1778–810.
Arbelo E, Brugada J, Blomström-Lundqvist C, Laroche C, Kautzner J, Pokushalov E, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J. 2017;38:1303–16.
Demers J, Ton AT, Huynh F, Thibault S, Ducharme A, Paradis P, et al. Atrial electrical remodeling in mice with cardiac-specific overexpression of Angiotensin II Type 1 receptor. J Am Heart Assoc. 2022;11:e023974.
Chen Y, Chen X, Li H, Li Y, Cheng D, Tang Y, et al. Serum extracellular vesicles containing MIAT induces atrial fibrosis, inflammation and oxidative stress to promote atrial remodeling and atrial fibrillation via blockade of miR-485-5p-mediated CXCL10 inhibition. Clin Transl Med. 2021;11:e482.
Lu X, Rudemiller NP, Wen Y, Ren J, Hammer GE, Griffiths R, et al. A20 in myeloid cells protects against hypertension by inhibiting dendritic cell-mediated T-cell activation. Circ Res. 2019;125:1055–66.
Tang LJ, Zhou YJ, Xiong XM, Li NS, Zhang JJ, Luo XJ, et al. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med. 2021;162:339–52.
Qin B, Zhou L, Wang F, Wang Y. Ubiquitin-specific protease 20 in human disease: Emerging role and therapeutic implications. Biochem Pharmacol. 2022;206:115352.
Lu X, Zhang Y, Zheng Y, Chen B. The miRNA-15b/USP7/KDM6B axis engages in the initiation of osteoporosis by modulating osteoblast differentiation and autophagy. J Cell Mol Med. 2021;25:2069–81.
Xu Q, Liu M, Gu J, Ling S, Liu X, Luo Z, et al. Ubiquitin-specific protease 7 regulates myocardial ischemia/reperfusion injury by stabilizing Keap1. Cell Death Discov. 2022;8:291.
Gong X, Li Y, He Y, Zhou F. USP7-SOX9-miR-96-5p-NLRP3 network regulates myocardial injury and cardiomyocyte pyroptosis in Sepsis. Hum Gene Ther. 2022;33:1073–90.
Huang YT, Cheng AC, Tang HC, Huang GC, Cai L, Lin TH, et al. USP7 facilitates SMAD3 autoregulation to repress cancer progression in p53-deficient lung cancer. Cell Death Dis. 2021;12:880.
Zhang N, Wang F, Zhang G, Zhang Q, Liu Y, Wang Q, et al. USP7 Promotes deubiquitination and stabilization of MyD88 to enhance immune responses. Front Immunol. 2022;13:900243.
Gao M, Qi Z, Deng M, Huang H, Xu Z, Guo G, et al. The deubiquitinase USP7 regulates oxidative stress through stabilization of HO-1. Oncogene. 2022;41:4018–27.
Gu YH, Ren KW, Wang Y, Wang SH, Yu XH, Xu LW, et al. Administration of USP7 inhibitor P22077 inhibited cardiac hypertrophy and remodeling in Ang II-induced hypertensive mice. Front Pharm. 2022;13:1021361.
Palazón-Riquelme P, Worboys JD, Green J, Valera A, Martín-Sánchez F, Pellegrini C, et al. USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep. 2018;19:e44766.
Liu Y, Lv H, Tan R, An X, Niu X-H, Liu Y-J, et al. Platelets promote Ang II (Angiotensin II)-induced atrial fibrillation by releasing TGF-β1 (Transforming Growth Factor-β1) and interacting with fibroblasts. Hypertension. 2020;76:1856–67.
Bi H-L, Zhang X-L, Zhang Y-L, Xie X, Xia Y-L, Du J, et al. The deubiquitinase UCHL1 regulates cardiac hypertrophy by stabilizing epidermal growth factor receptor. Sci Adv. 2020;6:eaax4826.
Shi K-N, Li P-B, Su H-X, Gao J, Li H-H. MK-886 protects against cardiac ischaemia/reperfusion injury by activating proteasome-Keap1-NRF2 signalling. Redox Biol. 2023;62:102706.
Yao C, Veleva T, Scott L, Cao S, Li L, Chen G, et al. Enhanced Cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–42.
Lv W, Zhang L, Cheng X, Wang H, Qin W, Zhou X, et al. Apelin inhibits angiotensin ii-induced atrial fibrosis and atrial fibrillation via TGF-β1/Smad2/α-SMA pathway. Front Physiol. 2020;11:583570.
Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: a translational perspective. Circ Res. 2020;127:51–72.
Zhang Y-L, Cao H-J, Han X, Teng F, Chen C, Yang J, et al. Chemokine receptor CXCR-2 initiates atrial fibrillation by triggering monocyte mobilization in mice. Hypertension. 2020;76:381–92.
Ao X, Ding W, Li X, Xu Q, Chen X, Zhou X, et al. Non-coding RNAs regulating mitochondrial function in cardiovascular diseases. J Mol Med. 2023;101:501–26.
De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–65.
Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325.
Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–88.
Carlson CR, Aronsen JM, Bergan-Dahl A, Moutty MC, Lunde M, Lunde PK, et al. AKAP18δ anchors and regulates CaMKII activity at Phospholamban-SERCA2 and RYR. Circ Res. 2022;130:27–44.
Luo X, Yu W, Liu Z, Pu Z, Liu T, Li Y, et al. Ageing increases cardiac electrical remodelling in rats and mice via NOX4/ROS/CaMKII-mediated calcium signalling. Oxid Med Cell Longev. 2022;2022:8538296.
Zuo K, Fang C, Liu Z, Fu Y, Liu Y, Liu L, et al. Commensal microbe-derived SCFA alleviates atrial fibrillation via GPR43/NLRP3 signaling. Int J Biol Sci. 2022;18:4219–32.
Mighiu AS, Recalde A, Ziberna K, Carnicer R, Tomek J, Bub G, et al. Inducibility, but not stability, of atrial fibrillation is increased by NOX2 overexpression in mice. Cardiovasc Res. 2021;117:2354–64.
Liu Y, Ding W, Wang J, Ao X, Xue J. Non-coding RNA-mediated modulation of ferroptosis in cardiovascular diseases. Biomed Pharmacother. 2023;164:114993.
Liu G, Liu Q, Yan B, Zhu Z, Xu Y. USP7 inhibition alleviates H2O2-induced injury in chondrocytes via inhibiting NOX4/NLRP3 pathway. Front Pharm. 2020;11:617270.
Xue Q, Yang D, Zhang J, Gan P, Lin C, Lu Y, et al. USP7, negatively regulated by miR-409-5p, aggravates hypoxia-induced cardiomyocyte injury. APMIS. 2021;129:152–62.
Dong X, Yang C, Luo Y, Dong W, Xu X, Wu Y, et al. USP7 attenuates endoplasmic reticulum stress and NF-κB signaling to modulate chondrocyte proliferation, apoptosis, and inflammatory response under inflammation. Oxid Med Cell Longev. 2022;2022:1835900.
Lu H, Yuan P, Ma X, Jiang X, Liu S, Ma C, et al. Angiotensin-converting enzyme inhibitor promotes angiogenesis through Sp1/Sp3-mediated inhibition of notch signaling in male mice. Nat Commun. 2023;14:731.
Li J, Wang S, Bai J, Yang X-L, Zhang Y-L, Che Y-L, et al. Novel role for the immunoproteasome subunit PSMB10 in Angiotensin II–induced atrial fibrillation in mice. Hypertension. 2018;71:866–76.
Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation—the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol. 2017;18:861–9.
Cau SB, Bruder-Nascimento A, Silva MB, Ramalho FNZ, Mestriner F, Alves-Lopes R, et al. Angiotensin-II activates vascular inflammasome and induces vascular damage. Vasc Pharmacol. 2021;139:106881.
Colleran A, Collins PE, O’Carroll C, Ahmed A, Mao X, McManus B, et al. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 promotes transcription. Proc Natl Acad Sci USA. 2013;110:618–23.
Nie L, Wang C, Liu X, Teng H, Li S, Huang M, et al. USP7 substrates identified by proteomics analysis reveal the specificity of USP7. Genes Dev. 2022;36:1016–30.
Gao G, Dudley SC. Redox regulation, NF-kappaB, and atrial fibrillation. Antioxid Redox Signal. 2009;11:2265–77.
Funding
This study’s completion was funded by grants from the National Natural Science Foundation of China (82170320 to H.-L.B.); the Natural Science Foundation of Liaoning Provincial (2021-MS-279 to H.-L.B.); the Dalian Science Fund for Distinguished Young Scholars (2023RJ020 to H.-L.B.; 2022RJ13 to X.-L.Y.); Dalian Medical University Interdisciplinary Research Cooperation Project Team Funding (JCHZ2023023 to H.-L.B.).
Author information
Authors and Affiliations
Contributions
H.-L. B. and X.-L. Y. conceived the project. Y. W., Y.-H. G., K.-W. R., and X. X. performed the experiments and analyzed the data, S.-H. W., X.-X. Z. and L. W. were responsible for human clinical studies and analyses. H.-L. B., X.-L. Y. and Y. W. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics
The Animal Care and Use Committee of Dalian Medical University assessed and authorized the laboratory study, which was conducted out in accordance with the US National Institutes of Health Studies involving human participants have been authorized by the First Affiliated Hospital of Dalian Medical University (PJ-KS-KY-2021-229).The animal study was assessed and authorised by the Animal Care and Use Committee of Dalian Medical University and carried out in accordance with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (publication no. 85-23, 1996). Studies involving human participants were approved by the First Affiliated Hospital of Dalian Medical University (PJ-KS-KY-2021-229).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Gu, YH., Ren, KW. et al. Administration of USP7 inhibitor p22077 alleviates Angiotensin II (Ang II)-induced atrial fibrillation in Mice. Hypertens Res 47, 1309–1322 (2024). https://doi.org/10.1038/s41440-024-01581-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01581-2
- Springer Nature Singapore Pte Ltd.