Abstract
The aim of this study was to assess the role of endothelial function measured by the reactive hyperemia index (RHI), arterial stiffness measured by the augmentation index (AIx), and Framingham’s cardiovascular disease (CVD) risk score (FRS) in kidney function decline in patients with chronic kidney disease (CKD). The RHI and AIx@75 (adjusted for 75 heart beats per minute), both derived from peripheral arterial tonometry (EndoPAT), were measured in 428 CKD patients aged 18 years old and older during hospitalization. We evaluated kidney function and its decline (incident ≥40% decline in estimated glomerular filtration rate [eGFR] or initiation of renal replacement therapy) associated with the RHI, AIx@75, and FRS during follow-up for a median of 36 months. The mean age of the participants was 56 years old, and 63.8% were men. In Spearman correlation analysis, the FRS, AIx@75, and RHI levels inversely correlated with eGFR. Over a median follow-up of 36 months, 122 participants experienced kidney function decline. In multivariate Cox analysis, only the FRS remained independently associated with the progression of kidney function (HR, 1.37; 95% CI, 1.14 to 1.64; P = 0.001). Multivariable-adjusted spline regression models showed a positive linear relationship between the FRS and the risk of kidney function decline (P-overall = 0.001, P-nonlinear = 0.701). However, adding the FRS to a model containing kidney function markers did not improve risk prediction for kidney outcome (category-free net reclassification improvement index [cf-NRI] = 0.179, P = 0.084; integrated discrimination improvement [IDI] = 0.017, P = 0.128). Additionally, the increased risk of the outcome associated with an elevated FRS was particularly evident among CKD patients with eGFR ≥60 ml/min/1.73 m2 (eGFR ≥ 60 ml/min/1.73 m2 vs.< 60 ml/min/1.73 m2, P for interaction = 0.022). Participants with higher FRS levels were at increased risk of kidney function decline, emphasizing the important role of traditional CVD risk factors in the progression of CKD.

Similar content being viewed by others
Introduction
The progression of chronic kidney disease (CKD) is closely related to cardiovascular disease (CVD), which accounts for the majority of deaths in patients with CKD [1]. Endothelial dysfunction, which is considered to be an independent predictor of cardiovascular events in CKD patients, contributes to multiple diseases, including arterial stiffness and kidney dysfunction [2, 3]. Arterial stiffness is an important pathophysiological mediator between cardiovascular disease and kidney disease. Aortic stiffness, which could lower the normal gradient leading to peripheral arterial stiffness, plays a detrimental role in the vascular beds of the kidney by increasing the pressure pulsatility [4, 5].
Previous studies have investigated the association between kidney function decline and endothelial dysfunction as well as arterial stiffness estimated through flow-mediated dilation (FMD), ankle-brachial index (ABI) and pulse wave velocity (PWV) measured at different arterial segments [6, 7]. Peripheral arterial tonometry (EndoPAT 2000, Caesarea, Itamar Medical, Israel) is a novel, noninvasive, automatic, reproducible, and less operator-dependent device for the measurement of vascular endothelial function and peripheral arterial stiffness in vivo. This device could quantify the changes in digital pulse volume in response to reactive hyperemia to assess peripheral endothelial function represented by the reactive hyperemia index (RHI) [8]. Additionally, the augmentation index (AIx) could be automatically calculated by EndoPAT from the digital arterial pulse waveform to evaluate peripheral arterial stiffness. To our knowledge, the application of EndoPAT has rarely been adopted in clinical research related to the risk of renal function decline in patients with CKD.
Framingham’s CVD risk score (FRS) is a widely accepted tool used to predict ischemic cardiac disease, peripheral vascular disease, and cerebrovascular disease in the general population by the simple evaluation of demographic, clinical, and biochemical parameters [9]. However, data are limited about the relationship between the FRS and kidney function decline in patients with CKD.
Therefore, the objectives of our study were to examine whether digital artery endothelial function and stiffness analyzed by EndoPAT as well as the FRS are associated with kidney function progression in CKD patients.
Materials and methods
Study population
This observational longitudinal cohort study was conducted in the Chinese PLA General Hospital, Beijing, China, from November 2017 to December 2019. Inclusion criteria were adults (≥18 years) with CKD (defined as abnormalities of kidney structure or function, present for >3 months, with implications for health [10]) not yet on dialysis and who underwent the measurement of EndoPAT. Individuals were excluded if they did not have available follow-up data. The study was approved by the Ethics Committee of the Chinese PLA General Hospital (No. S2017-038-01), and all patients gave written informed consent.
Assessments of clinical and biological parameters
Demographic data, the presence of comorbidities, smoking status and medications were obtained from clinical records. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg on repeated measurements or being on antihypertensive medication [11]. Diabetes was defined by the use of hypoglycemic agents, self-report, fasting plasma glucose ≥7.0 mmol/l, or an oral glucose tolerance test ≥11.1 mmol/l. Cardiovascular disease (CVD) was defined as a history of ischemic cardiac disease or heart failure and/or the presence of peripheral vascular disease and/or cerebrovascular disease. Blood and urine samples were obtained on the first day of hospitalization. Serum creatinine, 24 h urine protein quantities (24 h-UPRO), uric acid, triglycerides, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), parathyroid hormone (PTH), calcium, phosphorus, C-reactive protein (CRP) and interleukin-6 (IL-6) were measured, and the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Assessments of peripheral endothelial function and arterial stiffness
Endothelial function and peripheral arterial stiffness, measured by EndoPAT, were estimated using the RHI and AIx@75 (AIx corrected to a heart rate of 75 bpm), respectively.
All tests were performed only once in a quiet and dimly lit room at a temperature of 21–24 °C with the patients in the supine position. An inflatable blood pressure (BP) cuff was applied to one upper arm. Two finger probes were placed on the index finger of each hand to measure pulse volume (pulse amplitude), which was recorded for 5 min at baseline. The BP cuff was then inflated to 60 mmHg above systemic systolic BP or 200 mmHg, whichever was higher, to occlude the pulsatile arterial flow for 5 min. Following the test period, the BP cuff was released, and the pulse amplitude was measured for an additional 5 min. The RHI was calculated automatically and represents the ratio of the average amplitude of the PAT signal after cuff deflation and before cuff inflation, corrected for the nonoccluded arm. A higher RHI indicates better endothelial function. The AIx@75, a measure of peripheral arterial stiffness, was also automatically generated. The brachial artery BP was measured at least 20 min before the test was started.
Assessment of Framingham’s CVD risk
The FRS was calculated based on age, sex, smoking status, diabetes, treatment for hypertension, cholesterol, HDL, and SBP. The FRS provided a risk percentage of developing cardiovascular disease in the next 10 years [12].
Outcomes
The combined end point was a ≥ 40% decline in eGFR (CKD-EPI) since study entry or the initiation of renal replacement therapy (RRT). We selected the outcome of a ≥ 40% decline in eGFR over follow-up because this outcome is clinically and biologically significant [13]. RRT was defined as the initiation of hemodialysis, peritoneal dialysis, or renal transplantation. The follow-up period was defined as the time between data collection until the occurrence of the endpoint or the last follow-up. ΔeGFR was defined as the change in eGFR in ml/min/1.73 m2 per month. For patients starting RRT, eGFR was estimated as 5 ml/min/1.73 m2 at the first dialysis date. The study database was closed in October 2021.
Statistical analysis
Statistical analysis was performed using R version 4.2.1. Continuous variables were expressed as the mean ± SD when normally distributed and the median with interquartile range (IQR) when nonnormally distributed. Categorial variables were expressed using frequencies or numbers of positive cases. We categorized participants by the levels of the FRS and compared the distribution of demographic, clinical, and laboratory characteristics using the chi-squared test for categorical variables and Kruskal‒Wallis or ANOVA tests for continuous variables, as appropriate. A Spearman correlation test was used to evaluate the relationships among the AIx@75, RHI, FRS, eGFR, 24 h-UPRO and ΔeGFR. To minimize bias that might occur if participants with missing data were excluded from analyses, we used multiple imputation, based on 5 replications and chained equations, to account for missing data on CRP and IL-6. Cox proportional hazards regression analyses were performed to determine the independent predictive value of the AIx@75, RHI and FRS on the progression of kidney function. For multivariable models, we used data on age, sex, markers of kidney function (eGFR and 24 h-UPRO), history of CVD, hypertension, diabetes and inflammatory biomarkers (CRP and IL-6). The proportional hazards assumption of the Cox regression was tested, and we found no violations. The adjusted model using a restricted cubic spline with 3 knots was constructed to flexibly display the association between the hazards of renal function decline and continuous covariate of the FRS. Furthermore, we used C-index estimation to evaluate the predictive performance of the AIx@75, RHI and FRS for the renal end point and calculated the category-free net reclassification improvement (cf-NRI) and the integrated discrimination improvement (IDI) to examine the net effects of adding the AIx@75, RHI and FRS for predicting kidney disease progression. The IDI was computed from 1000 bootstrap samples. Subgroup analyses were also performed to explore whether the association between the FRS and kidney function decline was different between patients on the basis of age, sex, baseline CKD status, diabetes and hypertension. For all analyses, P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 428 subjects were included in this study (Fig. 1), and the baseline characteristics are shown in Table 1. The median age was 56 years, men accounted for 63.8%, and the median serum creatinine and 24 h-UPRO were 112.8 μmol/L and 2.11 g, respectively. Compared with participants with lower levels of FRS, those with higher levels had a higher AIx@75 and 24 h-UPRO levels and lower eGFR levels (P < 0.05).
Associations among the AIx@75, RHI, FRS, eGFR, 24 h-UPRO and ΔeGFR
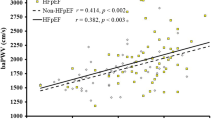
The AIx@75 level was inversely correlated with eGFR (r = −0.22, p < 0.001) and ΔeGFR (r = -0.16, p < 0.001) and positively correlated with the RHI (r = 0.35, p < 0.001), FRS (r = 0.35, p < 0.001) and 24 h-UPRO (r = 0.12, p < 0.05). The FRS level was inversely correlated with eGFR (r = −0.22, p < 0.001) and ΔeGFR (r = −0.16, p < 0.01) and positively correlated with 24 h-UPRO (r = 0.22, p < 0.001; Fig. 2).
Correlations among the AIx@75, RHI, FRS, eGFR, 24 h-UPRO, and ΔeGFR. ***P < 0.001, **P < 0.01, *P < 0.05. AIx@75, augmentation index adjusted for 75 heart beats per minute; RHI reactive hyperemia index, FRS Framingham’s cardiovascular disease risk score, eGFR, estimated glomerular filtration rate, 24 h-UPRO 24 h urine protein quantity
Association between the levels of RHI, AIx@75, and FRS and kidney function decline
After a median follow-up of 36 months (95% CI, 34 to 37, reverse Kaplan‒Meier method), a ≥ 40% decline in eGFR occurred in 56 participants, and 66 patients received RRT. As shown in Table 2, univariate Cox regression revealed that a higher risk of kidney function decline was significantly associated with higher quartiles of the AIx@75 and FRS. After further adjustments for kidney function markers, history of CVD, and inflammatory biomarkers (Model 2), a 1-SD increase in the FRS remained independently associated with kidney function decline (HR, 1.37; 95% CI, 1.14 to 1.64, P = 0.001). Furthermore, compared with the first quartile, multivariable-adjusted HRs for kidney function decline were 2.15 (95% CI, 1.19 to 3.87, P = 0.012) and 2.35 (95% CI, 1.28 to 4.31, P = 0.007) for the third and fourth FRS quartiles, respectively. However, the association for a 1-SD higher AIx@75 was no longer statistically significant. In Model 3, after adjustment for established risk factors that the FRS contained, including age, sex, hypertension, and diabetes, the association of the AIx@75 with kidney function decline remained nonsignificant. Multivariable-adjusted restricted cubic spline analyses suggested that high levels of the FRS were associated with an increased risk of kidney function decline (Fig. 3).
Multivariable adjusted hazard ratios for kidney function decline according to the levels of the FRS on a continuous scale. Solid red lines are multivariable-adjusted hazard ratios, with dashed black lines showing 95% confidence intervals derived from restricted cubic spline regressions with three knots. Purple curves show the fraction of the population with different levels of FRS. Analyses are adjusted for eGFR and 24 h-UPRO
C-statistics, cf-NRI, and IDI for the assessment of the AIx@75, RHI, and FRS in predicting kidney function decline
We examined whether adding the AIx@75, RHI, and FRS to a base model consisting of eGFR and 24 h-UPRO could improve the predictive power for kidney function decline. As shown in Table 3, the c-statistic value of the base model was 0.797 (95% CI, 0. 752 to 0. 842). The addition of the FRS to the base model significantly increased the c-statistic value to 0.805 (95% CI, 0.762 to 0.848). The cf-NRI resulting from FRS inclusion was 0.179 (95% CI, −0.024 to 0.383, P = 0.084), and the IDI was 0.017 (95% CI, −0.005 to 0.051, P = 0.128), showing that the predictive value for kidney function decline did not increase when the FRS was considered in addition to the base model.
Subgroup analyses
The study cohort was stratified according to age, sex, eGFR, 24 h-UPRO, and the presence of diabetes and hypertension. As shown in Fig. 4, the increased risk of kidney function decline associated with elevated FRS levels was particularly evident among individuals with eGFR ≥ 60 ml/min/1.73 m2 (eGFR ≥ 60 ml/min/1.73 m2: HR, 2.66; 95% CI, 1.72 to 4.10; eGFR < 60 ml/min/1.73 m2: HR, 1.25; 95% CI, 1.05 to 1.49; P-interaction = 0.022). No statistically significant interactions were observed across the subgroups defined according to age, sex, 24 h-UPRO, and the presence of diabetes and hypertension.
Discussion
This study investigated whether three noninvasive CVD risk markers, namely, the RHI (a marker of peripheral endothelial function), AIx@75 (a marker of peripheral arterial stiffness), and FRS (traditional risk factor for CVD risk), were independently associated with kidney function decline in patients with CKD stages 1 to 5. The major finding of this prospective, observational longitudinal study was that the FRS was an independent predictor of renal function decline over a median follow-up period of 36 months after adjustment for several confounding variables, including markers of kidney function, history of CVD, and inflammatory biomarkers. We also found that the positive association between elevated FRS and kidney function decline was particularly evident in patients with eGFR ≥ 60 ml/min/1.73 m2.
CKD-specific alterations, such as the accumulation of uremic toxins, chronic inflammation, mineral metabolism disorder, and oxidative stress, could increase cardiovascular risk through vascular alterations, including endothelial dysfunction and arterial stiffness, accompanied by a synchronous decline in kidney function [1, 14]. The association between reduced eGFR and endothelial dysfunction, arterial stiffness, and cardiovascular risk factors is expected; however, no consensus has been reached. The significant association of a higher RHI with impaired renal function in our study is a paradoxically unexpected but interesting result. Wang et al. found that the RHI seemed to be inversely correlated with eGFR (worse renal function with a better RHI) with a marginal p value (p = 0.089) in a study of 252 patients with non-dialysis-dependent CKD stages 1 to 5 [15]. Cerqueira et al. studied 120 predialysis patients with CKD stages 1 to 5 and found that there was a lack of a significant association of a lower RHI with reduced eGFR, and the RHI in healthy subjects did not differ significantly from the patients in the CKD group [16]. In contrast, Hirata et al. found a significantly lower LnRHI in CKD patients (n = 383) than in non-CKD patients (n = 474) [3]. The inconsistent correlations of the RHI have been reported not only with kidney function but also with age, blood pressure, and serum uric acid [17,18,19,20,21]. The result that CKD patients with a higher RHI are more likely to have lower eGFR in our study can be explained by the following reasons. Heterogeneity in clinical participants among studies might cause a difference in the relationship between the RHI and kidney function. While the RHI has been validated as a marker of endothelial function, it reflects alterations in flow and digital microvascular dilatation that may be affected by nitric oxide (NO) [22]. Most of the participants in our study were taking antihypertensive medication, which can affect endothelial function by increasing the production or activity of NO [23]. Our previous study reported that a higher natural logarithm RHI (LnRHI) was associated with more severe intrarenal arteriolosclerosis, and a similar finding also suggested that patients with more severe diabetic retinopathy (DR) had enhanced endothelial function assessed by the RHI [24, 25]. The exaggerated reactive hyperemic responses in patients with more severe microvascular disease or kidney disease may represent endothelial dysfunction rather than better endothelial function. FMD has been widely used to evaluate endothelial function in vivo by describing any vasodilatation of an artery following an increase in luminal blood flow and internal-wall shear stress [26], but the shear stress stimulus cannot be assessed with the EndoPAT technique. Tajima et al. showed that there was no significant correlation between FMD and the RHI [27]. Thus, the RHI may not fully describe endothelial function. The association between the RHI and kidney function should be further investigated in large-sized independent studies.
Arterial stiffness has been demonstrated to be an important and common risk factor for cardiovascular events in patients with CKD. CKD could contribute to the progression of arterial stiffness owing to several pathogenic factors, including chronic inflammation, oxidative stress, mineral metabolism disorder, and uremic toxins [4]. Arterial stiffness in turn could aggravate the damage to renal function by imposing excess transmission of pressure pulsatility on microvascular beds [6]. Arterial stiffness leads to the early merging of reflected waves into incident waves by increasing the pulse wave velocity (PWV), resulting in an increase in AIx [9]. Thus, the AIx reflects arterial stiffness. Previous studies have reported inconsistent associations between arterial stiffness and eGFR [28,29,30], and no research has assessed the relationship between arterial stiffness evaluated by the AIx and kidney function. In our study, we found that higher AIx@75 levels were associated with lower baseline eGFR. As in the prior cross-sectional study, we found that greater peripheral vascular stiffness measured by the AIx@75 was associated with more severe intrarenal arteriolosclerosis determined by renal biopsy [24]. The role of arterial stiffness in renal function decline, which has been evaluated in several studies, is still controversial. In a study with 2053 Japanese subjects, elevated brachial-ankle PWV in individuals with normal renal function or early CKD was an independent risk factor for renal function decline [31]. In a study of 3666 participants, Sedaghat et al. found that carotid-femoral PWV was associated with a steeper decline in renal function after a follow-up time of 11 years. However, the meta-analysis they conducted incorporated their findings [6]. Madero et al. reported that aortic PWV was associated with the incidence of CKD but not rapid kidney function in a study of 2129 older adults with a median follow-up duration of 8.9 years [32]. In contrast, the Framingham Heart Study showed that arterial stiffness correlated with the incidence of albuminuria but not CKD [33]. In our study, we found that arterial stiffness evaluated by the AIx@75 was not significantly associated with kidney function decline. These inconsistent results could be explained by the differences in the study population, follow-up time or other possible causes that may be more dominant in affecting the progression of renal function.
The FRS is well-validated in predicting cardiovascular risk and is widely used in clinical practice [12]. Since the progression of CKD and CVD share similar traditional risk factors, including hypertension, diabetes, and dyslipidemia [1], the role of the FRS in renal function and its progression deserves to be investigated. Similar to our result, Jin et al. reported a significant inverse association between eGFR and FRS [34]. The FRS has been commonly used to evaluate CVD risk in CKD patients, and Lee et al. reported that the FRS could effectively predict the incidence of CKD in a study of 9080 subjects with normal renal function [35]. To our knowledge, no study has reported whether the FRS could perform equally well and accurately in predicting the progression of CKD. The result that a higher FRS is associated with a higher risk of renal function decline suggests that traditional cardiovascular risk plays an important role in kidney function progression. We also found that among CKD patients with eGFR ≥ 60 ml/min/1.73 m2, the association between elevated FRS and kidney function decline was particularly evident. The reason for this result can be explained by the fact that more factors caused by the decline in renal function, which outweigh the cardiovascular risk factors, affect kidney function progression.
Some limitations of this study deserve mention. First, we relied on measurement at a single time point during hospitalization, which may not be an ideal time for risk prediction, as the patients’ health status and medication therapy could affect the investigated markers. Second, we lack data on FMD and PWV, which are considered the gold standard measurements for endothelial function and arterial stiffness, respectively, and the relationships between different methods need to be determined. Third, the patient population was relatively small since our study covered all stages of CKD. Fourth, we acknowledge that due to the observational design of our study, causality conclusions cannot be drawn.
Perspective of Asia
While the FRS is widely used in clinical practice and has been validated in large cohorts of patients with various diseases [12], some differences in the predictive power of the FRS for the incidence of CKD in the general population and the incidence of CVD in CKD patients have been reported among different ethnicities [9, 35, 36]. Given the paucity of research on the correlation between the FRS and kidney function progression in CKD patients, further studies are needed to confirm our findings in other ethnicities.
Conclusion
This study shows that the FRS was independently associated with the progression of kidney function after adjusting for additional confounders, including markers of kidney function and inflammation biomarkers. The predictive value of the FRS was significantly improved in CKD patients with a GFR ≥ 60 ml/min/1.73 m2. Our study highlights the importance of considering and managing the traditional risk factors for CVD in patients with CKD.
References
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease, and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52.
Bhatraju PK, Zelnick LR, Shlipak M, Katz R, Kestenbaum B. Association of soluble TNFR-1 concentrations with long-term decline in kidney function: the multi-ethnic study of atherosclerosis. J Am Soc Nephrol. 2018;29:2713–21.
Hirata Y, Sugiyama S, Yamamoto E, Matsuzawa Y, Akiyama E, Kusaka H, et al. Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol. 2014;173:481–6.
Zanoli L, Lentini P, Briet M, Castellino P, House AA, London GM, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol. 2019;30:918–28.
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008;105:1652–60.
Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, et al. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. 2015;10:2190–7.
Peralta CA, Jacobs DR Jr., Katz R, Ix JH, Madero M, Duprez DA, et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2012;59:41–9.
Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–41.
Mukai H, Svedberg O, Lindholm B, Dai L, Heimburger O, Barany P, et al. Skin autofluorescence, arterial stiffness and Framingham risk score as predictors of clinical outcome in chronic kidney disease patients: a cohort study. Nephrol Dial Transpl. 2019;34:442–8.
Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1–S115.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53.
Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–35.
Dusing P, Zietzer A, Goody PR, Hosen MR, Kurts C, Nickenig G, et al. Vascular pathologies in chronic kidney disease: pathophysiological mechanisms and novel therapeutic approaches. J Mol Med (Berl). 2021;99:335–48.
Wang L, Huang X, He W, Liu W, Yang J. Digital microvascular reactivity does not decline with impaired renal function in chronic kidney disease. BMC Nephrol. 2019;20:288.
Cerqueira A, Quelhas-Santos J, Sampaio S, Ferreira I, Relvas M, Marques N, et al. Endothelial dysfunction is associated with cerebrovascular events in pre-dialysis CKD patients: A prospective study. Life. 2021;11.
Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117:2467–74.
Brant LC, Hamburg NM, Barreto SM, Benjamin EJ, Ribeiro AL. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort study. J Am Heart Assoc. 2014;3:e001279.
Koo BK, Chung WY, Moon MK. Peripheral arterial endothelial dysfunction predicts future cardiovascular events in diabetic patients with albuminuria: a prospective cohort study. Cardiovasc Diabetol. 2020;19:82.
Yang WI, Park S, Youn JC, Son NH, Lee SH, Kang SM, et al. Augmentation index association with reactive hyperemia as assessed by peripheral arterial tonometry in hypertension. Am J Hypertens. 2011;24:1234–8.
Harada M, Fujii K, Yamada Y, Tsukada W, Tsukada M, Hashimoto K, et al. Relationship between serum uric acid level and vascular injury markers in hemodialysis patients. Int Urol Nephrol. 2020;52:1581–91.
Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 2006;101:545–8.
Schiffrin EL. Circulatory therapeutics: use of antihypertensive agents and their effects on the vasculature. J Cell Mol Med. 2010;14:1018–29.
Li Y, Yang Y, Wang W, Miao H, Zhang L, Cai G, et al. Peripheral arterial stiffness is correlated with intrarenal arteriolosclerosis according to biopsies from patients with kidney disease. Nephrology. 2020;25:371–8.
Lim LS, Ling LH, Cheung CM, Ong PG, Gong L, Tai ES, et al. Relationship of systemic endothelial function and peripheral arterial stiffness with diabetic retinopathy. Br J Ophthalmol. 2015;99:837–41.
Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12.
Tajima E, Sakuma M, Tokoi S, Matsumoto H, Saito F, Watanabe R, et al. The comparison of endothelial function between conduit artery and microvasculature in patients with coronary artery disease. Cardiol J 2020;27:38–46.
Kim ED, Tanaka H, Ballew SH, Sang Y, Heiss G, Coresh J, et al. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2018;72:682–90.
Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–9.
Savant JD, Betoko A, Meyers KE, Mitsnefes M, Flynn JT, Townsend RR, et al. Vascular stiffness in children with chronic kidney disease. Hypertension 2017;69:863–9.
Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J, et al. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 2010;212:345–50.
Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, et al. Association of arterial rigidity with incident kidney disease and kidney function decline: the Health ABC study. Clin J Am Soc Nephrol. 2013;8:424–33.
Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol. 2009;20:2044–53.
Jin B, Bai X, Han L, Liu J, Zhang W, Chen X. Association between kidney function and Framingham global cardiovascular disease risk score: a Chinese longitudinal study. PLoS One. 2014;9:e86082.
Lee C, Yun HR, Joo YS, Lee S, Kim J, Nam KH, et al. Framingham risk score and risk of incident chronic kidney disease: A community-based prospective cohort study. Kidney Res Clin Pr. 2019;38:49–59.
Chen SC, Su HM, Tsai YC, Huang JC, Chang JM, Hwang SJ, et al. Framingham risk score with cardiovascular events in chronic kidney disease. PLoS One. 2013;8:e60008.
Funding
This study was supported by the Natural Science Foundation of China (NSFC) (82170686), the National Key Research and Development (R&D) Program of China (2018YFA0108803), and the Science and Technology Project of Beijing, China (D181100000118004).
Author information
Authors and Affiliations
Contributions
GY. Cai and YS. Li contributed to the research idea and study design; YS. Li, XR. Guo and S. Liang collected the data; YS. Li and P. Chen performed the statistical analyses; YS. Li, XR. Guo, Y. Zheng, and P. Li contributed to the interpretation of the results; and GY. Cai, J. Wu and XM. Chen provided supervision or mentorship.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Guo, X., Liang, S. et al. Endothelial function, arterial stiffness and Framingham risk score in chronic kidney disease: A prospective observational cohort study. Hypertens Res 46, 868–878 (2023). https://doi.org/10.1038/s41440-022-01141-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01141-6
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
The importance of lifestyle modification for hypertension in Asia
Hypertension Research (2023)
-
Era of biomarker-based disease risk management
Hypertension Research (2023)